Abstract
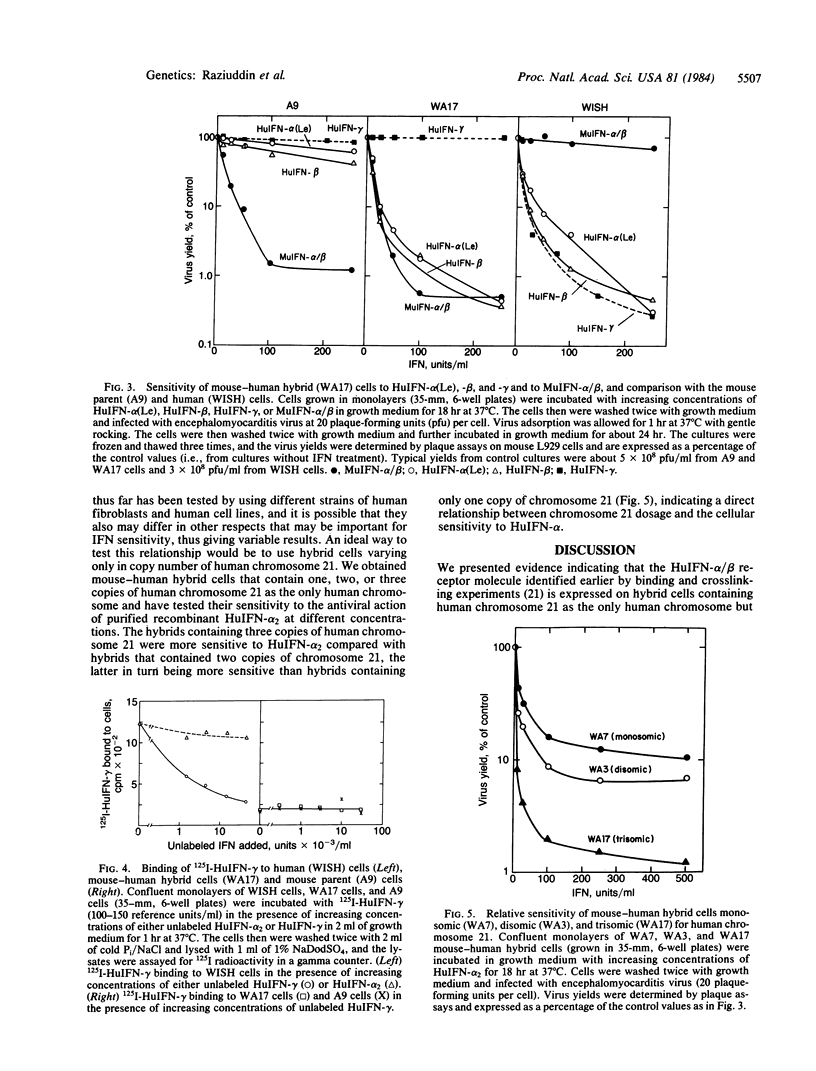
We examined the proposed role of human chromosome 21 in determining the cellular sensitivity to human alpha, beta, and gamma interferons (HuIFN-alpha, -beta, and -gamma) and the expression of the receptors for the HuIFNs with the use of mouse-human hybrid cells containing human chromosome 21. Hybrid cells (WA17) containing three copies of human chromosome 21 showed specific displaceable binding of 125I-labeled HuIFN-alpha 2 (125I-HuIFN-alpha 2), which was not observed with mouse parent (A9) cells. Crosslinking of 125I-HuIFN-alpha 2 bound to WA17 cells with disuccinimidyl suberate yielded a complex of Mr approximately equal to 150,000 similar to the 125I-HuIFN-alpha 2-receptor complex obtained with human cells as described earlier. Such a complex was not obtained with mouse parent (A9) cells or with hybrid cells containing certain other human chromosomes but not chromosome 21. Mice inoculated with mouse-human hybrid cells containing human chromosome 21 produce antibodies that block the antiviral action of HuIFN-alpha and -beta on human cells. Such antibodies could immunoprecipitate the 125I-HuIFN-alpha 2-receptor complex obtained from human cells but not free 125I-HuIFN-alpha 2, indicating that these antibodies were directed against the receptor. WA17 hybrid cells were highly sensitive to the antiviral action of HuIFN-alpha 2, -alpha (Le) and -beta but were completely insensitive to HuIFN-gamma. Furthermore, 125I-HuIFN-gamma showed specific binding to human WISH cells but not to WA17 hybrid cells or A9 mouse cells. The results indicate that the receptors for HuIFN-alpha and -beta but not for HuIFN-gamma are specified by human chromosome 21. Hybrid cells containing one, two, or three copies of human chromosome 21 were found to be increasingly sensitive to HuIFN-alpha 2, indicating that a chromosome 21-specified component (possibly the HuIFN-alpha receptor) may be a limiting factor in the cellular sensitivity to HuIFN-alpha.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Blanchard B. High affinity binding of 125I-Labeled mouse interferon to a specific cell surface receptor. II. Analysis of binding properties. Virology. 1981 Dec;115(2):249–261. doi: 10.1016/0042-6822(81)90108-2. [DOI] [PubMed] [Google Scholar]
- Aguet M., Gröbke M., Dreiding P. Various human interferon alpha subclasses cross-react with common receptors: their binding affinities correlate with their specific biological activities. Virology. 1984 Jan 15;132(1):211–216. doi: 10.1016/0042-6822(84)90105-3. [DOI] [PubMed] [Google Scholar]
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Alhadeff B., Velivasakis M., Siniscalco M. Simultaneous identification of chromatid replication and of human chromosomes in metaphases of man-mouse somatic cell hybrids. (With 1 color plate). Cytogenet Cell Genet. 1977;19(4):236–239. doi: 10.1159/000130814. [DOI] [PubMed] [Google Scholar]
