The complex pathophysiology of cancer, involving several hundreds of genes still remains a poorly understood area of biomedicine. This is reflected in the clinic where management of cancer frequently suffers from misdiagnosis and highly undesirable off-target side effects in treatment, obvious in chemotherapy. Consequently, there is great unmet need for improved diagnostic and therapeutic methods. In recent years, ion channels and transporters (including exchangers, pumps and associated enzymes), collectively referred to here as ‘ion transport mechanisms’ (ITMs) have emerged as novel mechanisms driving the cancer process, thus offering novel clinical possibilities. This is the first state-of-the-art volume dedicated to the involvement and clinical potential of ITMs in cancer.
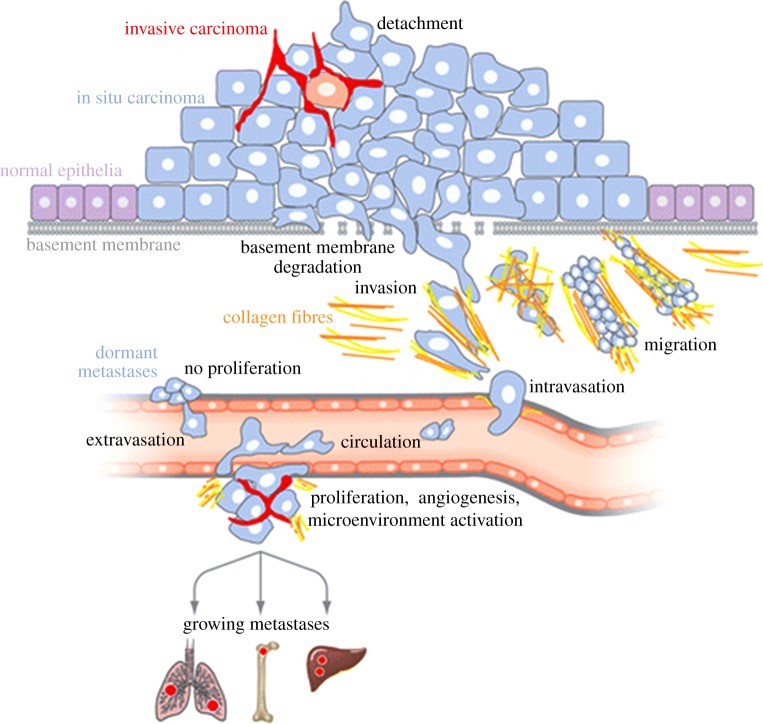
Most simply, cancer can be defined as ‘uncontrolled growth’ which can occur anywhere in the body! The initial abnormality is thought to arise from genetic damage to ‘tumour suppressor’ genes and/or cancer-promoting oncogenes, resulting in over-proliferative activity and formation of a primary tumour. Growth is accompanied by tight control of cell volume, and as the tumour reaches a size of 0.5–1 mm, the middle becomes hypoxic. This reduced level of oxygen is a trigger for the expression of a further wave of genes which induces angiogenesis (formation of new blood vessels), among other cellular changes, all together leading to more aggressive behaviour. Angiogenesis enables the growing tumour to continue receiving metabolic support via the enhanced blood supply while getting rid of waste by-products. Proliferating cells can steadily accumulate mutations and the gradual loss of homoeostasis may be reflected in ‘unsociable’ behaviour whereby the cells, with their ‘contact inhibition’ lost, start invading their surroundings. Invasive behaviour of cancer cells relies upon detachment both from each other and the substrate, enhanced motility (often directional) and digestion of the cells’ surroundings via release of proteolytic enzymes. Once migrating cancer cells enter the circulation (blood or lymph), they can spread around the body, aided by chemotactic and possibly electrical gradients. Migration is physically and metabolically stressful, however, and many circulating tumour cells undergo apoptosis and die. Surviving cells may exit the circulation and lodge at distant sites where they may remain dormant for some time (even years) or re-proliferate to form secondary tumours straight away. The overall process of secondary tumour formation is known as ‘metastasis’ and this is the main cause of death in cancer patients (figure 1).
Figure 1.
A schematic model of the cancer (carcinoma) process leading to metastasis. The initial transformation of normal epithelial cells results in carcinoma in situ. With reduced adhesiveness and enhanced migratory behaviour, tumour cells progress to an invasive stage. After degradation of the basement membrane, cells invade the surrounding stroma, migrate and ‘intravasate’ into lymph or blood circulation. Surviving cells arrest in the capillaries of a distant organ. There, cells may remain dormant without reproliferating for considerable time. Alternatively, the tumour cells may exit the circulation (‘extravasate’) and lead to formation of secondary tumours occurs after proliferation, induction of angiogenesis and microenvironment activation. Modified from Bacac & Stamenkovic [1]. (Online version in colour.)
