Abstract
Anoctamin 1 (TMEM16A, Ano1) is a recently identified Ca2+-activated chloride channel and a member of a large protein family comprising 10 paralogues. Before Ano1 was identified as a chloride channel protein, it was known as the cancer marker DOG1. DOG1/Ano1 is expressed in gastrointestinal stromal tumours (GIST) and particularly in head and neck squamous cell carcinoma, at very high levels never detected in other tissues. It is now emerging that Ano1 is part of the 11q13 locus, amplified in several types of tumour, where it is thought to augment cell proliferation, cell migration and metastasis. Notably, Ano1 is upregulated through histone deacetylase (HDAC), corresponding to the known role of HDAC in HNSCC. As Ano1 does not enhance proliferation in every cell type, its function is perhaps modulated by cell-specific factors, or by the abundance of other anoctamins. Thus Ano6, by regulating Ca2+-induced membrane phospholipid scrambling and annexin V binding, supports cellular apoptosis rather than proliferation. Current findings implicate other cellular functions of anoctamins, apart from their role as Ca2+-activated Cl− channels.
Keywords: anoctamin 1, anoctamin 6, TMEM16A, TMEM16F, cancer, head and neck stromal cell carcinoma
1. Introduction
Anoctamin 1 (Ano1, TMEM16A) is a novel Ca2+-activated chloride channel (CaCC) with important physiological functions in epithelial cells and other cell types [1–4]. It was also shown to be activated during cell swelling, probably secondary to an increase in intracellular Ca2+ [5]. While some detected a role of Ca2+-dependent anoctamins, such as Ano1 or Ano6, to volume regulation, others did not [5,6]. Upregulation of endogenous Ca2+-activated Cl− channels in proliferating cells has been observed recently, but the role of these channels for proliferation has remained unclear [7,8]. Before Ano1 was identified as CaCC, it was already known as a protein that is coexpressed with the morphogen sonic hedgehog during embryonic development [9]. Ano1 was also known as DOG1, a protein strongly expressed in gastrointestinal stromal tumours and head and neck squamous cell carcinoma (HNSCC). Expression of DOG1 correlates with poor outcome in oesophageal squamous cell cancer [10–12]. Interstitial cells of Cajal (ICC) are another predominant site for Ano1 expression [13–15]. It has been reported that mice lacking Ano1 had fewer proliferating ICC [16]. Moreover, additional data suggested that Ano1 regulates proliferation at the G1/S transition of the cell cycle. Assuming such a pro-proliferative role, targeting of Ano1 has been proposed as a novel method for the treatment of malignant tumours [7,17,18].
2. Ano1 is located on the 11q13 amplicon
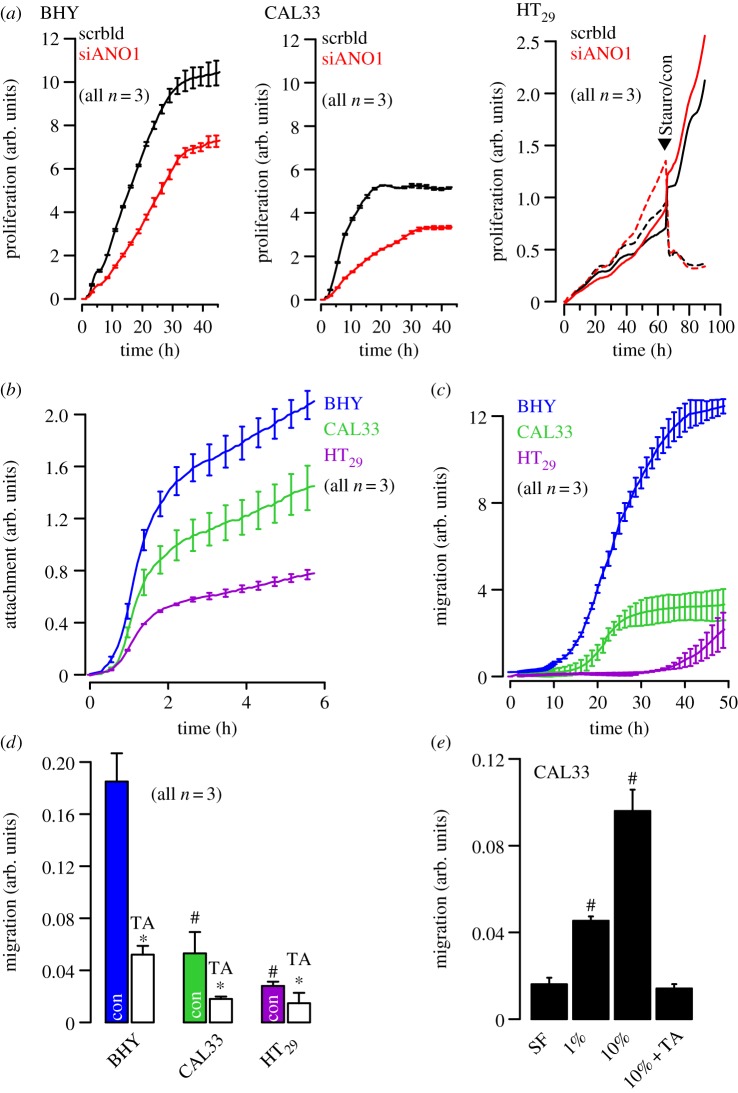
The coding sequence of Ano1 is located within the 11q13 region, a chromosomal locus that is frequently amplified in a number of different human cancers, such as urinary bladder cancer, breast cancer and HNSCC [19]. The 11q13 amplicon contains a stretch of proteins related to cell cycle, proliferation and apoptosis (cyclin D1, ORAOV1, FGF19, FGF4, TMEM16A, Fas-associated via death domain, PPFIA1, cortactin) [20]. The complex structure of this amplicon has mostly been studied in breast cancer, where multiple genes have been suggested as driver genes [21,22]. These findings implied a link between Ano1 expression, cell-cycle regulation and proliferation, which