Abstract
Incubation temperature influences a suite of traits in avian offspring. However, the mechanisms underlying expression of these phenotypes are unknown. Given the importance of thyroid hormones in orchestrating developmental processes, we hypothesized that they may act as an upstream mechanism mediating the effects of temperature on hatchling phenotypic traits such as growth and thermoregulation. We found that plasma T3, but not T4 concentrations, differed among newly hatched wood ducks (Aix sponsa) from different embryonic incubation temperatures. T4 at hatching correlated with time spent hatching, and T3 correlated with hatchling body condition, tarsus length, time spent hatching and incubation period. In addition, the T3 : T4 ratio differed among incubation temperatures at hatch. Our findings are consistent with the hypothesis that incubation temperature modulates plasma thyroid hormones which in turn influences multiple aspects of duckling phenotype.
Keywords: maternal effects, parental care, triiodothyronine
1. Introduction
The early developmental environment is important in shaping offspring phenotype, and parents have tremendous influence over this environment. In birds, incubation temperature, which is largely influenced by parental behaviour, is an important determinant of phenotype in precocial [1–8] and altricial [9] species, affecting many avian hatchling characteristics that have implications for the offspring's ability to survive and reproduce. For example, in wood ducks (Aix sponsa) artificial incubation at variable, naturally occurring temperatures influenced plasma corticosterone concentration, locomotor performance, thermoregulation, energy expenditure, immune function, growth and survival of ducklings [1–4,7]. In general, performance is lower in ducklings incubated at low temperatures. However, a mechanism responsible for temperature-dependent effects on avian offspring phenotype has not been determined.
One potential mechanism behind the temperature-dependent effects on offspring phenotype is altered thyroid hormone production and/or function. Thyroid hormones are critical for early development in vertebrates [10]. Thyroxine (T4), the primary secretory product of the thyroid gland, is converted to 3,5,3′-triiodothyronine (T3) by 5′-monodeiodinases in target tissues; T3 has 10–15 times the biological activity of T4 [10,11]. In precocial birds, plasma thyroid hormone concentrations increase dramatically during late stages of incubation and are thought to be important for embryonic growth, tissue growth and differentiation, organ maturation and hatching [10,12]. Thyroid hormones continue to facilitate development post-hatch, influencing the critical transition to homeothermy, and stimulating immune cell proliferation, feeding and metabolism [10,11]. As thyroid hormones are integral to the above processes, many of which are influenced by incubation temperature [1–4,8], we hypothesized that the phenotypic effects of embryonic incubation temperature are mediated in part by thyroid hormones. Thus, we predicted that (i) ecologically relevant variation in incubation temperature would positively correlate with thyroid hormone concentrations of nestlings and (ii) thyroid hormones would positively correlate with nestling mass, body condition and incubation period, but negatively correlate with tarsus length because of protracted development [2,4]. To test our hypothesis, we incubated wood duck eggs at three, ecologically relevant temperatures, which have been shown to produce different phenotypic outcomes in this species [3]. We then measured hatchling body size and circulating plasma concentrations of T3 and T4 on days 0, 4 and 10 post-hatch.
2. Material and methods
(a). Study species
Wood ducks are an abundant dabbling duck that readily use nest-boxes [13]. Their egg-laying season begins in February and continues until mid-July. Average clutch size is 12 eggs [13]. Minimum, maximum and average temperatures of naturally incubated wood duck nests at our study site are 34.98°C, 38.70°C and 36.79°C, respectively (G. R. Hepp 2008, unpublished data).
(b). Egg collection and incubation
We monitored 92 nest-boxes on 12 isolated wetlands in SC, USA. We collected freshly laid eggs daily and incubated them at mean temperatures of 35.0°C, 35.9°C or 37.0°C in incubators (Grumbach, Germany) with two decreases in temperature per day to mimic females' recesses for foraging [6]. These three temperatures produced incubation periods of 37, 35 and 31 days, respectively [4]. Eggs were randomly assigned to an incubation temperature; for more details on IACUC-approved egg collection and incubation protocols, see the electronic supplementary materials and [1,2,4].
