Abstract
Increasing evidence shows that spermatogenesis is costly. As a consequence, males should optimize the use of their sperm to maximize their reproductive outputs in their lifetime. However, experimental evidence on this prediction is largely lacking. Here, we examine how a male moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) responds to the presence of rivals or additional mates and how such response influences his lifetime reproductive fitness. We show that when rival males are present around a copulating pair, the male ejaculates more sperm to win a sperm competition battle but in such an environment he inseminates fewer females, sires fewer offspring and lives shorter. The opposite is the case when additional females are present during copulation. These findings reveal that elevated reproductive expenditure owing to sperm competition intensity is made at the expense of longevity and future reproduction.
Keywords: Ephestia kuehniella, sperm allocation, sperm competition intensity, male lifetime reproductive fitness
1. Introduction
In many insect species, a female obtains more than sufficient sperm from a single mating to fertilize her full egg load [1,2]. However, the majority of females copulate multiply [3]. Males of many insect [2] and non-insect species [4] including humans [5] have evolved strategies to adjust ejaculate investment in response to sperm competition because spermatogenesis is costly. In the natural environment, sex ratio is temporally and spatially dynamic [6], which may provide information about the risk and intensity of sperm competition at a given time and space [7]. Theoretically [7], males should save sperm for future copulations when additional mates are present and ejaculate more sperm when rivals are present. Increasingly empirical studies [8–12] appear to support this hypothesis.
Recently, two independent studies on Drosophila species [13,14] show that males increase sperm allocation after perceiving or experiencing the presence of other males. Price et al. [13] also indicate that males exposed to rivals achieve higher offspring production. However, these studies have only assessed males for one copulation rather than for their lifetime reproductive fitness. So far, whether and how the above mentioned sperm allocation strategy benefits males in their lifetime reproductive outputs is still poorly understood.
Empirical studies reveal that elevated reproductive expenditure is associated with accelerated ageing and reduced lifespan [15]. For example, male investment in reproduction may be made at the expense of longevity and future reproduction [16,17]. Therefore, the increased reproductive expenditure on ejaculate in response to high intensity of sperm competition should accelerate ageing and reduce longevity in males [17]. However, experimental evidence for this hypothesis is still largely lacking.
In the lifespan of our study species, the Mediterranean flour moth (Ephestia kuehniella Zeller), males can copulate with up to nine different females, and females can copulate up to four times despite the fact that from a single copulation they receive more than enough sperm to fertilize their full egg load [2].
On the basis of the theoretical framework and empirical findings outlined above, we hypothesize that in E. kuehniella: (i) a male ejaculates more sperm when rivals are present but fewer sperm when additional mates are present, (ii) the increased ejaculate expenditure shortens his longevity and reduces the number of offspring he can sire in his lifespan and (iii) the reduced ejaculate expenditure allows him to inseminate more females and sire more offspring in his lifespan. This is the first study that tests male reproductive fitness in both individual and lifetime mating scenarios in response to sperm competition intensity.
2. Material and methods
(a). Insects
Ephestia kuehniella larvae were reared on a standard diet [2] (see electronic supplementary material). Mature pupae were weighed and kept individually in glass tubes until adult emergence to ensure virginity and age. Adults were not given food or water as they do not feed [18]. The insect colony was kept and all experiments were carried out at 25 ± 1°C, 70 ± 10% RH and under a 14 L : 10 D photoperiod regime.
Pupal weight was considered adult body weight in this study (see electronic supplementary material). Unless stated otherwise, all adults used in this study were 1-day-old virgin moths with average body weight [2]. Copulations occurred during the scotophase in plastic cylinders (8 cm diameter × 10 cm height). Illumination during observation was provided by a 30 W red light. The plastic cylinders were lined with porous plastic sheets for oviposition.
(b). Ejaculate expenditure in the presence of rivals or additional mates
To determine whether the presence of rivals or additional mates affected the number of sperm a male ejaculated in a given copulation, we set up three treatments. (i) The copulation occurred in the presence of rivals, where we released three male rivals to the cylinder immediately after the copulation had commenced in the previously released pair (RM). (ii) The copulation occurred in the presence of additional mates, where we released three females to the cylinder immediately after the copulation had commenced in the previously released pair (AF). (iii) Negative control where neither rivals nor additional mates were released to the copulating pair (NC). We dissected the copulated female under a stereo microscope (Olympus SZ III, Japan) immediately after the copulation ended, and counted sperm she received using the methods outlined in Cook & Wedell [19]. Thirty replicates were performed in each treatment (see electronic supplementary material).
