Abstract
The global increase in the production of domestic farmed fish in open net pens has created concerns about the resilience of wild populations owing to shifts in host–parasite systems in coastal ecosystems. However, little is known about the effects of increased parasite abundance on life-history traits in wild fish populations. Here, we report the results of two separate studies in which 379 779 hatchery-reared Atlantic salmon smolts were treated (or not) against salmon lice, marked and released. Adults were later recaptured, and we specifically tested whether the age distribution of the returning spawners was affected by the treatment. The estimates of parasite-induced mortality were 31.9% and 0.6% in the River Vosso and River Dale stock experiments, respectively. Age of returning salmon was on average higher in treated versus untreated fish. The percentages of fish returning after one winter at sea were 37.5% and 29.9% for the treated and untreated groups, respectively. We conclude that salmon lice increase the age of returning salmon, either by affecting their age at maturity or by disproportionately increasing mortality in fish that mature early.
Keywords: age-at-maturation, sea lice, salmon, life-history traits
1. Introduction
One of the best-known and widely studied host–parasite systems among aquatic vertebrates is that of salmon and salmon lice. Atlantic salmon (Salmo salar L.) are naturally infected with salmon lice (Lepeophtheirus salmonis Krøyer, 1837) as they migrate from their natal river to the ocean to feed [1]. The production of domesticated farmed salmon in open net pens along the coast has led to an unnatural increase in the local host abundance [2]. Salmon lice have been linked to declines in wild salmon populations and have created concerns about the resilience of salmonids to the expanding aquaculture industry [3]. Increased mortality owing to salmon lice has been documented by recent studies comparing adult returns from released hatchery-reared juvenile salmon (smolts) that had been either treated or not treated against salmon lice [4,5].
Hosts employ a variety of strategies to reduce the harm caused by parasites, such as avoidance and immune responses. However, an alternate strategy is the alteration of important life-history traits, for example age at maturation [6,7]. In theory, hosts should alter their age at maturity depending on which life stage the parasite affects, the virulence of the parasite and the general life-history strategy of the host [8]. For example, in a host with indeterminate growth and correlated fecundity and size, for example most fish species, individuals should mature earlier if the parasite-induced effect on the host's probability of survival increases with age [9]. On the other hand, maturation should be delayed if growth is reduced [7]. Moreover, the age distribution of maturing individuals may also be affected by selective mortality. For example, there may be a trade-off between the host's immune response and its growth [10], potentially causing higher parasite-induced mortality among fast-growing and early maturing individuals. However, few studies have applied such theories to a commercially important species in a large-scale ecosystem.
Here, we report the results of two studies in which 379 779 hatchery-reared smolts were divided into groups that did or did not receive treatment against salmon lice and were then marked and released. We specifically examined the possible effects of lice treatment on the age distribution of the returning spawners.
2. Material and methods
From 2001 to 2012, two separate field experiments were conducted within the same fjord in the Vosso and Dale Rivers in western Norway (Vosso 60°64′ N, 5°95′ E; Dale 60°58′ N, 5°78′ E), an area with a large amount of salmonid farming activity [11]. Details regarding the Dale experiment have been reported elsewhere and will not be repeated here [5]. The Vosso smolt production was from eggs from the living gene bank in Eidfjord. The fish were reared through the smolt stage (1 year) in standard hatchery tanks at the Voss hatchery from 2001 to 2010 (except 2004). In 2010, smolts were also reared in a net-pen facility in Lake Evanger. Fish were randomly assigned to groups 10–14 days before release. The groups received either normal fish feed (untreated) or pellets containing emamectin benzoate (SLICE) (treated) at 50 µg kg−1 body weight day−1 for 8 days. The fish were sampled randomly and were weighed to confirm that there was no bias in the sorting of the fish. In 2005, Substance EX (Pharmaq, Norway) was applied (0.5 h bath at a concentration of 2 ppm) instead of SLICE in both rivers, and intraperitoneal injections of emamectin benzoate were administered in Dale from 2007 to 2010 [12]. All of the fish were marked according to their group using coded-wire tags after being anaesthetized with benzocaine or MS222.
The return rates from the fish released in both rivers were poor. Consequently, in addition to being released into the river, the smolts were also towed to various locations along the fjord migration route in the inner and outer fjord systems (30–50 and 100–120 km, respectively, from the river mouth; see electronic supplementary material) and were released. The release occurred during the period from 8 May to 5 June. Because the Vosso salmon were protected against fishing during the study period, they were primarily recaptured by operating large bag nets at two to three locations in the fjord.
