Abstract
The key morphological feature that distinguishes corbiculate bees from other members of the Apidae family is the presence of the corbicula (pollen basket) on the tibial segment of hind legs. Here, we show that in the honeybee (Apis mellifera), the depletion of the gene Ultrabithorax (Ubx) by RNAi transforms the corbicula from a smooth, bristle-free concave structure to one covered with bristles. This is accompanied by a reduction of the pollen press, which is located on the basitarsus and used for packing the pollen pellet as well as a loss of the orderly arrangement of the rows of bristles that form the pollen comb. All these changes make the overall identity of workers’ T3 legs assume that of the queen. Furthermore, in a corbiculate bee of a different genus, Bombus impatiens, Ubx expression is also localized in T3 tibia and basitarsus. These observations suggest that the evolution of the pollen gathering apparatus in corbiculate bees may have a shared origin and could be traced to the acquisition of novel functions by Ubx, which in Apis were instrumental for subsequent castes and behavioural differentiation.
Keywords: honeybee, pollen basket, Ubx
1. Introduction
Social insects are widely recognized for their complex behaviours and division of labour within the collective. The best-studied social species is the western honeybee, Apis mellifera, which has three castes: the queen, drones and workers, as is the case for all other Apis species. Workers are characterized by having a distinct feature, the corbicula (pollen basket) that they use for packing pollen and transporting it to the beehive. The corbicula is localized on the flattened and enlarged tibia of hind legs and is found in other eusocial bees, such as bumblebees and stingless bees. By contrast, this structure is much less elaborate or completely lacking in solitary and less socially complex bee species [1]. Therefore, the evolution of the pollen basket has been recognized as a major morphological innovation in social insects [2] and is tied to the development of more complex social behaviours within the Apidae.
Morphological diversification of insect hind legs has been associated with changes in the expression of the hox gene Ultrabithorax, Ubx [3–8]. Functional studies subsequently confirmed that Ubx directly regulates the size of individual leg segments in a number of insects [7,9,10]. These data, coupled with recent findings showing differential expression of Ubx between workers and queens [8], led us to test the hypothesis that this gene may play a functional role in the development of the pollen gathering apparatus in honeybees. In addition, we examined Ubx expression in the bumblebee, Bombus impatiens, to infer the generality of corbicular development in Apidae.
2. Material and methods
(a). RNA interference
Double-stranded RNA was prepared following a previously established protocol as described in Mahfooz et al. [9]. In short, two non-overlapping Ubx fragments directed to the 5′ and 3′ ends of the gene were injected independently to control for non-specific or off-target effects. The 309 bp long 5′ fragment was amplified with forward 5′ ACTCGTATTTTGAGCAGACTGCG and reverse 5′ GTCGAATACGAGGATGTCGTG primers. For the 3′ fragment, the following primers were used: forward 5′ ACCATACGTTCTACCCCTGGATG and reverse 5′ AGCAAGTCGAGGAACTAGCG, generating a 265 bp product. Ten clones of each fragment were recovered, sequenced and compared to each other as well as the existing Apis Ubx gene (GenBank accession no. XM623986). The injections were performed on L2 and L3 stages of worker larvae with a Picospritzer II (Parker). Approximately 2 µl of Ubx dsRNA (5 µg µl−1) was injected (duration of 60 ms and nitrogen pressure at 20 psi) in five independent trials. We verified the extent of Ubx depletion by RT-PCR analysis (electronic supplementary material, figure S1). After injection, the larvae were maintained and reared following an established protocol [11]. Overall, a total of 270 L2–L3 larvae were injected, 38 (14%) of which survived to adulthood. Among the surviving adults, 14 (36%) displayed either a strong or moderate phenotype. These survival rates are in line with similar Ubx studies in Tribolium in which larval injections caused lethality at the pupal stage, while embryonic injections had 20% survival [5]. In addition, mortality rates of 50% or more have been reported for RNAi experiments in Apis [12,13], irrespective of the method used (injections or feeding). To further address non-specific effects, we injected a previously cloned 710 bp fragment of the jellyfish green fluorescent protein [14], and followed the previously described control protocols in Apis [15]. A total of 50 L2–L3 stage larvae were injected, of which 15 individuals (30%) survived; all resulting adults showed wild-type phenotype.
