Abstract
Profound cell volume changes occur in primary brain tumours as they proliferate, invade surrounding tissue or undergo apoptosis. These volume changes are regulated by the flux of Cl− and K+ ions and concomitant movement of water across the membrane, making ion channels pivotal to tumour biology. We discuss which specific Cl− and K+ channels are involved in defined aspects of glioma biology and how these channels are regulated. Cl− is accumulated to unusually high concentrations in gliomas by the activity of the NKCC1 transporter and serves as an osmolyte and energetic driving force for volume changes. Cell volume condensation is required as cells enter M phase of the cell cycle and this pre-mitotic condensation is caused by channel-mediated ion efflux. Similarly, Cl− and K+ channels dynamically regulate volume in invading glioma cells allowing them to adjust to small extracellular brain spaces. Finally, cell condensation is a hallmark of apoptosis and requires the concerted activation of Cl− and Ca2+-activated K+ channels. Given the frequency of mutation and high importance of ion channels in tumour biology, the opportunity exists to target them for treatment.
Keywords: ion channel, ion transporter, glioma, migration, proliferation, apoptosis
1. Introduction
While initially thought to be the domain of excitable cells, over the past decades we have learned that ion channels are also important to support the basic biology of malignant and non-malignant cells. They are necessary for defined steps in cell-cycle progression, facilitate volume changes of migrating cells, and allow cellular condensation during programmed cell death. These biologies occur during development or following malignant transformation. Primary brain tumours, gliomas, are a model system in which the role of ion channels has been particularly well explored, and therefore this preparation will serve as a focus of this review; however, the main findings discussed have broad applicability.
Gliomas constitute a collection of primary brain tumours which are classified by the World Health Organization (WHO) according to their relative malignancy as grades I–IV [1], with increasing grades defining more malignant tumours. The highest grade gliomas, astrocytomas or glioblastoma multiforme (GBM), make up the majority (60%) of adult gliomas, and these carry a poor prognosis with an average survival time of less than 12 months [2,3]. Current treatments, which include surgical resection and temazolamide chemotherapy are largely ineffective as the infiltrative nature of the tumour makes complete resection difficult and recurrence common [4].
As is common in cancer, gliomas present with numerous genetic mutations in tumour suppressor and oncogenes [5] but surprisingly, ion channel mutations are also frequent with 90% of human GBM samples presenting with ion channel and transporter mutations [6]. In paediatric brain tumours, ion channel/transporters are upregulated in 33% and downregulated in 48% of cases [7]. Hence ion channels are emerging as potential genes involved in the aetiology of gliomas, and therefore as potential future therapeutic targets.
The most defining features of gliomas, and many other cancers, are uncontrolled proliferation, enhanced invasiveness and abnormal cell death. In the following paragraphs, we will examine the role of ion channels in these three biologies in more detail.
2. Proliferation
During development, all body tissues expand by well-orchestrated proliferation, and some cell types retain the ability to divide throughout life. To assure a proper coordination of cell supply and loss, this process is highly regulated. A potential role for ion channels in cell proliferation has been well documented across many cell types, including numerous cancers [8]. Most of these studies found that drugs, presumed to be specific ion channel blockers, also inhibit cell-cycle progression and often arrest proliferation at distinct stages of the cell cycle. Of channels investigated, both Cl− channels and K+ channels have emerged as important pathways to facilitate cell volume changes.
(a). Chloride channels
Surprisingly, resting Cl− conductance is partially responsible for the relatively depolarized resting membrane potential (RMP) that characterizes glioma cells [9]. Glioma cells accumulate Cl− to approximately 100 mM [10] through the activity of the sodium/potassium/chloride cotransporter NKCC1 [11]. As a result, any opening of Cl− channels causes the efflux of Cl− [10]. Cl− channels blockers inhibit glioma proliferation, and this affect appears largely owing to preventing cell volume changes required for mitosis (figure 1).
Figure 1.
Proliferation. (a) Cells undergoing proliferation use Cl− channels, specifically ClC-3, and multiple K+ channels to efflux ions, which cause water efflux through aquaporins (AQP). This causes cell rounding and shrinkage with an increase in membrane thickness. Downstream, cell shrinkage may regulate macromolecular crowding and activation or auto-activation of kinases or other enzymes, as shown with the black diamonds representing intracellular proteins and red triangles representing membrane-bound proteins. ClC-3 activation requires phosphorylation by CaMKII (CMK) after Ca2+ activation. (b) Cell rounding and decrease in cells size can be visualized by increased GFP intensity at M phase. The membrane is labelled with DiI to visualize the increase in membrane thickness. Adapted with permission from Habela et al. [12].
Specifically, as cells prepare for division, they undergo an increase in size as they double their membrane and intracellular constituents prior to separating into two daughter cells. Using time-lapse confocal microscopy, Habela et al. [12] followed individual cells through several complete divisions. These studies discovered that rather than gradually increasing in size, the mother cell undergoes a cell volume condensation immediately preceding M phase, a process termed ‘premitotic condensation’ (PMC) [12]. During this time, the cell rounds and the plasma membrane thickness increases, presumably through folds, as cell volume decreases, resulting in a greater than 2× increase in the membrane : cytoplasm ratio at M phase (figure 1). Across the population, cells converged to the same cell-type-specific volume at M phase, regardless of starting size [12]. A hypertonically induced decrease in cell size decreased cell-cycle length, increased number of cell divisions, increased rate of shrinkage, and decreased time spent at or near minimal volume are all consistent with the idea that condensation or cell shrinkage is required for proliferation [12]. This process is not restricted to gliomas but was also observed in primary culture of immature spinal cord astrocytes and HEK-293 cells [12,13], suggesting that PMC is a universal phenomenon of dividing cells.