(c). Effect of ejaculate expenditure on male longevity and lifetime reproductive success
To test whether the presence of rivals or additional mates during all copulations a male had affected his longevity and the total number of offspring he sired, we set up three treatments as the above experiment (RM, AF and NC). For all treatments, we offered a 1-day-old virgin female to the male for copulation each day until he died. The rivals and additional mates were removed after copulation.
We then individually caged all copulated females for their lifespan in the above mentioned plastic cylinders immediately after copulation. We collected eggs daily and incubated them in Petri dishes (8.5 × 1.5 cm). We recorded the total number of eggs (fecundity) and fertilized eggs (fertility) a female laid in her lifetime. Those with black dots (larval heads) after 3 days of incubation were recorded as fertilized [20]. For each treatment, we also recorded the male longevity and number of copulations. Fifteen replicates were performed in RM and AF, and 20 in NC.
(d). Statistical analysis
A goodness-of-fit test was performed to test the data distribution. Data for the number of sperm ejaculated in the first experiment were analysed using ANOVA and those for other parameters in the second experiment analysed by MANOVA. All analyses were done using SAS9.1. Rejection level was set at α < 0.05. Unless stated otherwise, all values reported here are means ± s.e.
3. Results
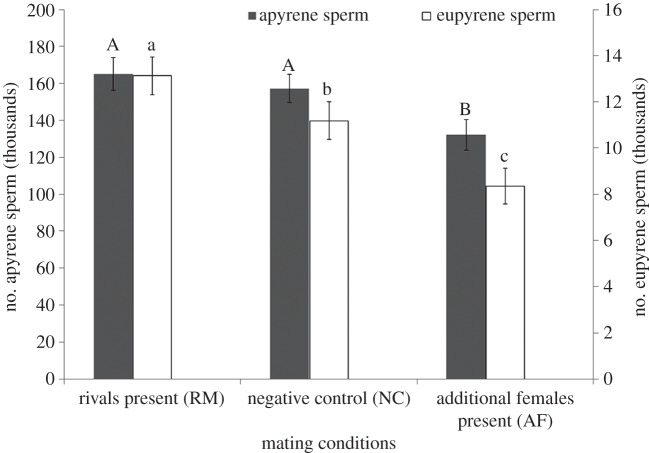
Males transferred significantly more eupyrene sperm (ANOVA: F1,84 = 6.86, p = 0.002) to females in the order of RM > NC > AF (figure 1). Males in both RM and NC transferred significantly more apyrene sperm than in AF (ANOVA: F1,83 = 4.42, p = 0.015) (figure 1). The number of apyrene sperm transferred was lower in NC than in RM but the difference between the two treatments was not significant (p > 0.05).
Figure 1.
Mean (±s.e.) number of apyrene and eupyrene sperm ejaculated by male E. kuehniella under different mating conditions. For each parameter, bars with different letters are significantly different (p < 0.05).
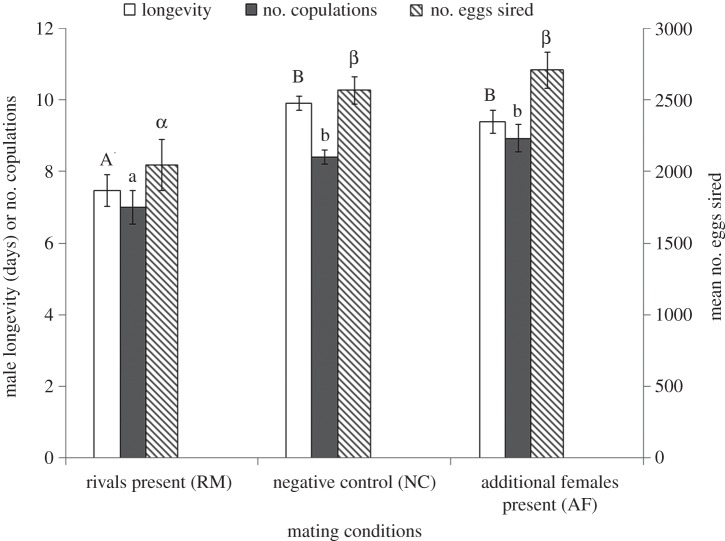
Males in RM lived significantly shorter, inseminated significantly fewer females and sired significantly fewer offspring in their lifespan than in AF and NC (MANOVA: F6,92 = 7.03, p < 0.0001 for overall dependent variables, F1,47 = 17.6, p < 0.0001 for longevity, F1,47 = 7.49, p = 0.002 for number of copulations and F1,47 = 6.62, p = 0.003 for number of offspring sired, figure 2).