For the purpose of this analysis, the data from each year were pooled to avoid dependency between releases within years (complete data set available in the electronic supplementary material). To test the effects of the lice treatment on fish survival, a paired t-test was used to analyse the natural log-transformed [4] paired recapture estimates of the control and treatment groups for each river. The parasite-induced mortality was calculated as 1 − exp(−estimated difference in log survival) [4]. The effects of the lice treatment on maturation and weight were analysed with a generalized linear mixed model (GLMM) [13]. To analyse maturation, we used a binomial error distribution to model the numbers of spawners returning after one winter (1SW) versus two winters (2SW). The effect of treatment on the weight of the 1SW group was tested using a Gaussian distribution. Year and river were considered random factors when determining the variation within each variable. All of the analyses were performed using R v. 2.15.2.
3. Results
In the Vosso experiment, the log-transformed survival was higher in the treated fish compared with the untreated fish (t8 = 2.53, p = 0.035), whereas no significant difference was observed in the Dale experiment (t9 = 0.03, p = 0.98). The parasite-induced mortality was estimated to be 31.9% (95% CI 3.4–52.0%) in Vosso and 0.6% (95% CI −50.5–34.3%) in Dale. In the Vosso experiment, more treated fish than untreated fish were recaptured every year except 2009 (table 1). The number of recaptures during 2009 amounted to 38.3% of all of the recaptures during the study period, which may be partly explained by the high marine survival rate throughout the northeast Atlantic that year [14].
Table 1.
Overview of releases and recaptures. Recaptures of untreated and treated individuals are specified for one sea-winter (1SW), two sea-winters (2SW), more than two sea-winters (3SW+) and per cent overall survival. Year signifies year of release (n.a. signifies not available).
| river | year | smolts released |
recaptures |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| untreated | treated | 1SW |
2SW |
3SW+ |
survival (%) |
||||||
| Dale | 2001 | 2294 | 6302 | 11 | 29 | 12 | 15 | 2 | 3 | 1.09 | 0.75 |
| Dale | 2002 | 5109 | 5362 | 45 | 62 | 8 | 20 | 0 | 8 | 1.04 | 1.68 |
| Dale | 2003 | 5600 | 5617 | 7 | 12 | 13 | 12 | 1 | 2 | 0.38 | 0.46 |
| Dale | 2004 | 5466 | 5501 | 11 | 21 | 25 | 16 | 5 | 1 | 0.75 | 0.69 |
| Dale | 2005 | 5242 | 5240 | 2 | 0 | 0 | 1 | 1 | 0 | 0.06 | 0.02 |
| Dale | 2006 | 6156 | 6143 | 4 | 1 | 11 | 7 | 7 | 5 | 0.36 | 0.21 |
| Dale | 2007 | 9700 | 9610 | 2 | 9 | 4 | 13 | 4 | 5 | 0.10 | 0.28 |
| Dale | 2008 | 7750 | 7745 | 9 | 7 | 22 | 27 | 10 | 10 | 0.53 | 0.57 |
| Dale | 2009 | 13 440 | 13 410 | 25 | 35 | 142 | 141 | 25 | 26 | 1.43 | 1.51 |
| Dale | 2010 | 8165 | 8115 | 23 | 36 | 57 | 74 | n.a. | n.a. | 0.98 | 1.36 |
| Vosso | 2001 | 8400 | 8675 | 2 | 2 | 1 | 2 | 0 | 1 | 0.04 | 0.06 |
| Vosso | 2002 | 14 100 | 14 100 | 8 | 10 | 2 | 5 | 2 | 2 | 0.09 | 0.12 |
| Vosso | 2003 | 11 625 | 11 950 | 6 | 33 | 5 | 23 | 2 | 3 | 0.11 | 0.49 |
| Vosso | 2005 | 14 103 | 14 834 | 1 | 3 | 12 | 24 | 9 | 4 | 0.16 | 0.21 |
| Vosso | 2006 | 9750 | 9750 | 0 | 0 | 1 | 4 | 2 | 1 | 0.03 | 0.05 |
| Vosso | 2007 | 14 400 | 15 130 | 3 | 9 | 27 | 26 | 17 | 22 | 0.33 | 0.38 |
| Vosso | 2008 | 14 948 | 15 197 | 4 | 10 | 31 | 37 | 46 | 55 | 0.54 | 0.67 |
| Vosso | 2009 | 12 850 | 13 000 | 21 | 25 | 242 | 225 | 127 | 104 | 3.04 | 2.72 |
| Vosso | 2010 | 17 500 | 17 500 | 57 | 89 | 93 | 76 | n.a. | n.a. | 0.86 | 0.94 |
The percentage of fish that returned after one winter at sea was significantly higher in the treated group (37.5%) than the untreated group (29.9%) (GLMM, treatment, z = 3.21, n = 38, p < 0.01; figure 1). Year explained a large proportion of the variance with model estimates ranging from 10.6 to 73.1% (untreated). There were no significant differences between the weights of the treated and untreated 1SW fish (GLMM, 2.07 versus 2.01 kg, 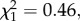 p > 0.05).
p > 0.05).