Ion channel activity directly affects cell volume, as flux of K+ and Cl− causes osmotically obligated water to follow (schematically depicted in figure 1). A specific requirement for Cl− channels in mitotic volume change was derived from subsequent studies using a combination of channel blockers and genetic tools to manipulate ion channel expression. In condensing M-phase cells, ClC-3 is enriched on the membrane [13], and patch-clamp recordings show an approximately twofold increase in Cl− conductance during M phase [12]. Cl− channel inhibitors or knockdown of ClC-3 each decreased currents, impaired PMC and lengthened the cell cycle [12,13]. This indicates that ClC-3 mediates Cl− efflux and accelerates PMC, ensuring a rapid progression through the cell cycle [12,13].
A number of studies in different glioma cells also point to other yet unidentified Cl− channels as likely contributors to the regulation of cell proliferation [14,15]. These too found that the rate of cell proliferation correlates with the rate of cell volume change following a bell-shaped curve, with both very high and very low volumes decreasing the rate of proliferation [14]. Similarly, proliferation rates were sensitive to volume changes, where experimentally altering osmolarity to induce swelling decreased proliferation rates, and shrinking cells increased proliferation [16].
These studies clearly establish cell volume regulation as a critical component of the cell cycle and identify one mechanism whereby Cl− channels directly participate. A number of theories mechanistically link volume changes to cell proliferation, including: alterations in the cytoskeleton [14], cell-size checkpoints [17], enhancement of intracellular nutrient concentration [17], regulation of gene expression [18] and macromolecular crowding [14,17]. Macromolecular crowding has been suggested to cause auto-activation of kinases [19] and crowding of effectors, especially kinases and phosphatases, enzymes and protein/electrolyte interactions [12,17,20]. Some consider cell volume to be a second messenger system, as small changes in volume create large changes in activity [20]. Clearly, we are only beginning to understand the effect(s) of cell volume changes in cell proliferation and much work remains.
(b). Potassium channels
K+ channels regulate proliferation of many cell types [21] and pharmacological blockers typically decrease cell proliferation [22,23]. Although the mechanism(s) linking K+ channel function to cell proliferation are not entirely understood, changes in RMP appear to represent an important mechanistic link, where it may affect cell-cycle checkpoints [24]. Importantly, gliomas and other cancers tend to have a more depolarized RMP [24].
To explore the mechanistic relationship between RMP and cell proliferation, an interesting comparison can be drawn between the function of KIR4.1 in both post-mitotic glial cells and dividing glioma cells. KIR4.1 is highly expressed on the membrane of differentiated astrocytes where it underlies uptake of K+. During development and after injury, astrocytes divide to produce scar tissue. This division is characterized by a depolarized RMP (from −80 to −40 mV) and is associated with decreased KIR4.1 activity [25]. Interestingly, dividing glioma cells have a persistently depolarized RMP (–40 mV), and lack membrane expression of KIR4.1 [21,26]. Manipulation of the RMP via modulation of functional membrane expression of KIR4.1 regulates proliferation in both cell types: blockade of the channel in mature astrocytes induced proliferation, and overexpression in glioma cells arrested proliferation [25,26]. The cell-cycle arrest in glioma cells could be overcome by blockade of the channels or by chronically depolarizing the cells with high K+ [26], demonstrating that a membrane depolarization is necessary to support cell proliferation.
How changes in voltage translate into cell-cycle re-entry remains elusive. However, RMP can regulate depolarization-dependent nuclear translocation, induce kinase activation and affect influx of mitogens through voltage-powered transporters [24]. Changes in RMP may also affect internal pH, nutrient transport, Ca2+ influx and integrin activation [17,24]. Clearly, further studies are warranted to elucidate the downstream mechanism for voltage regulation of glioma division.
As previously described for Cl− efflux, there are many outwardly rectifying K+ channels that may affect proliferation by altering cell volume. For example, inhibition of the voltage-gated K+ channels Kv1.3 and Kv1.5 decreases proliferation of glioma [17] and non-malignant glia [27]. Also, knockdown or inhibition of the ATP-sensitive K+ channels (KATP) decreases proliferation in vitro and slows tumour formation in xenografts, with cells arrested at G0/G1 phase of the cycle [28]. Use of an agonist or overexpression of the channel increases proliferation and promotes tumour development in vivo [28]. Three classes of Ca2+-activated K+ channels (KCa) have been identified in glioma cells: large-conductance (KCa1.1), intermediate-conductance (KCa3.1) and small-conductance (KCa2) [29]. Their role in regulating cell proliferation is unclear as some studies found evidence for a reduction of proliferation when KCa1.1 or KCa3.1 were inhibited [22,30,31] yet others found little to no effect [29].
Taken together, it is clear that both Cl− and K+ channels play a major role in the proliferation of glioma cells, though how they specifically affect downstream mechanisms is unclear. Future studies should investigate the specific mechanisms regarding ion channels in proliferation, with attention to determining the relative role of RMP versus cell volume.
3. Migration
Gliomas are particularly invasive cancers that have often already seeded metastases at the time of first presentation [32]. While most systemic cancers initially spread via a haematogeneous route, gliomas exclusively use individual cell movement through the extracellular spaces as they invade. This makes them an excellent model system in which to study the biology of cell movement within tissue.