Figure 2.
Mean (±s.e.) male longevity, number of lifetime copulations and number of eggs sired by males under different mating conditions. For each parameter, bars with different letters are significantly different (p < 0.05).
4. Discussion
This study shows that males in RM ejaculated more sperm than in AF (figure 1), supporting the theoretical predictions [7,10,11,21] that males can adjust ejaculate size in response to sperm competition intensity and opportunities for further copulations. In particular, our results resemble those of two recent studies on Drosophila species where Price et al. [13] and Garbaczewska et al. [14] show that males increased sperm allocation after perceiving or experiencing the presence of other males. This phenomenon may be attributed to the theoretical predictions and experimental findings that the paternity is determined by the relative number of competing sperm in females from different males [8].
Price et al. [13] indicate that the increased sperm allocation made by males exposed to rivals resulted in more offspring to be sired, and that such sperm allocation increase only involved fertilizing sperm with no change in non-fertilizing sperm. Therefore, Price et al. [13] suggest that the evolution of non-fertilizing sperm in flies may not be driven by sperm competition. However, our previous and current studies do not support the notion proposed by Price et al. [13] because in E. kuehniella (i) the increased allocation of sperm did not increase female fecundity and fertility [2], and (ii) both fertilizing and non-fertilizing sperm increased proportionally with the increase of sperm competition intensity (figure 1). We suggest that the symmetrical increase of non-fertilizing sperm may benefit males by reducing sperm competition intensity because non-fertilizing sperm may delay female remating [22].
Our results demonstrate that males in RM lived shorter, inseminated fewer females and sired fewer offspring in their lifetime than those in AF and NC (figure 2). This phenomenon may be attributed to the elevated reproductive expenditure [15] or physical stress caused by male rivals via interfering with the mating pairs. However, unlike many other insect orders lepidopteran adults are not aggressive [23]. In our study, both rivals and additional mates interacted with the mating pair by fanning wings around or antennal contact with the pair but these interactions were very brief (less than 3 min) compared with copulation duration of about 2 h [24] and mainly occurred at the beginning of introduction. We thus suggest that in response to high sperm competition intensity elevated reproductive expenditure rather than physical conflict plays the key role in the reduced lifetime reproductive fitness.
Acknowledgement
We thank K. van Epenhuijsen for his assistance on E. kuehniella colony establishment. We also thank the Handling Editor, N. Bennett, and two anonymous referees for their constructive comments, which have significantly improved the paper.
Funding statement
Research reported here was supported by Massey University Doctoral Research Scholarship, Massey University Research Fund (08/0169) and Natural Science Foundation of China (31160434).