has recently been demonstrated in HNSCC and other cancer cells [11,16,23–25]. Surprisingly, downregulation of Ano1 contributes to cerebrovascular remodelling by promoting basilar smooth muscle cell proliferation, which is through inhibition of expression of cyclin D1 and cyclin E [26]. We performed additional experiments and compared proliferation of two HNSCC (CAL33, BHY) and one colonic epithelial cell line (HT29) using an online impedance-based xCELLigence proliferation assay system [27] (figure 1a). Proliferation was clearly higher in the HNSCC cell lines (cf. scale), which express much higher levels of Ano1, when compared with HT29 cells (cf. western blot, figure 3a) [27]. In a previous study, we did not detect inhibition of proliferation of BHY cells by siRNA [27]. Using improved Stealth siRNA (cf. electronic supplementary material), these experiments were repeated. Proliferation was suppressed by siRNA-knockdown of Ano1 expression in HNSCC but not in HT29 cells. These results suggest that very effective suppression of Ano1 expression is necessary to eliminate the pro-proliferative effects of Ano1, suggesting that low expression of Ano1 is sufficient to induce this effect. While siRNA had no effect in HT29 cells, 1 µM staurosporine (figure 1a black arrow, dashed lines) inhibited proliferation and induced cell apoptosis in colonic carcinoma cells, as demonstrated in an earlier publication [28]. Thus, enhanced expression of Ano1 does not seem to enhance proliferation in every cell type [27,29]. However, there is increasing consent that high levels of Ano1 lead to enhanced cell motility, distal metastasis and poor prognosis [23,24,27,29]. Also in this study, cell attachment and migration was enhanced with increasing expression of Ano1 (BHY > CAL33 > HT29). It is further demonstrated that migration could be significantly inhibited by tannic acid, a blocker of Ano1 and other anoctamins (figure 1c,d). Moreover, migration was strongly dependent on the presence of serum in the migration chamber and was inhibited by the Ano1 inhibitor tannic acid (TA, figure 1e). Although proliferation, attachment and migration were positively correlated with expression of Ano1, this does not prove that Ano1 is directly responsible for the change of these properties.
Figure 1.
Ano1 controls proliferation: (a) Cell proliferation measured online by impedance-based xCELLigence proliferation assay system in HNSCC (BHY, CAL33) and colonic epithelial (HT29) cells (see electronic supplementary material). siRNA-knockdown of Ano1 expression reduced proliferation in both BHY and CAL33 cells, but slightly augmented proliferation in HT29 cells. Staurosporine (1 µg ml−1) strongly inhibited proliferation. (b) Attachment of BHY, CAL33 and H29 cells after seeding in xCELLigence chambers (see electronic supplementary material). (c) Migration of BHY, CAL33 and H29 cells after seeding in the xCELLigence migration chamber (see electronic supplementary material). (d) Summary of the rate of cell migration between 10 and 30 h after seeding and inhibition of migration by TA(10 µM). (e) Migration of CAL33 cells and effect of fetal calf serum and TA. Mean ± s.e.m. (number of cells). Symbol # denotes significant difference when compared with BHY and serum free, respectively (p < 0.05; ANOVA). Asterisks (*) denote significant inhibition by TA (p < 0.05; paired t-test). (Online version in colour.)