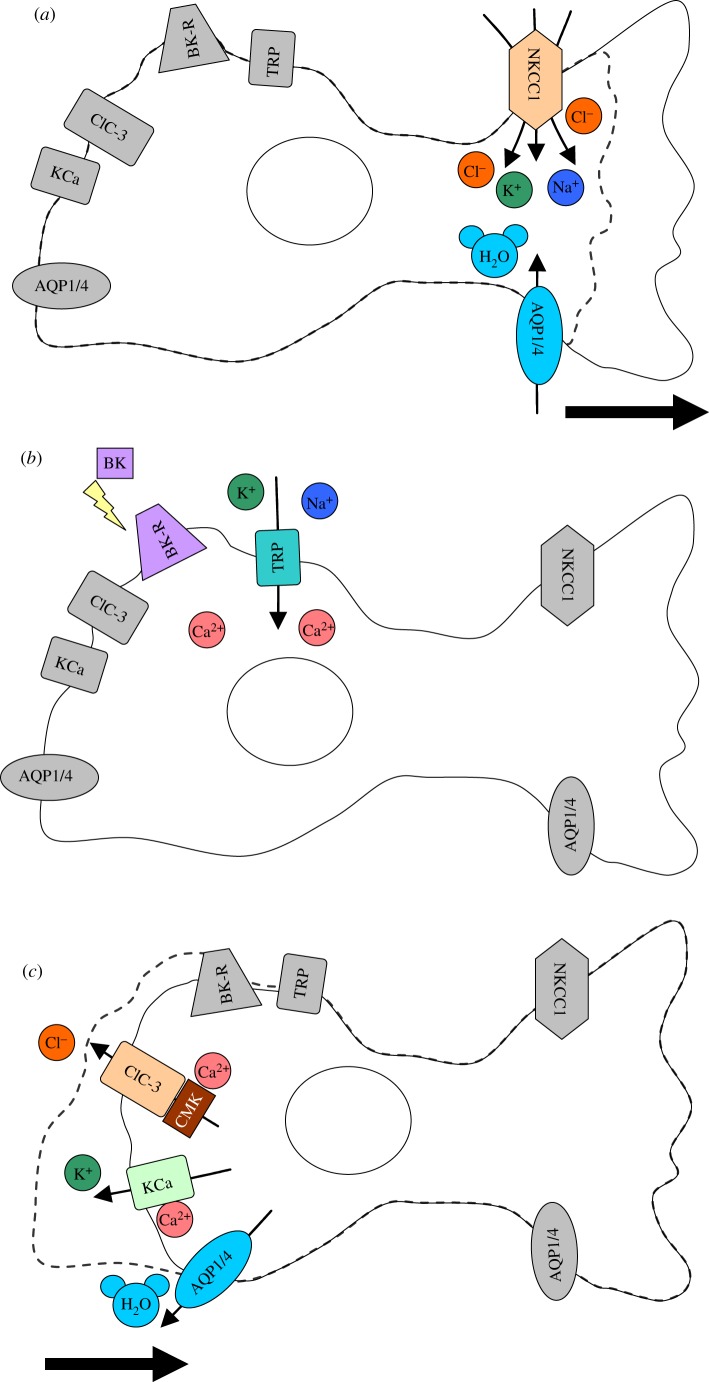
Molecular motors and rearrangement of the plasma membrane are fundamentally important in cell movement. Actin polymerization and myosin II contraction regulate the leading and trailing edges of the cell, respectively, to coordinate migration [33,34]. A role for ion channels in cell migration has been demonstrated for a variety of cell types, both cancerous and non-cancerous [35]. Ion channels can affect migration in multiple ways: by acting as a sensor for extracellular guidance cues [36], mediating the influx of Ca2+ that controls actin polymerization [37], and inducing shape and volume changes to adjust the moving cell to its environment [35]. The activity of ion channels may predominantly occur locally at the leading or trailing edges of the cell (figure 2) or occur globally, affecting the entire cell (figure 3). Below, we explore the role of some ion channels for which we have some understanding of how they participate in glioma migration.
Figure 2.
Local volume change during migration. (a) Migrating cells require extension of a lamellapodium or leading edge in order to migrate. This is facilitated by local volume increase through inflow of water through AQP. The water flows into the cell due to an osmotic gradient created by ion channels and transporters, such as NKCC1, causing ion influx. The dotted line indicates where the cell originally started before process extension. Bold arrow indicates direction of cell movement. (b) Many channels involved in migration require increases in Ca2+. Ca2+ sources may vary with the cell type or ion channel of interest. For example, TRP channels are non-specific cation channels which allow influx of Ca2+, and the bradykinin receptor (BK-R) also increases intracellular Ca2+. (c) Specific activation of ion channels, such as ClC-3 or KCa channels, at the trailing edge of the cells allows ion and water efflux, effectively shrinking only the end of the cell and causing a retraction. This is another important step in forward movement of cells. The dotted line represents the cells' original starting shape before retraction. Bold arrow indicates direction of cell movement. CMK = CaMKII.
Figure 3.