References
- 1.Thornhill R, Alcock J. 1983. The evolution of insect mating systems, p. 576 Cambridge, UK: Harvard University Press [Google Scholar]
- 2.Xu J, Wang Q. 2009. Male moths undertake both pre- and in-copulation mate choice based on female age and weight. Behav. Ecol. Sociobiol. 63, 801–808 (doi:10.1007/s00265-009-0713-x) [Google Scholar]
- 3.Arnqvist G, Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164 (doi:10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre J-F, Ramm SA, Hurst JL, Stockley P. 2011. Social cues of sperm competition influence accessory reproductive gland size in a promiscuous mammal. Proc. R. Soc. B 278, 1171–1176 (doi:10.1098/rspb.2010.1828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shackelford TK, Goetz AT. 2007. Adaptation to sperm competition in humans. Curr. Dir. Psychol. Sci. 16, 47–50 (doi:10.1111/j.1467-8721.2007.00473.x) [Google Scholar]
- 6.Casula P, Nichols JD. 2003. Temporal variability of local abundance, sex ratio and activity in the Sardinian chalk hill butterfly. Oecologia 136, 374–382 (doi:10.1007/s00442-003-1288-2) [DOI] [PubMed] [Google Scholar]
- 7.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (doi:10.1016/S0169-5347(02)02533-8) [Google Scholar]
- 8.Parker GA, Ball MA, Stockley P, Gage MJG. 1997. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. Lond. B 264, 1793–1802 (doi:10.1098/rspb.1997.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholls EH, Burke T, Birkhead TR. 2001. Ejaculate allocation by male sand martins, Riparia riparia. Proc. R. Soc. Lond. B 268, 1265–1270 (doi:10.1098/rspb.2001.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934 (doi:10.1086/656840) [DOI] [PubMed] [Google Scholar]
- 11.delBarco-Trillo J. 2011. Adjustment of sperm allocation under high risk of sperm competition across taxa: a meta-analysis. J. Evol. Biol. 24, 1706–1714 (doi:10.1111/j.1420-9101.2011.02293.x) [DOI] [PubMed] [Google Scholar]
- 12.Parker GA, Lessells CM, Simmons LW. 2013. Sperm competition games: a general model for precopulatory male-male competition. Evolution 67, 95–109 (doi:10.1111/j.1558-5646.2012.01741.x) [DOI] [PubMed] [Google Scholar]
- 13.Price TAR, Lize A, Marcello M, Bretman A. 2012. Experience of mating rivals causes males to modulate sperm transfer in the fly Drosophila pseudoobscura. J. Insect Physiol. 58, 1669–1675 (doi:10.1016/j.jinsphys.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 14.Garbaczewska M, Billeter JC, Levine JD. 2013. Drosophila melanogaster males increase the number of sperm in their ejaculate when perceiving rival males. J. Insect Physiol. 59, 306–310 (doi:10.1016/j.jinsphys.2012.08.016) [DOI] [PubMed] [Google Scholar]
- 15.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453 (doi:10.1111/j.1365-2435.2008.01417.x) [Google Scholar]
- 16.Scharf I, Peter F, Martin OY. 2013. Reproductive trade-offs and direct costs for males in arthropods. Evol. Biol. 40, 169–184 (doi:10.1007/s11692-012-9213-4) [Google Scholar]
- 17.Bretman A, Westmancoat JD, Gage MJG, Chapman T. 2013. Costs and benefits of lifetime exposure to mating rivals in male Drosophila melanogaster. Evolution 67, 2413–2422 (doi:10.1111/evo.12125) [DOI] [PubMed] [Google Scholar]
- 18.Norris MJ. 1934. Contributions towards the study of insect fertility-III. Adult nutrition, fecundity, and longevity in the genus Ephestia (Lepidoptera, Phycitidae). Proc. Zool. Soc. Lond. 104, 333–360 (doi:10.1111/j.1469-7998.1934.tb07756.x) [Google Scholar]
- 19.Cook PA, Wedell N. 1996. Ejaculate dynamics in butterflies: a strategy for maximizing fertilization success? Proc. R. Soc. Lond. B 263, 1047–1051 (doi:10.1098/rspb.1996.0154) [Google Scholar]
- 20.Xu J, Wang Q, He XZ. 2007. Influence of larval density on biological fitness of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). N Z Plant Prot. 60, 199–202 [Google Scholar]
- 21.Ball MA, Parker GA. 2007. Sperm competition games: the risk model can generate higher sperm allocation to virgin females. J. Evol. Biol. 20, 767–779 (doi:10.1111/j.1420-9101.2006.01247.x) [DOI] [PubMed] [Google Scholar]
- 22.Cook PA, Wedell N. 1999. Non-fertile sperm delay female remating. Nature 397, 486 (doi:10.1038/17257) [Google Scholar]
- 23.Scott JA. 1974. Mate-locating behavior of butterflies. Am. Midl. Nat. 91, 103–117 (doi:10.2307/2424514) [Google Scholar]
- 24.Xu J, Wang Q. 2010. Mechanisms of last male precedence in a moth: sperm displacement at ejaculation and storage sites. Behav. Ecol. 21, 714–721 (doi:10.1093/beheco/arq044) [Google Scholar]