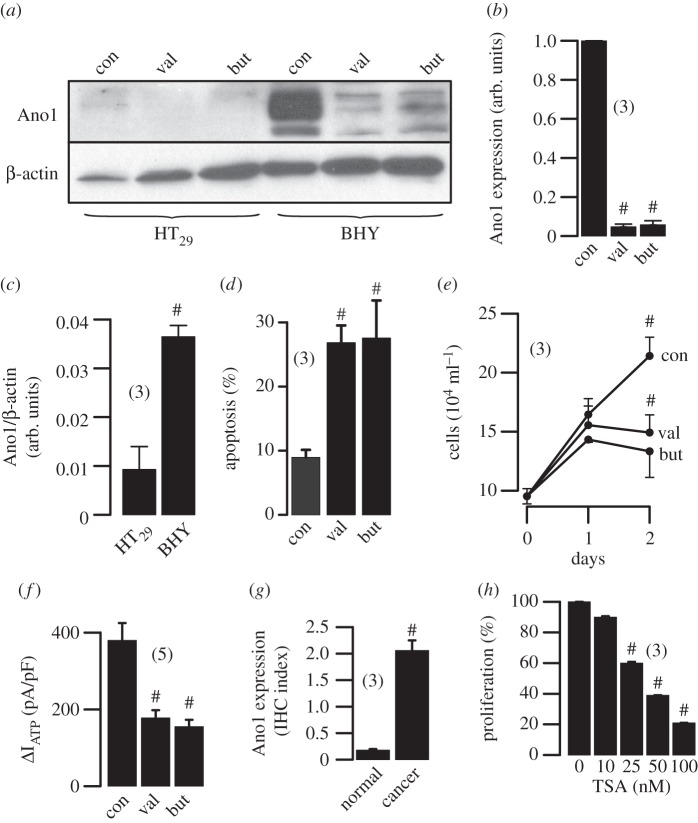
Figure 3.
HDAC regulates expression of Ano1: (a,b) western blots and densitometric analysis indicating expression of Ano1 in HT29 colonic epithelial and BHY HNSCC cells, and inhibition by HDAC inhibitors valproic acid (3 mM) and butyrate (4 mM). ß-actin was used as a loading control. (c) Real-time PCR analysis of Ano1-mRNA expression in HT29 and BHY cells. (d,e) Induction of apoptosis and inhibition of proliferation of BHY cells by valproic acid and butyric acid, as measured by apoptosis assays (see electronic supplementary material) and cell counting. (f) Whole cell Ano1 Cl− currents (Vc = +100 mV) activated by an increase in intracellular Ca2+ owing to stimulation with the purinergic agonist ATP (100 µM). (g) Expression of Ano1 in human HNSCC samples and normal tissue as measured by immunohistochemistry (cf. electronic supplementary material). (h) Inhibition of proliferation of UM-SCC cancer cells by various concentrations of TSA. Mean ± s.e.m. (number of cells). Symbol # denotes significant difference when compared with control, normal tissue, HT29 cells or absence of HDAC inhibitors, respectively (p < 0.05; unpaired t-test).