(c). Duckling hatching and husbandry
We monitored eggs for pipping and hatching approximately hourly between 06.00 and 21.00 h, to calculate the amount of time needed for a duckling to complete the hatching process (i.e. hatch duration), a vulnerable developmental window that could be influenced by thyroid hormones. Only ducklings of known incubation length were included in this study. At hatching, we measured duckling body weight (g), tarsus length (mm) and culmen length (mm), and calculated body condition of ducklings as the residual of body weight regressed against tarsus. Ducklings were housed in groups of two to three according to previous methods ([1,2,4]; and see the electronic supplementary material).
(d). Blood collection
We collected blood samples following previous methods [1] from each duckling to quantify plasma T3 and T4 concentration at 0, 4 and 10 days post-hatch. Sampling on day 0 occurred within 0.25 and 4.70 h of hatching. Sampling on days 4 and 10 occurred between 13.00 and 18.00 h.
(e). Analysis of plasma T3 and T4 by radioimmunoassay
Plasma concentrations of total T3 and T4 were measured by radioimmunoassays (RIAs) following previous methods [14] with minor modifications. Primary antiserums were purchased from AbCam (anti-T3, ab30803; anti-T4, ab30833-200) and high specific activity tracers from Perkin-Elmer ([125]T3 NEX110X; [125]T4, NEX111X). All samples were run in single RIAs for each hormone, and intraassay variation was 3.6% for T3 and 5.9% for T4. Analysis of three plasma pools demonstrated that wood duck plasma produced dilution curves that were parallel to the standards. Plasma samples were run in duplicate at two dilutions in each assay.
(f). Statistics
To avoid pseudoreplication and account for parental effects, we only included one randomly selected duckling per female per incubation temperature (n = 14–20 ducklings/incubation temperature/age); the remaining ducklings were used in other experiments. All statistical analyses were run in SAS v.9.1 (SAS Institute Inc., Cary, NC, USA) or Microsoft Excel and statistical significance was recognized at α < 0.05.
We tested for differences in mass, tarsus length and body condition (residuals of mass versus tarsus) at hatching among incubation temperatures using three ANCOVAs (egg mass and lay sequence were covariates). Models contained incubation temperature, hatchling sex and egg storage time (i.e. prior to incubation) as main effects. We also ran simplified models only containing incubation temperature (main effect) and egg mass (covariate). We examined the influence of incubation temperature on plasma T3, T4 and the T3 : T4 ratio (all log10-transformed) of ducklings using three repeated measures ANOVAs (SAS proc mixed; repeated factor = age). Satterthwaite's approximation was used in order to calculate degrees of freedom in the T3 and T4 models to account for mild violations of homoscedasticity. We ran models including duckling sex (main effect), age (repeated factor), lay sequence (covariate), cage mate (random effect), time from hatching to sampling on Day 0 (covariate) and egg storage time (main effect) in the models, as well as simplified models only containing age and incubation temperature. We also regressed plasma T3, T4 and T3 : T4 measured on day 0 with measures of body size (body weight and tarsus length), body condition at hatching, hatch duration, incubation period and the time after hatching that blood samples were collected. For details on hatching success and incubation period for eggs incubated in 2011, see [4].
3. Results
Incubation temperature, sex, lay sequence and egg storage time did not affect duckling body mass at hatching (in all cases p ≥ 0.38). However, both tarsus length and body condition at hatching varied with incubation temperature in the directions we predicted (tarsus (mm): F2,42 = 8.3, p < 0.001; LSMeans: 35.0°C = 18.9, 35.9°C = 18.8, 37.0°C = 17.9; body condition: F2,42 = 4.1, p = 0.024; LSMeans: 35.0°C = −0.87, 35.9°C = −0.01, 37.0°C = 1.09), but were not influenced by any of the other variables (in all cases p ≥ 0.44). As egg mass increased so did duckling body mass (F2,42 = 125, p < 0.001), tarsus length (F2,42 = 33.2, p < 0.001) and body condition (F2,42 = 37.5, p < 0.001) at hatching. For mass, tarsus and body condition, the simple models only containing incubation temperature and egg mass produced similar statistical outcomes.