The central role played by particular ITMs in the pathophysiology of cancer is indicated, in the first instance, by their appearance consistently in all cancers studied thus far. Ion transport occurs throughout the multi-stage cancer process, from the initial transformation of a normal cell into a cancer cell to metastasis. In fact, all ‘hallmarks’ of cancer involve or can potentially involve ITMs [2–4]. In this overall regard, ITMs possess some remarkable characteristics. For example, a typical membrane potential is equivalent to a voltage gradient of some |107 V m−1|, a huge force that can impact upon every protein in the membrane. Even red blood cells which do not have a nucleus still have a membrane potential. Voltage-gated sodium channels (VGSCs) permeate ions at an incredible rate of some 104 ions ms−1 (in single file), confirming their supreme design. It is not a surprise, therefore, that up to 40% of a cell's energy is spent maintaining the trans-membrane Na+ and K+ electrochemical gradients, essential for healthy electrophysiological signalling. Most cancers are carcinomas, i.e. they originate from epithelial cells. Ion transport is well known to occur in epithelia and underlie processes like secretion, and solute and water transport. However, the question of whether abnormalities in such ion transport may lead to cancer has only been addressed relatively recently. Another surprising discovery is the aberrant expression in carcinomas of voltage-gated ion channels, normally associated with ‘excitable’ cells. Expression of ITMs is controlled by hormones and growth factors which are well known to play significant roles in the cancer process. In other words, ITMs are functionally associated with ‘mainstream’ cancer mechanisms. Regulation of ITMs in cancer is a dynamic process, subject to the varying conditions in the microenvironment of the tumour at different stages, as disease progresses.
In this one volume, for the first time, we have assembled 16 articles, all written by international experts, demonstrating how ITMs can contribute to the cancer process and what clinical potential they may offer. We cover the cancer process systematically from primary to secondary tumourigenesis. Throughout, each contribution also discusses how the mechanism(s) in hand might be exploited both in diagnosis and therapy. Diagnostically, for example, as expression of VGSCs is an initial, integral part of the acquisition of metastatic potential in human colon cancer, they could serve as an early, functional biomarker [5]. The expression of carbonic anhydrase IX is another example of an ITM with clinical potential for renal carcinoma [6]. As regards therapy, it has been estimated that more than 10% of existing marketed drugs act upon ion channels [7]. Furthermore, new ion channel screening technologies continue to be developed to discover new drugs [8]. Importantly, therefore, such drugs may ‘repurposed’ for use against cancer. Such a strategy has already been proposed for VGSC expression in metastasis [9].
In conclusion, it is clear that application of electrophysiology and ionic imaging, complemented by molecular biology, for the first time to tumour cells has generated not just exciting new findings and concepts but also novel clinical potential. Thus, there is the real possibility of exploiting ITMs for early, functional diagnosis of cancer and controlling it non-toxically.
References
- 1. Bacac M, Stamenkovic I. 2008. Metastatic cancer cell. Annu. Rev. Pathol. 3, 221–247. ( 10.1146/annurev.pathmechdis.3.121806.151523) [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 3. Fraser SP, Pardo L. 2008. Ion channels: functional expression and therapeutic potential in cancer. Colloquium on ion channels and cancer. EMBO Rep. 9, 512–515. ( 10.1038/embor.2008.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prevarskaya N, Skryma R, Shuba Y. 2010. Ion channels and the hallmarks of cancer. Trends Mol Med 16, 107–121. ( 10.1016/j.molmed.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 5. House CD, et al. 2010. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 70, 6957–6967. ( 10.1158/0008-5472.CAN-10-1169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. 2012. Targeting the metabolic microenvironment of tumors. Adv. Pharmacol. 65, 63–107. ( 10.1016/B978-0-12-397927-8.00004-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clare JJ. 2010. Targeting ion channels for drug discovery. Discov. Med. 9, 253–260. [PubMed] [Google Scholar]
- 8. Martina M. 2012. Ion channel screening: advances in technologies and analysis. Front. Pharmacol. 3, 86 ( 10.3389/fphar.2012.00086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djamgoz MBA, Onkal R. 2013. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Patents Anticancer Drug Discov. 8, 66–84. [DOI] [PubMed] [Google Scholar]