3. Role of Ano1 for metastasis
How does Ano1 control the ability of tumour cells to migrate and form distal metastasis? The relationship between ion channel currents, cell volume regulation, migration and metastasis is well established [30–33]. Previous findings indicate that Ano1 is activated during hypotonic cell swelling and contributes to regulatory volume decrease (RVD), which certainly requires a rise in intracellular Ca2+ [5,27,34]. A rise in intracellular Ca2+ may activate Ano1 together with K+ channels to release intracellular K+ and Cl− ions. Resulting osmotic loss of intracellular water will cause rapid cell shrinkage and allow passage through narrow gaps like those formed by endothelial cells [33,35] (figure 2). Ca2+-dependent activation of Ano1 would support cell shrinkage at the rear end of migrating cells, thereby further reducing cell volume and facilitating diapedesis. Inhibition of Cl− channels impedes cell volume changes and thereby compromises tumour cell invasion. This has been demonstrated for Ano1 [27] as well as other Cl− channels [33]. Importantly, cell migration requires constant depolymerization and repolymerization of the actin cytoskeleton, which permanently changes cell-matrix adhesions [36,37]. Our earlier findings suggested that Ano1 Cl− currents are controlled by the actin cytoskeleton [38]. This was supported by a subsequent report indicating that Ano1 associates with the signalling/scaffolding proteins ezrin, radixin, moesin and RhoA, which are known to connect plasma membrane proteins to the cytoskeleton [39]. Moreover, results from a two hybrid split ubiquitin screening suggested interaction of Ano1 with a number of proteins related to cell attachment and migration, such as zyxin, fibulin 1, S100A11, twinfilin and catenin (unpublished results from the Kunzelmann laboratory, K. Kunzelman 2012, figure 2).
Figure 2.
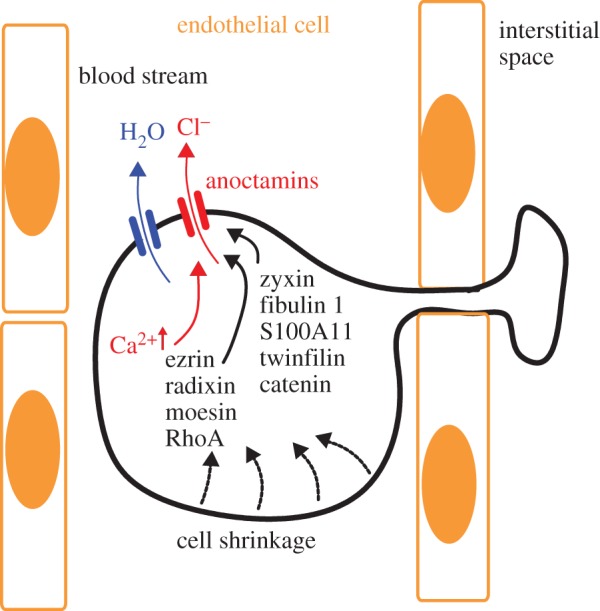
Anoctamins facilitate cell volume decrease and support diapedesis and cell migration: model for diapedesis and migration of a tumour cell expressing anoctamin Cl− channels, which allow Cl− release and osmotic cell shrinkage. Ano1 is regulated by actin and possibly by a number of proteins related to signalling/scaffolding, such as ezrin, radixin, moesin and proteins related to cell attachment and migration, such as zyxin, fibulin 1, S100A11, twinfilin and catenin. (Online version in colour.)
It was shown that members of the Rho GTPase family exert effects on cell shape and motility by regulating actin cytoskeleton; the activation of Rac1 organizes cortical actin cytoskeleton and promotes formation of lamellipodia at the leading edge, a hallmark of a motile cell, while the activation of RhoA at the rear influences acto-myosin complexes to allow retraction of the trailing end. Spatial and temporal regulation of the activity at each end create an unequal distribution of membranous, cytoskeletal and cytoplasmic contents to induce a highly polarized, motile shape that is suitable for movement and metastasis [32]. Thus, the presence of Ano1 at the plasma membrane, its ability to regulate cell shape and volume, and its connections to cytoplasmic/cytoskeletal elements is likely to contribute to cell movement and metastasis.