Whole cell volume change during migration. (a) Glioma cells travel through small extracellular spaces to migrate through the brain, and must shrink their volume in order to do so. Many ion channels involved in migration require activation by Ca2+. Sources include the bradykinin receptor (BK-R) and TRP channels. (b) Activation of channels such as KCa and ClC-3 cause outflow of ions, which triggers outflow of water through AQP. This causes whole cell shrinkage, which is 30–35% of the total cell volume. The dotted line represents the original cell size before volume change. CMK = CaMKII; the grey ECM denotes the extracellular barriers cells must go through. (c) To maintain electroneutrality, cells must efflux both Cl− and K+, which may be activated by similar signalling. For example, KCa3.1 (green), ClC-3 (blue) and BK-R (red) colocalize on glioma cells, meaning that BK-R could provide a specific source of Ca2+ for simultaneous activation of both channels. Adapted from Cuddapah et al. [38].
(a). Potassium channels
A retraction of the trailing edge of the cell is required for forward movement (figure 2), which in many other cell types, is accomplished by the actions of KCa3.1 [35]. Being Ca2+-activated, this channel transduces changes in intracellular Ca2+ into changes in membrane potential and K+ flux. KCa3.1 may act similarly in gliomas, as the ligands bradykinin and CXCL12 have been implicated in activation of KCa3.1 by increasing intracellular Ca2+ [38,39]. Pharmacological inhibition or genetic knockdown of KCa3.1 disrupts chemotaxis towards either of these ligands [38,39]. KCa3.1 is also required for the migration of neuroblasts, one of the purported brain tumour precursor cells, along the RMS [40]. Together, these studies suggest that KCa3.1 may play a role in glioma migration along gradients of extracellular cues, possibly by facilitating the retraction of the trailing edge of the cell.
Inhibition of KCa1.1 may also play a role in local volume change [35]. Studies have confirmed that KCa1.1 inhibition decreases migration in vitro [41]. However, KCa1.1 channel activators yielded more heterogeneous results: increases, decreases or no change in migration [41–43]. The action of KCa1.1 may vary depending on the cell line or the type and concentration of activators, as studies used different ways to activate the channel, including drug application and ionizing radiation.
(b). Chloride channels
Typically, migratory gliomas are elongated and wedge-shaped [32], suggesting that invading gliomas undergo profound cell volume changes to squeeze through small extracellular spaces (figure 3). Direct measurements of volume changes during in vivo migration of glioma indeed show a 30–35% decrease in cell volume, independent of cell size [44]. Experiments using hypotonic media suggest that this decrease accounts for an almost complete loss of the free cytoplasmic water content. Hence, cells achieve a minimal size as they encounter barriers [44] through shedding all unbound water. Blocking Cl− channels inhibits both this volume decrease and cell migration [44]. Inhibition of NKCC1, which is responsible for establishing an outward gradient for Cl−, similarly impairs the dispersal of gliomas in vivo [11]. These data suggest that Cl− is used as an osmolyte that facilitates the extrusion of cytoplasmic water from the cells [44].
Unfortunately, there is a paucity of specific inhibitors for Cl− channels making it difficult to unequivocally identify the underlying channel. Non-specific Cl− channel inhibitors decrease in vitro and in situ migration [38,45,46] as does replacement of Cl− with impermeant anions [47]. The most likely molecular candidate to date is ClC-3, as shRNA knockdown of ClC-3 decreases in vitro migration [47]. Furthermore, chlorotoxin (Cltx), shown to reduce membrane expression of ClC-3, inhibits migration in vitro and in vivo [21]. A likely mechanism for ClC-3 inhibition of migration is dysregulation of cell volume, as non-specific inhibition of Cl− channels blocks RVD and inhibits migration in many glioma cell types [9,32].
In order to maintain electroneutrality in volume change, cells must efflux both K+ and Cl−. Given the previous evidence, we can theorize that Cl− efflux is largely via ClC-3, and K+ efflux via KCa channels, which commonly co-localize and are activated under similar conditions. As mentioned previously, KCa3.1 affects migration after activation by bradykinin, and the same study found colocalization of KCa3.1 and ClC-3 on the invadopodia [38,45] (figure 3). Furthermore, application of bradykinin activates both channels, and inhibition of either channel decreased migration [38,45]. Blocking both channels together almost completely inhibits migration in vitro, indicating that both ions are used for migration [38,45]. KCa1.1 has also been found to colocalize with ClC-3 on lipid rafts in the invadopodia, where changes in activation alter migration [48]. These data indicate that KCa and ClC-3 channels are probably involved in volume change during migration, and their colocalization may allow them to respond to similar downstream signals from growth factor activation, most probaly through Ca2+ activation as illustrated in figures 2 and 3.
4. Apoptosis
Apoptosis is one important pathway through which cell populations are regulated during development and disease. Also called programmed cell death, it is characterized by DNA fragmentation, chromatin condensation and cellular condensation termed apoptotic volume decrease (AVD). Apoptosis can occur through two distinct pathways, intrinsic and extrinsic. Intrinsic apoptosis is triggered by internal injury to the cell, such as nutrient deprivation or DNA damage and can be stimulated by staurosporine (STS) [19,49]. Extrinsic apoptosis is caused by surface binding to death receptors, such as CD95, and ligands include Fas and TRAIL [19,49].
In glioma cells, both intrinsic and extrinsic apoptosis are associated with AVD, which is caused by activation of K+ and Cl− channels leading to loss of water [19]. Blocking the volume decrease inhibits the activation of caspases and decreases DNA fragmentation, and a large and long enough volume decrease can initiate apoptosis, making AVD both necessary and sufficient [19].