(b). Antibody staining
FP6.87 mouse antibody was used to detect the Ubx staining in Bombus leg discs, as described in Mahfooz et al. [6].
3. Results
Honeybee workers transport pollen on the tibial segment of the T3 leg (figure 1a), which is distinguished by having a concave, naked cuticle region that defines the corbicula (figure 1b). Except for a single central bristle (white arrow) thought to stabilize the pollen pellet, this area is completely bristle-free. By contrast, the queen lacks a corbicula and has a tibia that is covered in bristles (figure 1b). The other segment that is involved in pollen gathering is the T3 basitarsus, which is located distal to the tibia. In workers, it features 11 neatly spaced rows of bristles that make up the pollen comb as well as a protrusion known as the pollen press (figure 1b, black arrowhead). Both of these features are absent in queens.
Figure 1.
(a) Adult worker bee collecting pollen, the white arrow indicates corbicula filled with pollen granules. (b) Effects of Ubx-RNAi on adult worker T3 leg morphology. The white arrow points to the central bristle in wild-type tibia, while white arrowheads point to ectopic bristles in the moderate phenotype. The pollen press on T3 basitarsus is indicated by a black arrowhead in worker bees, while the asterisk denotes the absence of this structure in queens. (Online version in colour.)
To determine the role of Ubx in corbicular development, two non-overlapping Ubx fragments were independently injected, yielding similar results; a moderate or strong T3 leg phenotype was observed in 36% of the emerging Ubx-RNAi adults (figure 1b). In moderate phenotypes, the tibial segment lost the central bristle while gaining several ectopic bristles (white arrowheads) in the previously naked cuticular area. In strongly affected individuals, the whole corbicular region became completely covered with bristles. Interestingly, the tibia's overall size and its distinct triangular shape remained unchanged indicating that the function of Ubx at this developmental stage is primarily in the suppression of bristle development (see electronic supplementary material, figure S2).
Within the T3 basitarsus (figure 1b), the distinct rows of bristles that make up the pollen comb were either reduced in number (moderate phenotype) or they completely lost their organization (strong phenotype). In addition, the pollen press was reduced in size (figure 1b, black arrowhead). These combined results in the T3 tibia and basitarsus reveal that Ubx plays a critical role in the development of the morphological features that enable pollen collection in Apis.
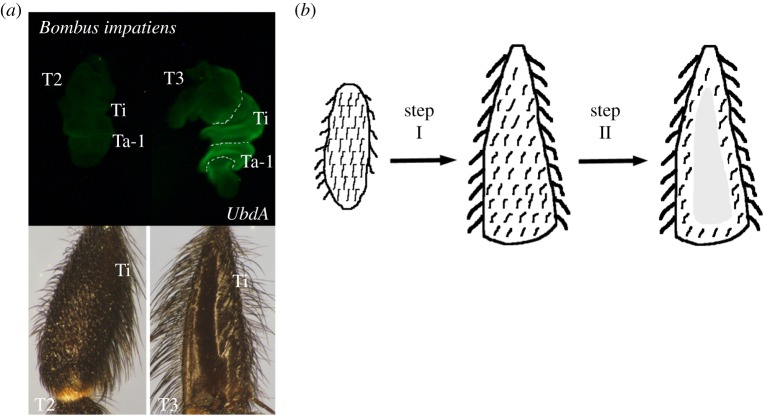
To determine whether this function of Ubx may exist in other corbiculate bees, we examined its expression in the leg discs of B. impatiens, which belongs to tribe Bombini (bumblebees). In this species as well, the T3 tibia and basitarsus are modified for pollen collection. As shown in figure 2a, the Ubx expression is restricted solely to these two segments suggesting that Ubx may play a general role in the formation of the bee's pollen gathering apparatus.