4. Regulation of expression of Ano1 by histone deacetylase and clinical implications
Recruitment of histone acetyltransferases and histone deacetylases (HDACs) is a key element in the dynamic regulation of genes controlling cellular proliferation and differentiation during normal development as well as carcinogenesis [40,41]. A number of anti-cancer treatments are based on the inhibition of HDAC. HDAC inhibitors promote expression of p21 in breast cancer cells, which inhibits the action of cyclin D1. HDAC inhibitors may therefore also be useful for the treatment of those HNSCC that show overexpression of Ano1 and concomitant activation of cyclin D1 [23]. In fact, HDAC inhibitors have already entered preclinical evaluation [42,43]. In recent experiments, we found that pronounced expression of Ano1 in the HNSCC cell line BHY was largely inhibited by treatment with the HDCA inhibitors valproic acid or butyric acid, along with inhibition of cell survival and Ano1-dependent whole cell currents (figure 3a–f). Although this does not prove that HDAC inhibitors act through downregulation of Ano1, these novel results again demonstrate a correlation between Ano1 expression and proliferation. Corresponding to the data discussed in figure 1, which did not identify a role Ano1 expression for proliferation of HT29 colonic epithelial cells, expression levels for Ano1 were much lower and were not affected by HDCA inhibitors. As pointed out, expression levels for Ano1 are much higher in HNSCC compared with normal tissues (figure 3g). We also found a dose-dependent inhibition of proliferation of another HNSCC cell line, UM-SCC cancer cells, by a third type of HDAC-inhibitor, trichostatin (TSA) (figure 3h). UM-SCC cells contain amplification of the Ano1 gene locus similar to BHY cells. The results confirm that the inhibitory effect of HDAC inhibitors on Ano1 expression is independent of the HNSCC cell line used. While valproic acid and butyric acid are rather broad, non-selective inhibitors of HDAC, TSA selectively suppresses class I/II, suggesting that Ano1 expression is regulated by these HDACs. Although the mechanisms by which TSA promotes loss of cell survival/growth in these cells is incompletely understood, the present results support the use of HDAC inhibitors for the treatment of HNSCC, which may act in part through inhibition of Ano1 expression.
5. Ano1 and sonic hedgehog
Interesting links exist between Ano1 and the sonic hedgehog (Hh) signalling pathway. Hh is coexpressed with Ano1 in the zone of polarizing activity in mouse limb buds during E10.5 and E11.5 [44]. Hh signalling controls many aspects of development and also regulates cell growth and differentiation in adult tissues. It is activated in a number of human malignancies. Hh and Wnt signalling frequently act together in controlling cell growth and tissue morphogenesis. Hh is also active in ‘embryonic cancers’ such as basal cell carcinoma of the skin, stromal cancer [45,46] and also during epithelial to mesenchymal transition. Hh expression has been shown to be upregulated in the neoplastic or inflammatory intestine when stem cells compensate for epithelial damage, while suppression of hedgehog signalling by cyclopamine has been shown to induce apoptosis [47,48]. It will be interesting to learn more about the correlations between Hh signalling, Ano1 expression and cancer.
6. Ano1 required for terminal differentiation?
In cell types others than GIST, Ano1 has been reported to be inhibitory on cell proliferation and expression of Ano1 was related to cellular differentiation [26,49]. These somewhat surprising results were supported by our own results indicating inhibition of cell proliferation by Ano1 in colonic epithelial cells (figure 1a). Interestingly, one study identified Ano1 by expression cloning in oocytes from axolotl, a salamander that does not undergo terminal differentiation and metamorphosis, and therefore maintains an amazing ability to regenerate limbs and other parts of the body [4]. Terminal differentiation is missing in these animals because of a complete lack of thyroid hormones. Maybe more than a coincidence, plasma thyronine levels were found to be abnormally low in patients with advanced colon carcinoma [50]. It might be worth examining whether expression of Ano1 is regulated by these hormones.