Mean plasma T4 concentration decreased with age for all incubation temperature treatments (figure 1a; age: F2,73.4 = 25.12, p < 0.001). However, there was no influence of incubation temperature on plasma T4 concentration at any age (incubation temperature: F2,52.3 = 0.96, p = 0.39; incubation temperature × age: F4,75.4 = 0.61, p = 0.66). Similar to T4, plasma T3 concentrations decreased with age in all treatments (figure 1b: age: F2,75.5 = 163.52, p < 0.001). In addition, there was a significant age by incubation temperature interaction (F4,78.4 = 5.43, p < 0.001), in which ducklings from the lowest incubation temperature had significantly lower plasma T3 concentration on day 0 than ducklings from the higher incubation temperatures (post hoc slice by age: day 0, p < 0.001; day 4, p = 0.08; day 10, p = 0.94). However, by day 4, plasma T3 concentrations were similar among incubation temperatures. There was no effect of lay sequence, sex or egg storage time on T4 concentrations (in all cases p ≥ 0.58) or T3 (in all cases p ≥ 0.09), and models not containing these factors or cage mate yielded similar results (T4: age, p < 0.001; incubation temperature, p = 0.40; incubation temperature × age, p = 0.63; T3: age, p < 0.001; incubation temperature, p = 0.004; age × incubation temperature, p < 0.001).
Figure 1.
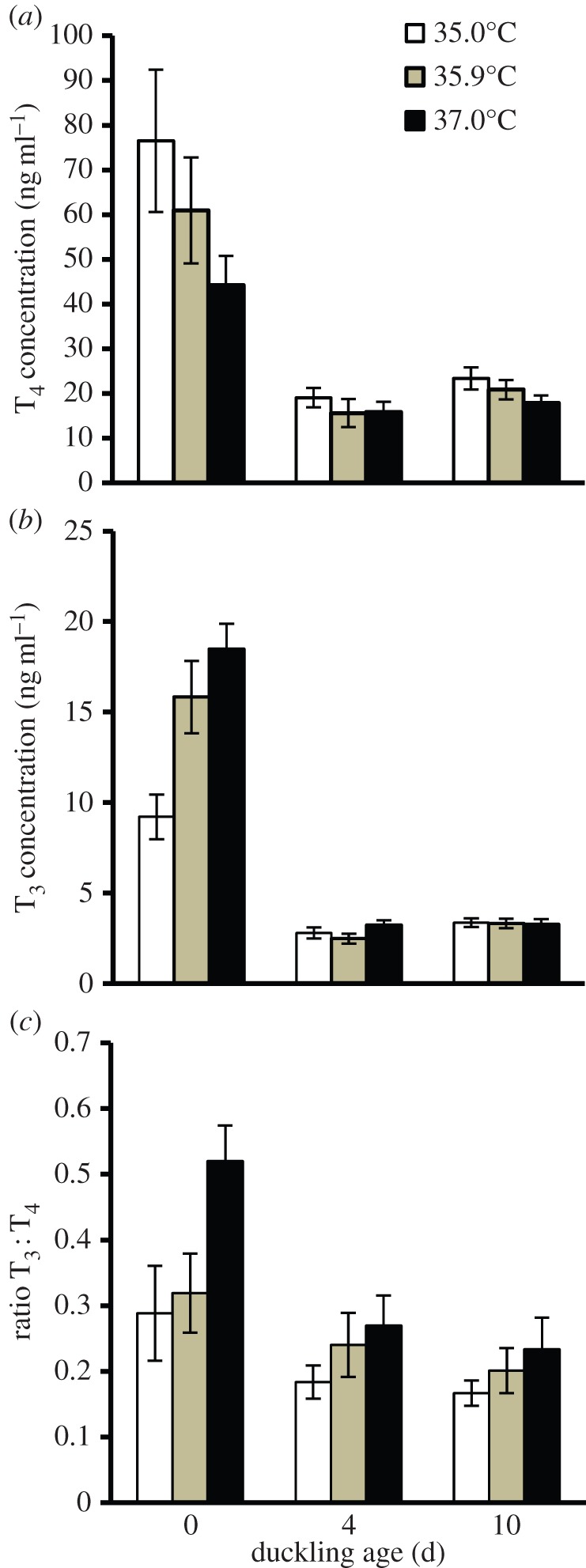
The relationship between incubation period and (a) plasma T4 concentration, (b) plasma T3 concentration and (c) plasma T3 : T4 ratio of ducklings sampled on days 0, 4 and 10 post-hatch. Eggs were incubated at either 35.0°C, 35.9°C or 37.0°C. n = 14–20 ducklings/age/incubation temperature. Error bars are ±1 s.e.m.