(a). Potassium channels
The effect of K+ channels in apoptosis differs depending on the cell type and channel studied [50]. In many glioma cells, inhibition of outwardly rectifying K+ channels with non-specific blockers causes apoptosis [22,23]. However, typically K+ efflux causes apoptosis through cell shrinkage; therefore, inhibition should decrease apoptosis [23]. Indeed, in some cell lines, sustained K+ channel activity is sufficient for apoptosis [50,51]. Confirming this idea, decreased TASK3 activity correlates with increased survival of glioma cells [52], and application of the TASK3 opener decreased survival of glioma cells, which was reversed with inhibitors [53].
Interestingly, the intrinsic and extrinsic pathways in glioma are regulated by different KCa channels [49], though there is disagreement on the effect of KCa inhibition on apoptosis. One study found that when both types of AVD are inhibited by high extracellular K+, KCa1.1 specifically regulates extrinsic apoptosis, as the inhibitor paxilline blocked AVD after being induced by TRAIL, but not STS [49]. KCa3.1 regulates intrinsic apoptosis, as the inhibitor TRAM-34 decreases AVD and caspase-3 activation after STS, but not TRAIL addition [49]. This study found overall that KCa inhibition decreases AVD, and presumably apoptosis [49]. However, other studies have found that KCa inhibition may increase apoptosis [54–56], though contradictions may be because of differences in inhibitors, cell types or perhaps different types of cell death, as inhibition of AVD alters apoptosis, but does not necessarily prevent death [19].
(b). Chloride channels
Cl− efflux is highly involved in glioma apoptosis, as inhibition of Cl− channels reduces AVD and caspase activation, both hallmarks of apoptosis [19]. Furthermore, simply inducing a large enough volume decrease in gliomas can stimulate apoptosis [19]. Although the molecular identity of the Cl− channel(s) has not been resolved, it is clear that Cl− channels are both necessary and sufficient for AVD and apoptosis [19].
As AVD is a common characteristic of apoptosis among many cell types, specific ion channels involved need to be further investigated. Similarly, downstream mechanisms linking ion channel function to AVD and apoptosis, such as macromolecular crowding, should be subject of further scrutiny as activation of channels involved in apoptosis could provide a useful therapeutic approach.
5. Therapy
Because ion channels are involved in a diversity of malignant characteristics of gliomas, they could be potential targets for therapy. However, creation of drugs targeting gliomas may be more complex than other cancers, as not only is it necessary to inhibit ion channels expressed highly on gliomas, and not the surrounding tissue, but the drugs must also cross the blood brain barrier. In using ion channels for glioma therapy, it would probably be necessary to activate or inhibit multiple channels together. This could include the inhibition of multiple similar channels in order to stop compensation by upregulation of other channels. It may also be beneficial to inhibit both K+ and Cl− channels together, as coordinated movement of both ions is required for volume change. Targeting channels that are consistently involved in multiple glioma processes could also be promising. For example, ClC-3 has been implicated in glioma migration and proliferation [12,57], and KCa channels are involved in migration [38,41,42,45] and apoptosis [49,54,56]. Furthermore, interference with ion channel activity may serve as adjuvant to other chemotherapeutic targets; because migration, proliferation and apoptosis are complex processes, multiple disruptions may be required to significantly slow the cancer.
Another possible therapeutic target is the regulation of ion channels by Ca2+, as multiple channels commonly involved in glioma biology require high levels of intracellular Ca2+ in order to be activated. As extensively discussed above, Cl− channels are involved in proliferation, migration and apoptosis of glioma cells. Well studied in glioma is ClC-3, which is a target of phosphorylation by CaMKII, a serine/threonine-specific protein kinase activated by Ca2+ [47]. In addition, KCa channels are activated by increases in intracellular Ca2+. Both KCa3.1 and KCa1.1 are important for apoptosis, proliferation and migration, though their effect is sometimes controversial, as previously discussed. Because multiple channels rely on intracellular Ca2+, it may be beneficial to target a common Ca2+ source, such as specific TRP channels. Many TRP channels are present in glioma cells and can regulate migration, proliferation and apoptosis, making them a possible therapeutic target. As TRPs are non-selective cation channels, their effects can be attributed to K+ and Na+ flux, but most studied is the role of Ca2+. TRPC1 specifically is important for cytokinesis in proliferation, and migration towards a chemoattractant EGF [57,58]. TRPC1 was also found to colocalize with ClC-3, where it provides Ca2+ for the channel and affects cell migration [57]. Activated by menthol, TRPM8 channel opening provides Ca2+ for KCa1.1, which also influence migration [59]. Targeting a specific Ca2+ source in glioma cells, such as TRPC1 or TRPM8, may prove useful as a drug therapy, and distinguishing the Ca2+ signals that affect specific ion channels in malignancy versus normal physiological processes would improve their standing as a therapeutic target.
One example of a drug in clinical trials is Cltx, which binds a protein complex on the cell surface that contains MMPs and ClC-3, and causes the internalization of the complex [60]. Cltx inhibits migration of glioma cells in vitro and in vivo in mice, probably caused by the inhibition of ClC-3 [21]. TM-601, a synthetic version of Cltx, has been used in phase I and II clinical trials where it was conjugated to 131I and administered intracavitary to adults with recurrent high grade gliomas [61]. They found no toxicity and few other adverse effects [62]. Because the clinical trials were in the early phases, there was not much study on efficacy of the drug, but they did find a possible anti-tumour effect [62], providing proof of principle that ion channels could serve as therapeutic targets in glioma.