Figure 2.
(a) UbdA expression in Bombus impatiens (bumblebee) leg discs showing expression in the tibia (Ti) and basitarsus (Ta-1) of T3 legs, which corresponds to the presence of a corbicula on the adult T3 leg below. (b) Proposed two-step model for the development of the corbicula. (Online version in colour.)
4. Discussion
The smooth bristle-free surface of corbicula in Apis (figure 1b) is reminiscent of the ‘naked valley’ region on the T2 legs in several Drosophila species [16]. In the latter instance though, the smooth cuticle is generated by the suppression of the trichomes, which are simple cuticular extensions produced by epidermal cells. However, the honeybee worker pollen basket is generated by inhibiting the development of bristles, which are sensory organs [8]. This suggests that the pollen basket in corbiculate bees results from a novel role of Ubx in the suppression of bristles on the T3 tibia. In honeybees, this new role of Ubx appears to be under the control of environmental factors (i.e. diet), which leads to varying hormonal levels, resulting in phenotypic plasticity giving rise to the different castes.
Two recent studies [8,17] examined the global changes in expression between Apis queens and workers and identified a number of genes that are differentially expressed between the castes. While the functional data are still lacking, some genes, such as grunge (gug) and Ataxin-2 (Atx2), play a role in the formation of bristles in Drosophila [18,19]. As Ubx has been shown to regulate the development of specific bristles in the fly thorax [20], gug and Atx2 represent prime candidates as possible Ubx targets. Another avenue to explore would be to characterize the role of Ubx in generating distinct T3 basitarsus phenotypes among the castes. As shown in figure 1b, while the basitarsus in workers is organized in 11 distinct rows of bristles, in Ubx-RNAi adults this pattern is completely disrupted and resembles the situation present in queens. The observed differences in RNAi phenotypes between the T3 tibia and basitarsus suggest the presence of two modes of regulation by Ubx. In the former, Ubx may have a direct role in bristle suppression (as it is expressed in workers but not in queens). In the latter, the role of Ubx in the spatial organization of bristles may be at the downstream target level instead (because its expression in the basitarsus is common to both castes).
As a way of furthering our understanding of the evolution of the corbicula, we propose a two-step process outlined in figure 2b. The first step involves the divergence of the T3 tibia from its foreleg counterparts and the establishment of its distinct morphology. This is accomplished by the enlargement of the segment in its distal half and the acquisition of a triangular shape. A similar differential enlargement is also seen in the hind legs of non-social bees suggesting that the T3 enlargement preceded the formation of the corbicula [1]. The second step involves the actual creation of the pollen basket, whose presence is a distinguishing trait between the castes (such as workers and queens). As shown in figure 1b, this step is directly regulated by Ubx through its repression of bristle development generating a naked cuticular region. The finding that Ubx expression in Bombus is also restricted to the T3 tibia and basitarsus raises the possibility that this gene may act as a common trigger in the development of the pollen gathering apparatus in corbiculate bees. Further comparative studies in non-corbiculate bees will be necessary to confirm this hypothesis, and in turn will provide insight of how the acquisition of novel functions by Ubx may have facilitated the evolution of the social behaviour.
Acknowledgements
We thank Dr Kim Skyrm from Koppert Biological Systems Inc. for supplying the Bombus impatiens larvae.
Funding statement
This work was supported by NIH grant no. GM071927 to A.P. and Wayne State University Graduate Enhancement Funds to V.M.