For all incubation temperatures, the T3 : T4 ratio was the highest at hatching and then decreased approximately 25–55% by days 4 and 10 (age: F2,68.3 = 8.81, p < 0.001). Ducklings from the lowest incubation temperature had 46% lower ratios than ducklings from the high incubation temperature, but ratios were similar by day 4 (incubation temperature: F2,44.9 = 5.71, p = 0.006; incubation temperature × age F4,69.9 = 0.83, p = 0.51; post hoc slice by age: day 0, p = 0.004; day 4, p = 0.18; day 10, p = 0.55). There was no effect of lay sequence, sex or egg storage time on ratios (in all cases p ≥ 0.35), and models not containing these factors or cage mate yielded similar results (age, p < 0.001; incubation temperature, p = 0.004; incubation temperature × age, p = 0.67).
At hatching, we did not detect a relationship between incubation period and plasma T4 concentration (figure 2a; r2 = 0.01, p = 0.46), but we did detect a strong negative correlation between incubation period and plasma T3 concentration (figure 2b: r2 = 0.39, y = −0.055x + 3.00, p < 0.001) and a mild negative correlation with T3 : T4 (r2 = 0.10, y = −4.162x + 35.8, p = 0.056). T3 : T4 ratios did not correlate with any other variable (for all variables r2 ≤ 0.04, p ≥ 0.21). The only significant correlation detected with T4 at hatching was a negative relationship with hatch duration (r2 = 0.14, y = −0.007x + 1.98, p = 0.007; for all other variables r2 ≤ 0.06, p ≥ 0.10). By contrast, plasma T3 at hatching negatively correlated with hatch duration (r2 = 0.14, y = −0.006x + 1.32, p = 0.012) and tarsus length (r2 = 0.11, y = −0.091x + 2.78, p = 0.031), and positively correlated with body condition (r2 = 0.13, y = 0.044x + 1.07, p = 0.017). We found no relationship between plasma T3 and duckling body weight (in both cases r2 ≤ 0.03, p ≥ 0.24).
Figure 2.
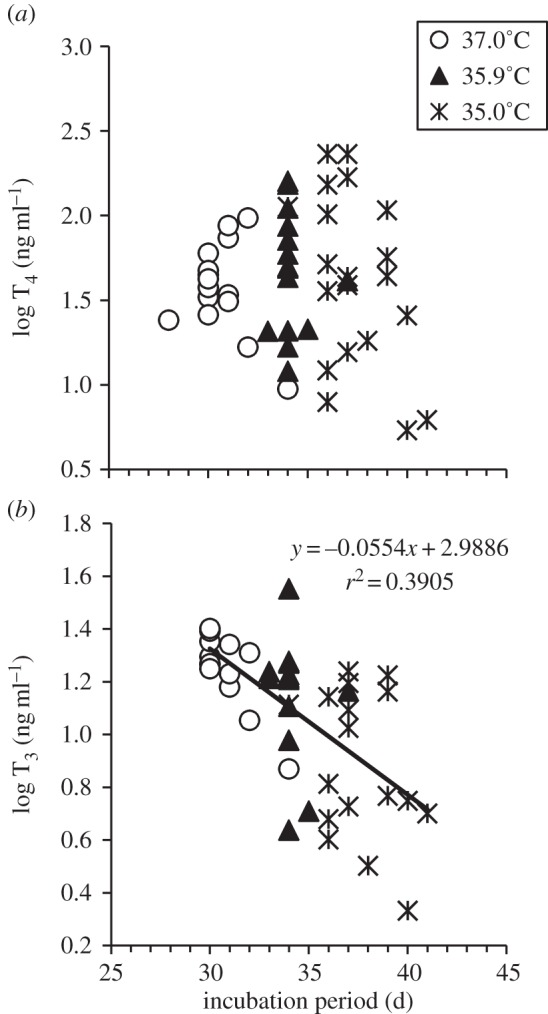
The relationship between incubation period and (a) plasma T4 concentration and (b) plasma T3 concentration of ducklings sampled within 0.25–4.70 h of hatching. Eggs were incubated at either 35.0°C, 35.9°C or 37.0°C. n = 14–20 ducklings/incubation temperature.