One possible way to expedite the process of finding glioma therapies is to use drugs that are already FDA approved. One example of this is targeting NKCC1, which accumulates Cl− in glioma cells and is important for migration [11]. Its inhibitor, bumetanide, is already approved by the FDA as a loop diuretic and is currently also being tested to treat neonatal seizures [63].
Although we have primarily discussed the role of ion channels in glioma progression, many similar channels are also involved in progression of other cancers. Therefore, similar therapeutics might be used for multiple different cancers. One example is tamoxifen, which inhibits Cl− channels [64] and is already given as a treatment of oestrogen-dependent cancers. Not only could this be a therapy for primary brain tumours, but also other cancers in which Cl− channels are involved. It is possible that future study investigating similar ion channel activity in other cancers will yield common therapeutic targets.
6. Conclusion
Given the above evidence, it is now clear that ion channels and transporters play a major role in glioma biology, and similar ion channels are probably employed to support the biology of other cancerous and non-cancerous cells. A frequently discussed downstream pathway of ion channel activation is volume change, as many aspects of glioma physiology are regulated by this coordinated ion and water efflux. For example, proliferation requires PMC before division, migration requires both local and whole cell volume changes, and apoptosis requires AVD for completion of the pathways. Because this is a common theme, it is important to study the downstream effects of volume change. One common hypothesis is that macromolecular crowding activates signalling pathways, but this has not been studied in glioma cells in depth. Furthermore, changes in cell size can also alter protein synthesis, gene transcription and cytoskeletal arrangements [65]. There are many possible outcomes of volume change in the cell; therefore, the specific pathways should be investigated.
Mutations in ion transporters are also common in glioma [6,7]. Ion transporters, such as NKCC1, move ions against their gradient to set up a driving force which allows efflux of K+ and Cl− when the ion channels open. Mutations in ion transporters alter the driving force for certain ions, which could facilitate tumour progression; however, future studies should specifically investigate the identity and mechanism of transporters involved in glioma biology.
Ca2+ regulation of channels is a consistent feature in many aspects of gliomas and is a likely candidate to coordinate activation K+ and Cl− currents, which are required for water efflux and volume change. This has been demonstrated for cells stimulated with various ligands. For example, in glioma cells, the bradykinin-induced increase in Ca2+ activated both KCa3.1 and a Cl− efflux, which were used in migration [38]. Further studies should investigate the spatial and temporal aspects of Ca2+ changes within the cell, and how these are coordinated to activate channels and affect cell activity. Judging from the large number of ion channels that are altered in glioma and affect the basic biological processes, it may be useful to classify some of the channels as oncogenes [66]. It is clear that ion channels are a worthy target of future investigation, not only therapeutically, but also as a model system for the general behaviour of all cells.
Funding statement
This work was supported by NIH-RO1-NS031234, RO1NS-036692 and T32 GM008111.
References
- 1.Kleihues P, Burger PC, Scheithauer BW. 1993. The new WHO classification of brain tumours. Brain Pathol. 3, 255–268. ( 10.1111/j.1750-3639.1993.tb00752.x) [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. 2006. Cancer statistics, 2006. CA Cancer J. Clin. 56, 106–130. ( 10.3322/canjclin.56.2.106) [DOI] [PubMed] [Google Scholar]
- 3.Huncharek M, Muscat J. 1998. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 18, 1303–1311. [PubMed] [Google Scholar]
- 4.Zalutsky MR. 2005. Current status of therapy of solid tumors: brain tumor therapy. J. Nucl. Med. 46(GeneRIF), 49. [PubMed] [Google Scholar]
- 5.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. 2001. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 15, 1311–1333. ( 10.1101/gad.891601) [DOI] [PubMed] [Google Scholar]
- 6.Molenaar RJ. 2011. Ion channels in glioblastoma. ISRN Neurol. 2011, 590249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masselli M, et al. 2012. Deregulation of ion channel and transporter encoding genes in pediatric gliomas. Front Oncol. 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becchetti A. 2011. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 301, C255–C265. ( 10.1152/ajpcell.00047.2011) [DOI] [PubMed] [Google Scholar]
- 9.Ransom CB, O'Neal JT, Sontheimer H. 2001. Volume-activated chloride currents contribute to the resting conductance and invasive migration of human glioma cells. J. Neurosci. 21, 7674–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habela CW, Ernest NJ, Swindall AF, Sontheimer H. 2009. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J. Neurophysiol. 101, 750–757. ( 10.1152/jn.90840.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas BR, Sontheimer H. 2010. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res. 70, 5597–5606. ( 10.1158/0008-5472.CAN-09-4666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habela CW, Sontheimer H. 2007. Cytoplasmic volume condensation is an integral part of mitosis. Cell Cycle 6, 1613–1620. ( 10.4161/cc.6.13.4357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habela CW, Olsen ML, Sontheimer H. 2008. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J. Neurosci. 28, 9205–9217. ( 10.1523/JNEUROSCI.1897-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouzaire-Dubois B, Malo M, Milandri JB, Dubois JM. 2004. Cell size-proliferation relationship in rat glioma cells. Glia 45, 249–257. ( 10.1002/glia.10320) [DOI] [PubMed] [Google Scholar]