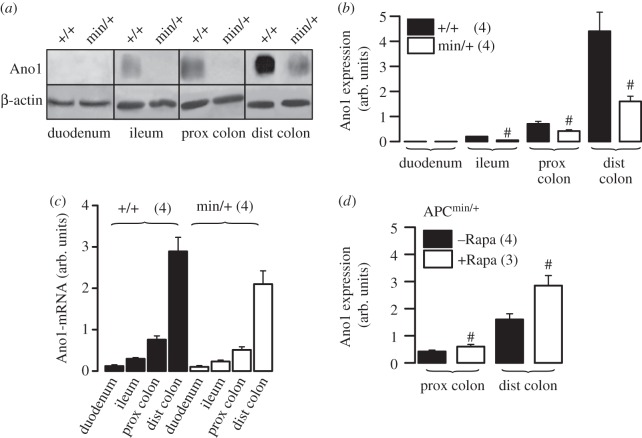
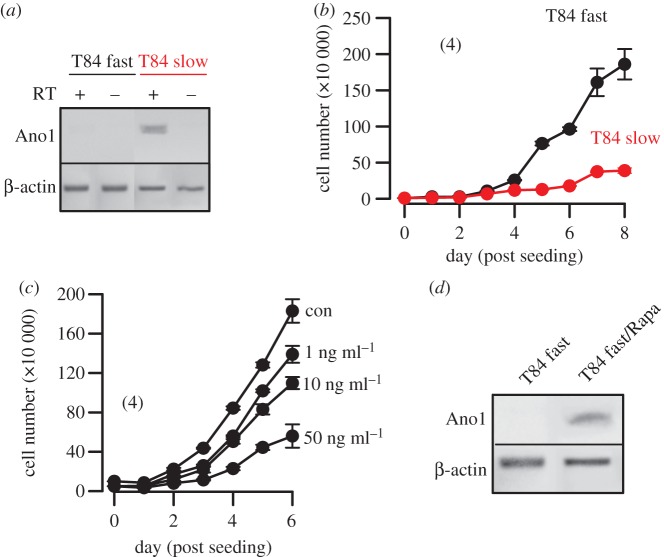
We found evidence for regulation of Ano1 expression by the tumour suppressor adenomatosis polyposis coli (APC) in mouse colon. Ano1 is expressed in mouse ileum, proximal and particularly distal colon, but its expression is largely attenuated in APCmin/+ mice. APCmin/+ mice demonstrate reduced tumour suppressive activity by APC, resulting in upregulation of mTOR, thus leading to numerous large intestinal polyps and ultimately cancer (figure 4a,b) [51]. APCmin/+ mice develop particularly large polyps in the distal colon, where we detected a pronounced decrease in Ano1 expression. Notably, the mTOR-inhibitor rapamycin increased Ano1 expression in both proximal and distal colon (figure 4d). This inverse correlation between low Ano1 levels and upregulation of mTOR [51] suggests that Ano1 may be inhibitory on proliferation of mouse intestinal epithelial cells, similar to HT29 cells. Interestingly, a fast growing subclone of T84 colonic epithelial cells (T84 fast) is lacking expression of Ano1, when compared with the slowly growing parental cells (T84 slow) [28] (figure 5a,b). Notably, treatment of fast growing T84 cells with the mTOR-inhibitor rapamycin reduced proliferation and induced expression of Ano1 (figure 5c,d). These data support the concept that effects of Ano1 on cell survival are cell-type dependent [26,29].
Figure 4.
APC/mTOR controls expression of Ano1 in mouse intestine: (a,b) western blot analysis of expression of Ano1 in duodenum, ileum and colon of APCmin/+ and wild-type mice. (c) Semiquantitiative analysis (see electronic supplementary material) of Ano1-mRNA expression in duodenum, ileum and colon of APCmin/+ and wild-type mice. (d) Treatment with the mTOR-inhibitor rapamycin [51] increased expression of Ano1 in the intestine of APCmin/+ mice. Mice were fed 40 mg kg−1 Sirolimus (Wyeth Pharmaceuticals, Collegeville, PA, USA) in their chow to reach blood levels of 12 ± 2 ng ml−1 rapamycin (n = 66). Mean ± s.e.m. (number of cells). Symbol # denotes significant difference when compared with +/+ or –Rapa, respectively (p < 0.05; unpaired t-test).
Figure 5.
APC/mTOR controls expression of Ano1 and proliferation of T84 cells: (a) RT-PCR analysis of Ano1-mRNA expression in fast growing and slowly growing T84 colonic carcinoma cells. +/− RT indicates the presence of or the absence of reverse transcriptase in the reaction. (b) Growth curves for fast growing and slowly growing T84 cells. (c) Effect of treatment with rapamycin on proliferation of T84 fast cells. (d) RT-PCR analysis of Ano1-mRNA expression in fast growing T84 cells in the absence and the presence of rapamycin. The rapamycin concentration used was 50 ng ml−1. Mean ± s.e.m. (number of cells). (Online version in colour.)
7. Other anoctamins correlated to cancer
Apart from Ano1 and Ano2, much less information is available for other anoctamins. Although a deeper understanding is currently lacking, it should be mentioned that various anoctamins have a role during murine embryogenesis [9]. Ano7 (TMEM17G, NGEP) has been detected in prostate cancer [52,53]. Studies indicate that the long version of NGEP is present on the plasma membrane of overexpressing LNCaP cells and is highly concentrated at cell–cell contact regions [53]. A splice form of Ano6 was identified that was associated with metastatic capability of mammary cancers in mouse and was related to poor prognosis of patients with breast cancer [54]. Notably, Ano6 has recently been associated with membrane phospholipid scrambling and cell shrinkage and therefore seems to be correlated to apoptosis rather than proliferation and cancer [55–59].