- Anderson P., Yip Y. K., Vilcek J. Specific binding of 125I-human interferon-gamma to high affinity receptors on human fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11301–11304. [PubMed] [Google Scholar]
- Bobrow M., Cross J. Differential staining of human and mouse chromosomes in interspecific cell hybrids. Nature. 1974 Sep 6;251(5470):77–79. doi: 10.1038/251077a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Dutkowski R., Darlington G. J. Localization and quantitation of human superoxide dismutase using computerized 2-D gel electrophoresis. Biochem Biophys Res Commun. 1981 Sep 30;102(2):675–681. doi: 10.1016/s0006-291x(81)80185-4. [DOI] [PubMed] [Google Scholar]
- Chany C., Vignal M., Couillin P., Van Cong N., Boué J., Boué A. Chromosomal localization of human genes governing the interferon-induced antiviral state. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3129–3133. doi: 10.1073/pnas.72.8.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley M., Billiau A. Responsiveness of human cells trisomic for chromosome 21 to the antiviral action of human immune interferon. Antiviral Res. 1982 May;2(1-2):97–102. doi: 10.1016/0166-3542(82)90029-8. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., McManus N. H., Epstein L. B., Branca A. A., D'Alessandro S. B., Baglioni C. Direct evidence that the gene product of the human chromosome 21 locus, IFRC, is the interferon-alpha receptor. Biochem Biophys Res Commun. 1982 Aug;107(3):1060–1066. doi: 10.1016/0006-291x(82)90629-5. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Epstein C. J. Localization fo the gene AVG for the antiviral expression of immune and classical interferon to the distal portion of the long arm of chromosome 21. J Infect Dis. 1976 Jun;133 (Suppl):A56–A62. doi: 10.1093/infdis/133.supplement_2.a56. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Joshi A. R., Sarkar F. H., Gupta S. L. Interferon receptors. Cross-linking of human leukocyte interferon alpha-2 to its receptor on human cells. J Biol Chem. 1982 Dec 10;257(23):13884–13887. [PubMed] [Google Scholar]
- Le J., Prensky W., Yip Y. K., Chang Z., Hoffman T., Stevenson H. C., Balazs I., Sadlik J. R., Vilcek J. Activation of human monocyte cytotoxicity by natural and recombinant immune interferon. J Immunol. 1983 Dec;131(6):2821–2826. [PubMed] [Google Scholar]
- Lubiniecki A. S., Jones V., Eatherly C. Cytogenetic analysis of the sensitivity to anti-viral and anti-cell growth activities of human fibroblast interferon in aneuploid human tumor cell lines. Brief report. Arch Virol. 1979;60(3-4):341–346. doi: 10.1007/BF01317505. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Aguet M., Gressner I. Binding of 125I-labelled human alpha interferon to human lymphoid cells. Int J Cancer. 1981 Nov 15;28(5):575–582. doi: 10.1002/ijc.2910280508. [DOI] [PubMed] [Google Scholar]