- 15.Ullrich N, Sontheimer H. 1997. Cell cycle-dependent expression of a glioma-specific chloride current: proposed link to cytoskeletal changes. Am. J. Physiol. 273, C1290–C1297. [DOI] [PubMed] [Google Scholar]
- 16.Rouzaire-Dubois B, Bostel S, Dubois JM. 1999. Evidence for several mechanisms of volume regulation in neuroblastoma x glioma hybrid NG108–15 cells. Neuroscience 88, 307–317. ( 10.1016/S0306-4522(98)00236-X) [DOI] [PubMed] [Google Scholar]
- 17.Pardo LA. 2004. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda). 19, 285–292. ( 10.1152/physiol.00011.2004) [DOI] [PubMed] [Google Scholar]
- 18.Burg MB, Kwon ED, Kultz D. 1996. Osmotic regulation of gene expression. FASEB J. 10, 1598–1606. [DOI] [PubMed] [Google Scholar]
- 19.Ernest NJ, Habela CW, Sontheimer H. 2008. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J. Cell Sci. 121, 3–7. ( 10.1242/jcs.017483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Häussinger D. 1998. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306. [DOI] [PubMed] [Google Scholar]
- 21.Sontheimer H. 2008. An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. (Maywood). 233, 779–791. ( 10.3181/0711-MR-308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin LS, Park CC, Zitnay KM, Sinha M, DiPatri AJ, Jr, Perillan P, Simard JM. 1997. 4-Aminopyridine causes apoptosis and blocks an outward rectifier K+ channel in malignant astrocytoma cell lines. J. Neurosci. Res. 48, 122–127. () [DOI] [PubMed] [Google Scholar]
- 23.Yang KB, Zhao SG, Liu YH, Hu EX, Liu BX. 2009. Tetraethylammonium inhibits glioma cells via increasing production of intracellular reactive oxygen species. Chemotherapy 55, 372–380. ( 10.1159/000235730) [DOI] [PubMed] [Google Scholar]
- 24.Blackiston DJ, McLaughlin KA, Levin M. 2009. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8, 3519–3528. ( 10.4161/cc.8.21.9888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. 2001. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J. Neurophysiol. 85, 1719–1731. [DOI] [PubMed] [Google Scholar]
- 26.Higashimori H, Sontheimer H. 2007. Role of Kir4.1 channels in growth control of glia. Glia 55, 1668–1679. ( 10.1002/glia.20574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin LT, Fu YJ, Xu QL, Yang J, Liu ZL, Liang AH, Fan X-J, Xu C-G. 2007. Potential biochemical therapy of glioma cancer. Biochem. Biophys. Res. Commun. 362, 225–229. ( 10.1016/j.bbrc.2007.07.167) [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Li B, Li W, Guo H, Zou F. 2009. ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis 30, 737–744. ( 10.1093/carcin/bgp034) [DOI] [PubMed] [Google Scholar]
- 29.Abdullaev IF, Rudkouskaya A, Mongin AA, Kuo YH. 2010. Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation. PLoS ONE 5, e12304 ( 10.1371/journal.pone.0012304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z. 2004. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 448, 274–286. ( 10.1007/s00424-004-1258-5) [DOI] [PubMed] [Google Scholar]
- 31.Basrai D, Kraft R, Bollensdorff C, Liebmann L, Benndorf K, Patt S. 2002. BK channel blockers inhibit potassium-induced proliferation of human astrocytoma cells. Neuroreport 13, 403–407. ( 10.1097/00001756-200203250-00008) [DOI] [PubMed] [Google Scholar]
- 32.Sontheimer H. 2004. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol. Neurobiol. 29, 61–71. ( 10.1385/MN:29:1:61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YL. 1985. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J. Cell Biol. 101, 597–602. ( 10.1083/jcb.101.2.597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaira PT, Taylor DL. 1993. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J. Cell Biol. 120, 1381–1391. ( 10.1083/jcb.120.6.1381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab A, Fabian A, Hanley PJ, Stock C. 2012. Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. ( 10.1152/physrev.00018.2011) [DOI] [PubMed] [Google Scholar]
- 36.Gurnett CA, Hedera P. 2007. New ideas in epilepsy genetics: novel epilepsy genes, copy number alterations, and gene regulation. Arch. Neurol. 64, 324–328. ( 10.1001/archneur.64.3.324) [DOI] [PubMed] [Google Scholar]
- 37.Veksler A, Gov NS. 2009. Calcium-actin waves and oscillations of cellular membranes. Biophys. J. 97, 1558–1568. ( 10.1016/j.bpj.2009.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuddapah VA, Turner KL, Seifert S, Sontheimer H. 2013. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J. Neurosci. 33, 1427–1440. ( 10.1523/JNEUROSCI.3980-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sciaccaluga M, et al. 2010. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 299, C175–C184. ( 10.1152/ajpcell.00344.2009) [DOI] [PubMed] [Google Scholar]