Figure 1.
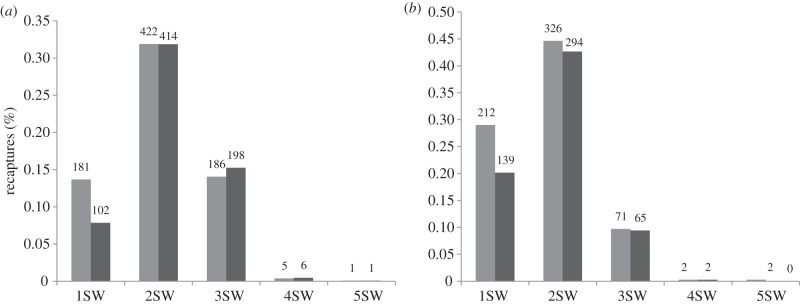
Bar plot of the per cent recapture of treated (light grey) and untreated (dark grey) salmon, divided into sea-winters (SW), for the (a) Vosso and (b) Dale experiments. The number of recaptures is indicated above the bars.
4. Discussion
This study demonstrates that protection against salmon lice during the first weeks at sea can shift the age distribution of the returning spawners toward 1SW salmon. This is the first documentation of an effect of salmon lice on the age of returning salmon in a long-term field study.
Life-history theory predicts that for a host with indeterminate growth and correlated fecundity and size, individuals should mature earlier if the parasite-induced effect on the host's probability of survival increases with age [9]. However, salmon lice are not likely to affect the host's probability of survival more in older age classes because the growth of the parasite is determinate and the rates of infection by new parasites in the open ocean are low [15]. This study suggests that salmon lice infestation increases the age of the returning salmon, either by increased age at maturity or by a reduction in the survival of individuals that mature early. For example, individuals that grow more rapidly may have reduced immune defences [10], creating a higher likelihood of succumbing to salmon lice infections. These two hypotheses are not mutually exclusive.
Life-history models predict that age at maturity should be delayed when growth conditions become less favourable [7], which has been supported by experimental studies of Atlantic salmon [16]. Whereas mortality is thought to occur when the infection of salmon smolts exceeds approximately 10 lice per fish (or 0.3 lice g−1) [17], stress-related responses have been documented in salmon smolts infected with as few as three lice [18]. The observed shift in the age distribution of the returning salmon may therefore be coupled with behavioural or endocrine-induced changes in energy acquisition that affect the timing of maturity [19]. This theory is consistent with observations that salmon lice may reduce the growth of salmonids [20] and with the clear negative relationship between growth and mean age at return exhibited by local wild salmon populations during different years of the experimental period [5].
Krkošek et al. [4] conducted a meta-analysis of five release studies and concluded that the overall parasite-induced mortality was 39%. This finding is similar to our estimated results from the Vosso experiment. However, their results were based on data that included 0.89% individuals maturing after more than one winter at sea (multiple-sea-winter, MSW). By contrast, the present dataset consists of 75.6% MSW and suggests that salmon lice can also affect the age of fish at maturity. This finding may have consequences for the interpretation of mortality estimates based on 1SW fish and may also bias results if catch efforts vary between years. Furthermore, our finding may have consequences for studies that use the proportion of 1SW fish as a proxy for feeding conditions in the ocean [21].
Acknowledgements
We thank G.O. Henden and the staff at Voss and Dale hatchery for all their work during this study.
Funding statement
This work was partly funded by the Norwegian Directorate for Nature Management, the Hordaland County Council, BKK, FHF, the Norwegian Research Council and the Institute of Marine Research.