References
- 1.Michener CD. 2000. The bees of the world. Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- 2.Cardinal S, Danforth BN. 2011. The antiquity and evolutionary history of social behavior in bees. PLoS ONE 6, e21086 (doi:10.1371/journal.pone.0021086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes CL, Kaufman TC. 2002. Hox genes and the evolution of the arthropod body plan. Evol. Dev. 4, 459–499 (doi:10.1046/j.1525-142X.2002.02034.x) [DOI] [PubMed] [Google Scholar]
- 4.Struhl G. 1982. Genes controlling segmental specification in the Drosophila thorax. Proc. Natl Acad. Sci. USA 79, 7380–7384 (doi:10.1073/pnas.79.23.7380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomoyasu Y, Wheeler SR, Denell RE. 2005. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature 433, 643–647 (doi:10.1038/nature03272) [DOI] [PubMed] [Google Scholar]
- 6.Mahfooz NS, Li H, Popadic A. 2004. Differential expression patterns of the hox gene are associated with differential growth of insect hind legs. Proc. Natl Acad. Sci. USA 101, 4877–4882 (doi:10.1073/pnas.04012161010401216101[pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khila A, Abouheif E, Rowe L. 2009. Evolution of a novel appendage ground plan in water striders is driven by changes in the hox gene Ultrabithorax. PLoS Genet. 5, e1000583 (doi:10.1371/journal.pgen.1000583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomtorin AD, Barchuk AR, Moda LM, Simoes ZL. 2012. Hox gene expression leads to differential hind leg development between honeybee castes. PLoS ONE 7, e40111 (doi:10.1371/journal.pone.0040111PONE-D-12-00549[pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahfooz N, Turchyn N, Mihajlovic M, Hrycaj S, Popadic A. 2007. Ubx regulates differential enlargement and diversification of insect hind legs. PLoS ONE 2, e866 (doi:10.1371/journal.pone.0000866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern DL. 2003. The hox gene Ultrabithorax modulates the shape and size of the third leg of Drosophila by influencing diverse mechanisms. Dev. Biol. 256, 355–366 (doi:10.1016/s0012-1606(03)00035-6) [DOI] [PubMed] [Google Scholar]
- 11.Huang ZY. 2009. A standarized procedure for in vitro rearing of Honey Bee larvae. See http://www.cdpr.ca.gov/docs/registration/reevaluation/larval_protocol.pdf [Google Scholar]
- 12.Wolschin F, Mutti NS, Amdam GV. 2011. Insulin receptor substrate influences female caste development in honeybees. Biol. Lett. 7, 112–115 (doi:10.1098/rsbl.2010.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucharski R, Maleszka J, Foret S, Maleszka R. 2008. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830 (doi:10.1126/science.1153069) [DOI] [PubMed] [Google Scholar]
- 14.Hrycaj S, Chesebro J, Popadic A. 2010. Functional analysis of Scr during embryonic and post-embryonic development in the cockroach, Periplaneta americana. Dev. Biol. 341, 324–334 (doi:10.1016/j.ydbio.2010.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page RE, Jr, Scheiner R, Erber J, Amdam GV. 2006. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr. Top. Dev. Biol. 74, 253–286 (doi:10.1016/S0070-2153(06)74008-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern DL. 1998. A role of Ultrabithorax in morphological differences between Drosophila species. Nature 396, 463–466 (doi:10.1038/24863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barchuk AR, Cristino AS, Kucharski R, Costa LF, Simoes ZL, Maleszka R. 2007. Molecular determinants of caste differentiation in the highly eusocial honeybee Apis mellifera. BMC Dev. Biol. 7, 70 (doi:10.1186/1471-213X-7-70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erkner A, et al. 2002. Grunge, related to human Atrophin-like proteins, has multiple functions in Drosophila development. Development 129, 1119–1129 [DOI] [PubMed] [Google Scholar]
- 19.Al-Ramahi I, et al. 2007. dAtaxin-2 mediates expanded Ataxin-1-induced neurodegeneration in a Drosophila model of SCA1. PLoS Genet. 3, e234 (doi:10.1371/journal.pgen.0030234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozowski M, Akam M. 2002. Hox gene control of segment-specific bristle patterns in Drosophila. Genes Dev. 16, 1150–1162 (doi:10.1101/gad.219302) [DOI] [PMC free article] [PubMed] [Google Scholar]