4. Discussion
We demonstrated that biologically relevant differences in incubation temperature affect plasma thyroid hormone concentrations of bird hatchlings from a natural population. Plasma thyroid hormone concentrations in ducklings from all incubation temperatures were within the concentrations measured for other precocial bird hatchlings [9,10,15]. We found that ducklings from the lowest incubation temperature had reduced plasma T3 concentrations and lower T3 : T4 ratios at day 0 compared with ducklings from the higher incubation temperatures (figure 1). In addition, plasma T3 at hatching was negatively correlated with incubation period (figure 2b), which is strongly influenced by incubation temperature [4]. As the bird thyroid gland secretes very little T3 [11,16], our findings suggest that the cool incubation temperatures could be reducing deiodination of T4 into T3, perhaps through reduced expression of liver deiodinases which increase rapidly around the time of hatching [11]. Additional possibilities for altered T3 may include differences in hormone clearance or degradation, or differential abundance of T3 binding proteins (e.g. transthyretin) that influence T3 degradation [11].
Reductions in T3, the more biologically active thyroid hormone, could be influencing many other phenotypic traits of ducklings from the low incubation temperature which might explain their reduced physiological performance (e.g. slower growth and poor thermoregulatory ability) relative to ducklings from higher incubation temperatures. Consistent with this hypothesis, ducklings with low T3 hatched later with larger structural size, but in lower body condition, than their counterparts from the high incubation temperature. This suite of effects is attributable to the more protracted growth period associated with slower development at low temperatures [2,4]. In addition, some of the effects of incubation temperature on phenotype appear to attenuate with age [1,7], which could correspond with the convergence of duckling plasma T3 concentration among incubation temperatures by day 4. Other studies also support the importance of incubation temperature for thyroid expression, although the direction of effects can differ across species [9,17]. For example, lower incubation temperatures caused an increase in plasma T4 of hatchling snapping turtles incubated at different temperatures [17]. Studies in poultry reveal that exposing embryos to higher incubation temperatures on specific days during incubation when the thyroid and adrenal axes are developing reduced plasma T3 and T4 of hatchlings (e.g. [9]). Although not measured in this study, covariations in other hormones (e.g. testosterone) could also influence embryonic development and hatchling phenotype (e.g. [18]).
Our findings suggest that changes in plasma thyroid hormone concentration may serve as an upstream mechanism for the effects of incubation temperature on traits like thermoregulation, metabolism, growth and immune responses. Ultimately, incubation temperature may modulate gene expression during development of the thyroid axis, including deiodinase expression or enzyme activity. Future research on gene expression, administration of exogenous thyroid hormones, and/or blocking of thyroid hormone production will help elucidate potential causal relationships between incubation temperature, thyroid hormones and expression of variable phenotypes in birds.
Acknowledgements
We thank the students and technicians who assisted on this project.
We followed an approved IACUC proposal (11-056-FIW), and eggs were collected under SCDNR permit G-08-07 and USFWS permit MB748024-0.
Data accessibility
Data can be accessed at http://dx.doi.org/10.5061/dryad.hb87k [19].
Funding statement
Funding came from NSF grants IOB-0615361 to G.R.H. and W.A.H., NSF grant IOS 0922583 to R.J.D. and DDIG (DEB-1110386) to S.E.D. and W.A.H.