- Revel M., Bash D., Ruddle F. H. Antibodies to a cell-surface component coded by human chromosome 21 inhibit action of interferon. Nature. 1976 Mar 11;260(5547):139–141. doi: 10.1038/260139a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Sarkar F. H., Gupta S. L. Receptors for human gamma interferon: binding and crosslinking of 125I-labeled recombinant human gamma interferon to receptors on WISH cells. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5160–5164. doi: 10.1073/pnas.81.16.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slate D. L., Ruddle F. H. Antibodies to chromosome 21 coded cell surface components can block response to human interferon. Cytogenet Cell Genet. 1978;22(1-6):265–269. doi: 10.1159/000130951. [DOI] [PubMed] [Google Scholar]
- Slate D. L., Ruddle F. H. Sensitivity of human-mouse somatic cell hybrids to human interferon. Cytogenet Cell Genet. 1978;22(1-6):270–274. doi: 10.1159/000130952. [DOI] [PubMed] [Google Scholar]
- Slate D. L., Shulman L., Lawrence J. B., Revel M., Ruddle F. H. Presence of human chromosome 21 alone is sufficient for hybrid cell sensitivity to human interferon. J Virol. 1978 Jan;25(1):319–325. doi: 10.1128/jvi.25.1.319-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980 Sep 19;209(4463):1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- Tan U. H. Chromosome-21-dosage effect on inducibility of anti-viral gene(s). Nature. 1975 Jan 24;253(5489):280–282. doi: 10.1038/253280a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Schneider E. L., Tischfield J., Epstein C. J., Ruddle F. H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974 Oct 4;186(4158):61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J., Tucker G., Epstein L. B., Epstein C. J. Interferon induction of (2'-5') oligoisoadenylate synthetase in diploid and trisomy 21 human fibroblasts: relation to dosage of the interferon receptor gene (IRFC). Hum Genet. 1983;65(2):108–111. doi: 10.1007/BF00286644. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Yonehara-Takahashi M., Ishii A., Nagata S. Different binding of human interferon alpha 1 and alpha 2 to common receptors on human and bovine cells. Studies with recombination interferons produced in Escherichia coli. J Biol Chem. 1983 Aug 10;258(15):9046–9049. [PubMed] [Google Scholar]
- Zhang Z. X., De Clercq E., Heremans H., Verhaegen-Lewalle M., Content J. Antiviral and anticellular activities of human and murine type I and type II interferons in human cells monosomic, disomic, and trisomic for chromosome 21. Proc Soc Exp Biol Med. 1982 May;170(1):103–111. doi: 10.3181/00379727-170-41405. [DOI] [PubMed] [Google Scholar]
- Zoon K., Zur Nedden D., Arnheiter H. Specific binding of human alpha interferon to a high affinity cell surface binding site on bovine kidney cells. J Biol Chem. 1982 May 10;257(9):4695–4697. [PubMed] [Google Scholar]