- 40.Turner KL, Sontheimer H. 2013. KCa3.1 modulates neuroblast migration along the rostral migratory stream (RMS) in vivo. Cereb. Cortex. ( 10.1093/cercor/bht090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraft R, Krause P, Jung S, Basrai D, Liebmann L, Bolz J, Patt S. 2003. BK channel openers inhibit migration of human glioma cells. Pflugers Arch. 446, 248–255. [DOI] [PubMed] [Google Scholar]
- 42.Weaver AK, Bomben VC, Sontheimer H. 2006. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 54, 223–233. ( 10.1002/glia.20364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinle M, Palme D, Misovic M, Rudner J, Dittmann K, Lukowski R, Ruth P, Huber SM. 2011. Ionizing radiation induces migration of glioblastoma cells by activating BK K+ channels. Radiother. Oncol. 101, 122–126. ( 10.1016/j.radonc.2011.05.069) [DOI] [PubMed] [Google Scholar]
- 44.Watkins S, Sontheimer H. 2011. Hydrodynamic cellular volume changes enable glioma cell invasion. J. Neurosci. 31, 17 250–17 259. ( 10.1523/JNEUROSCI.3938-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catacuzzeno L, Aiello F, Fioretti B, Sforna L, Castigli E, Ruggieri P, Tata AM, Calogero A, Franciolini F. 2011. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell Physiol. 226, 1926–1933. ( 10.1002/jcp.22523) [DOI] [PubMed] [Google Scholar]
- 46.Soroceanu L, Manning TJ, Jr, Sontheimer H. 1999. Modulation of glioma cell migration and invasion using Cl− and K+ ion channel blockers. J. Neurosci. 19, 5942–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuddapah VA, Sontheimer H. 2010. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J. Biol. Chem. 15, 11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. 2009. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 16, 66–93. ( 10.2174/092986709787002835) [DOI] [PubMed] [Google Scholar]
- 49.McFerrin MB, Turner KL, Cuddapah VA, Sontheimer H. 2012. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am. J. Physiol. Cell Physiol. 303, C1070–C1078. ( 10.1152/ajpcell.00040.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. 2005. Ion channels in cell proliferation and apoptotic cell death. J. Membr. Biol. 205, 147–157. ( 10.1007/s00232-005-0780-5) [DOI] [PubMed] [Google Scholar]
- 51.Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Bulbins E. 2000. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol. Biochem. 10, 417–428. ( 10.1159/000016367) [DOI] [PubMed] [Google Scholar]
- 52.Lehen'kyi V, Shapovalov G, Skryma R, Prevarskaya N. 2011. Ion channels and transporters in cancer. 5. Ion channels in control of cancer and cell apoptosis. Am. J. Physiol. Cell Physiol. 301, C1281–C1289. ( 10.1152/ajpcell.00249.2011) [DOI] [PubMed] [Google Scholar]
- 53.Meuth SG, Herrmann AM, Ip CW, Kanyshkova T, Bittner S, Weishaupt A, Budde T, Wiendl H. 2008. The two-pore domain potassium channel TASK3 functionally impacts glioma cell death. J. Neurooncol. 87, 263–270. ( 10.1007/s11060-008-9517-5) [DOI] [PubMed] [Google Scholar]
- 54.Weaver AK, Liu X, Sontheimer H. 2004. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J. Neurosci. Res. 78, 224–234. ( 10.1002/jnr.20240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang KH, Chen ML, Chen HC, Huang YW, Wu TY, Chen YJ. 1999. Enhancement of radiosensitivity in human glioblastoma U138MG cells by tetrandrine. Neoplasma 46, 196–200. [PubMed] [Google Scholar]
- 56.Khalid MH, Shibata S, Hiura T. 1999. Effects of clotrimazole on the growth, morphological characteristics, and cisplatin sensitivity of human glioblastoma cells in vitro. J. Neurosurg. 90, 918–927. ( 10.3171/jns.1999.90.5.0918) [DOI] [PubMed] [Google Scholar]
- 57.Cuddapah VA, Turner KL, Sontheimer H. 2013. Calcium entry via TRPC1 channels activates chloride currents in human glioma cells. Cell Calc. 53, 187–194. ( 10.1016/j.ceca.2012.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bomben VC, Sontheimer H. 2010. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia 58, 1145–1156. ( 10.1002/glia.20994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wondergem R, Ecay TW, Mahieu F, Owsianik G, Nilius B. 2008. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem. Biophys. Res. Commun. 372, 210–215. ( 10.1016/j.bbrc.2008.05.032) [DOI] [PubMed] [Google Scholar]
- 60.Deshane J, Garner CC, Sontheimer H. 2003. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J. Biol. Chem. 278, 4135–4144. ( 10.1074/jbc.M205662200) [DOI] [PubMed] [Google Scholar]
- 61.Hockaday DC, Shen S, Fiveash J, Raubitschek A, Colcher D, Liu A, Alvarez V, Mamelak AN. 2005. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 46, 580–586. [PubMed] [Google Scholar]
- 62.Mamelak AN, et al. 2006. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J. Clin. Oncol. 24, 3644–3650. ( 10.1200/JCO.2005.05.4569) [DOI] [PubMed] [Google Scholar]
- 63.Kahle KT, Barnett SM, Sassower KC, Staley KJ. 2009. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na+-K+-2Cl− cotransporter NKCC1. J. Child Neurol. 24, 572–576. ( 10.1177/0883073809333526) [DOI] [PubMed] [Google Scholar]
- 64.Zhang JJ, et al. 1994. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J. Clin. Invest. 94, 1690–1697. ( 10.1172/JCI117514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waldegger S, Steuer S, Risler T, Heidland A, Capasso G, Massry S, Lang F. 1998. Mechanisms and clinical significance of cell volume regulation. Nephrol. Dial. Transplant. 13, 867–874. ( 10.1093/ndt/13.4.867) [DOI] [PubMed] [Google Scholar]
- 66.Huber SM. 2013. Oncochannels. Cell Calc. 53, 241–255. ( 10.1016/j.ceca.2013.01.001) [DOI] [PubMed] [Google Scholar]