References
- 1.DuRant SE, Hepp GR, Moore IT, Hopkins BC, Hopkins WA. 2010. Slight differences in incubation temperature affect early growth and stress endocrinology of wood duck (Aix sponsa) ducklings. J. Exp. Biol. 213, 45–51 (doi:10.1242/jeb.034488) [DOI] [PubMed] [Google Scholar]
- 2.DuRant SE, Hopkins WA, Hepp GR. 2011. Energy expenditure of developing wood duck (Aix sponsa) embryos is related to incubation temperature. Physiol. Biochem. Zool. 84, 451–457 (doi:10.1086/661749) [DOI] [PubMed] [Google Scholar]
- 3.DuRant SE, Hopkins WA, Hepp GR, Walters JR. 2013. Ecological, evolutionary, and conservation implications of incubation-temperature-dependent phenotypes in birds. Biol. Rev. 88, 499–509 (doi:10.1111/brv.12015) [DOI] [PubMed] [Google Scholar]
- 4.DuRant SE, Hopkins WA, Carter AW, Stachowiak CM, Hepp GR. 2014. Incubation conditions are more important in determining early thermoregulatory ability than post-hatch resource conditions in a precocial bird. Physiol. Biochem. Zool. 86, 410–420 (doi:10.1086/671128) [DOI] [PubMed] [Google Scholar]
- 5.Eiby YA, Wilmer JW, Booth DT. 2008. Temperature-dependent sex-biased embryo mortality in a bird. Proc. R. Soc. B 275, 2703–2706 (doi:10.1098/rspb.2008.0954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hepp GR, Kennamer RA, Johnson MH. 2006. Maternal effects in wood ducks: incubation temperature influences incubation period and neonate phenotype. Funct. Ecol. 20, 307–314 (doi:10.1111/j.1365-2435.2006.01108.x) [Google Scholar]
- 7.Hopkins BC, DuRant SE, Hepp GR, Hopkins WA. 2011. Incubation temperature influences locomotor performance in young wood ducks (Aix sponsa). J. Exp. Zool. 315, 274–279 (doi:10.1002/jez.673) [DOI] [PubMed] [Google Scholar]
- 8.Nord A, Nilsson JA. 2011. Incubation temperature affects growth and energy metabolism in blue tit nestlings. Am. Nat. 5, 639–651 (doi:10.1086/662172) [DOI] [PubMed] [Google Scholar]
- 9.Piestun Y, Halevy O, Shinder D, Ruzal M, Druyan S, Yahav S. 2011. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Therm. Biol. 36, 469–474 (doi:10.1016/j.jtherbio.2011.08.003) [Google Scholar]
- 10.McNabb FMA, Scanes CG, Zeman M. 1998. Endocrine control of development. In Avian growth and development (eds Starcks JM, Rickefs RE.), pp. 174–202 New York, NY: Oxford University Press [Google Scholar]
- 11.Darras V, Verhoelst C, Reyns G, Kühn E, Van der Geyten S. 2006. Thyroid hormone deiodination in birds. Thyroid 16, 25–35 (doi:10.1089/thy.2006.16.25) [DOI] [PubMed] [Google Scholar]
- 12.Wilson CM, McNabb FMA. 1997. Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. Gen. Comp. Endocr. 107, 153–165 (doi:10.1006/gcen.1997.6906) [DOI] [PubMed] [Google Scholar]
- 13.Bellrose FC, Holm DJ. 1994. Ecology and management of the wood duck. Mechanicsburg, PA: Stackpole Books [Google Scholar]
- 14.Burger MF, Denver RJ. 2002. Plasma thyroid hormone concentrations in a wintering passerine bird: Their relationship to geographic variation, environmental factors, metabolic rate and body fat. Physiol. Biochem. Zool. 75, 187–199 (doi:10.1086/338955) [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Medan MS, Watanabe G, Taya K. 2005. Developmental changes of plasma inhibin, gonadotropins, steroid hormones, and thyroid hormones in male and female Shao ducks. Gen. Comp. Endocr. 143, 161–167 (doi:10.1016/j.ygcen.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 16.Decuypere E, Van As P, Van der Geyten S, Darras V. 2005. Thyroid hormone availability and activity in avian species: a review. Domest. Anim. Endocrin. 29, 63–77 (doi:10.1016/j.domaniend.2005.02.028) [DOI] [PubMed] [Google Scholar]
- 17.O'Steen S, Janzen FJ. 1999. Embryonic temperature affects metabolic compensation and thyroid hormones in hatchling snapping turtles. Physiol. Biochem. Zool. 72, 520–533 (doi:10.1086/316690) [DOI] [PubMed] [Google Scholar]
- 18.Eising CM, Müller W, Groothuis TGG. 2006. Avian mothers create different phenotypes by hormone deposition in their eggs. Bio. Lett. 2, 20–22 (doi:10.1098/rsbl.2005.0391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuRant SE, Carter AW, Denver RJ, Hepp GR, Hopkins WA.2013. Data from Are thyroid hormones mediators of incubation temperature-induced phenotypes in birds? Dryad Digital Repository. See http://dx.doi.org/10.5061/dryad.hb87k .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be accessed at http://dx.doi.org/10.5061/dryad.hb87k [19].