References
- 1.Tully O, Nolan DT. 2002. A review of the population biology and host–parasite interactions of the sea louse Lepeophtheirus salmonis (Copepoda: Caligidae). Parasitology 124, S165–S182 (doi:10.1017/S0031182002001889) [DOI] [PubMed] [Google Scholar]
- 2.Jansen PA, Kristoffersen AB, Viljugrein H, Jimenez D, Aldrin M, Stien A. 2012. Sea lice as a density-dependent constraint to salmonid farming. Proc. R. Soc. B 279, 2330–2338 (doi:10.1098/rspb.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello MJ. 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 22, 475–483 (doi:10.1016/JPt.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 4.Krkošek M, Revie CW, Gargan PG, Skilbrei OT, Finstad B, Todd CD. 2013. Impact of parasites on salmon recruitment in the Northeast Atlantic Ocean. Proc. R. Soc. B 280, 20122359 (doi:10.1098/rspb.2012.2359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skilbrei OT, Finstad B, Urdal K, Bakke G, Kroglund F, Strand R. 2013. Impact of early salmon louse, Lepeophtheirus salmonis, infestation and differences in survival and marine growth of sea-ranched Atlantic salmon, Salmo salar L, smolts 19972009. J. Fish Dis. 36, 249–260 (doi:10.1111/Jfd.12052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochberg ME, Michalakis Y, Demeeus T. 1992. Parasitism as a constraint on the rate of life-history evolution. J. Evol. Biol. 5, 491–504 (doi:10.1046/j.1420-9101.1992.5030491.x) [Google Scholar]
- 7.Stearns SC, Koella JC. 1986. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40, 893–913 (doi:10.2307/2408752) [DOI] [PubMed] [Google Scholar]
- 8.Michalakis Y, Hochberg ME. 1994. Parasitic effects on host life-history traits: a review of recent studies. Parasite 1, 291–294 [DOI] [PubMed] [Google Scholar]
- 9.Agnew P, Koella JC, Michalakis Y. 2000. Host life history responses to parasitism. Microbes Infect. 2, 891–896 (doi:10.1016/S1286-4579(00)00389-0) [DOI] [PubMed] [Google Scholar]
- 10.van der Most PJ, de Jong B, Parmentier HK, Verhulst S. 2011. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct. Ecol. 25, 74–80 (doi:10.1111/j.1365-2435.2010.01800.x) [Google Scholar]
- 11.Skilbrei OT, Wennevik V. 2006. The use of catch statistics to monitor the abundance of escaped farmed Atlantic salmon and rainbow trout in the sea. ICES J. Mar. Sci. 63, 1190–1200 (doi:10.1016/j.icesjms.2006.05.005) [Google Scholar]
- 12.Glover KA, Samuelsen OB, Skilbrei OT, Boxaspen K, Lunestad BT. 2010. Pharmacokinetics of emamectin benzoate administered to Atlantic salmon, Salmo salar L, by intra-peritoneal injection. J. Fish Dis. 33, 183–186 (doi:10.1111/j.1365-2761.2009.01099.x) [DOI] [PubMed] [Google Scholar]
- 13.Bates D, Maechler M, Bolker B.2011. lme4: linear mixed-effects models using S4 classes. R Package v. 0.999375-42. See http://CRANR-project.org/package=lme4 .
- 14.ICES 2013. Report of the working group on North Atlantic Salmon (WGNAS), 3–12 April 2013. Copenhagen, Denmark: ICES CM 2013/ACOM:09, p. 380 [Google Scholar]
- 15.Jacobsen JA, Gaard E. 1997. Open-ocean infestation by salmon lice (Lepeophtheirus salmonis): Comparison of wild and escaped farmed Atlantic salmon (Salmo salar L.). ICES J. Mar. Sci. 54, 1113–1119 (doi:10.1006/jmsc.1997.0288) [Google Scholar]
- 16.Taranger GL, et al. 2010. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 165, 483–515 (doi:10.1016/j.ygcen.2009.05.004) [DOI] [PubMed] [Google Scholar]
- 17.Holst JC, Jakobsen P, Nilsen F, Holm M, Asplin L, Aure J. 2003. Mortality of seaward-migrating post-smolts of Atlantic salmon due to salmon lice infection in Norwegian salmon stocks. In Salmon at the edge (ed. Mills D.), pp. 136–137 Oxford, UK: Blackwell Publishing [Google Scholar]
- 18.Nolan DT, Reilly P, Bonga SEW. 1999. Infection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in postsmolt Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 56, 947–959 (doi:10.1139/cjfas-56-6-947) [Google Scholar]
- 19.Thorpe JE, Mangel M, Metcalfe NB, Huntingford FA. 1998. Modelling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evol. Ecol. 12, 581–599 (doi:10.1023/A:1022351814644) [Google Scholar]
- 20.Skilbrei OT, Wennevik V. 2006. Survival and growth of sea-ranched Atlantic salmon, Salmo salar L, treated against sea lice before release. ICES J. Mar. Sci. 63, 1317–1325 (doi:10.1016/j.icesjms.2006.04.012) [Google Scholar]
- 21.Otero J, Jensen AJ, L'Abee-Lund JH, Stenseth NC, Storvik GO, Vøllestad LA. 2012. Contemporary ocean warming and freshwater conditions are related to later sea age at maturity in Atlantic salmon spawning in Norwegian rivers. Ecol. Evol. 2, 2192–2203 (doi:10.1002/Ece3.337) [DOI] [PMC free article] [PubMed] [Google Scholar]