8. Role of Ano6 in phospholipid scrambling and apoptosis
The distribution of lipids in the outer and inner leaflets of plasma membranes is asymmetrical: while phosphatidylcholine is mainly found in the outer leaflet, phosphatidylserine is present in the inner surface. During signalling events such as activation of platelets or cellular apoptosis, the distribution is rapidly altered leading to exposure of phosphatidylserine at the outer surface. A lipid transporter with phospholipid scrambling activity was proposed to be responsible for this process; however, a convincing candidate protein with such ability was not identified until recently. Surprisingly, a member of the TMEM16 family, TMEM16F (Ano6) was shown to contain phospholipid scrambling activity, when activated by a large increase in intracellular Ca2+ [58]. Moreover, reconstitution experiments in Ano6-deficient thymocytes suggested that other anoctamins such as Ano3, Ano4, Ano7 and Ano9 retain the ability to function as calcium-dependent phospholipid scramblase [60].
These results came as a surprise, since Ano6 has been characterized as a Ca2+-activated Cl− channel. Moreover, Ca2+-activated Cl− currents were also observed after overexpression of Ano4, 7 and 9 [6,55,56,61,62]. However, Cl− channel and scramblase activity were shown to be independent [55]. Moreover, we demonstrated recently that Ano6 is activated during cell swelling and by pro-apoptotic stimuli and therefore contributes to both RVD as well as apoptotic volume decrease [5,55,56]. Notably, the amplicon 11q13 that contains the Ano1 gene, not only carries genes that control proliferation but also FADD, a gene associated with apoptosis. Thus, anoctamins can also be regarded as a novel family of regulators of cell proliferation and apoptosis, which may be of particular relevance during development, activation of immune cells such as lymphocytes, dendritic cells [63] and macrophages, and in particular types of cancer.
9. Conclusion
Functional analysis of Ano1 and the other members of the anoctamin family have just begun. These proteins were recognized initially as cancer-associated proteins and are now discussed in the context of ion conductance, volume regulation and phospholipid scrambling. Because ion movement, RVD, lipid scrambling and migration are related and are dysfunctional in cancer and metastasis, anoctamins have great potential as therapeutic drugs. Thus, it will be exciting to analyse how inhibitors of anoctamins affect cancer progression, metastasis and the prognosis particularly of patients with head and neck cancer.
Acknowledgements
We thank Prof. L. Bubendorf (Department of Pathology, University of Basel/Switzerland for supplying the HNSCC cell lines.
Funding statement
This work was supported by Deutsche Krebshilfe, Sander Stiftung Förderprojekt no. 2013.031.1 and DFG SFB699/A7. U.D. was supported in part by a Career Development Award from the Department of Veterans Affairs, and funds from the PNC foundation.
References
- 1.AlDehni F, Spitzner M, Martins JR, Barro Soria R, Schreiber R, Kunzelmann K. 2009. Role of bestrophin for proliferation and in epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 20, 1556–1564. ( 10.1681/ASN.2008090987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caputo A, et al. 2008. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594. ( 10.1126/science.1163518) [DOI] [PubMed] [Google Scholar]
- 3.Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. 2009. Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J. Biol. Chem. 284, 28 698–28 703. ( 10.1074/jbc.M109.012120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder BC, Cheng T, Jan YN, Jan LY. 2008. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029. ( 10.1016/j.cell.2008.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K. 2009. TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 284, 28 571–28 578. ( 10.1074/jbc.M109.010074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu T, Iehara T, Sato K, Fujii T, Sakai H, Okada Y. 2013. TMEM16F is a component of a Ca2+-activated Cl− channel but not a volume-sensitive outwardly rectifying Cl− channel. Am. J. Physiol. Cell Physiol. 304, C748–C759. ( 10.1152/ajpcell.00228.2012) [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Perrett S, et al. 2001. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl Acad. Sci. USA 98, 1182–1187. ( 10.1073/pnas.98.3.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber R. 2005. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J. Membr. Biol. 205, 129–137. ( 10.1007/s00232-005-0778-z) [DOI] [PubMed] [Google Scholar]
- 9.Rock JR, Harfe BD. 2008. Expression of TMEM16 paralogs during murine embryogenesis. Dev. Dyn. 237, 2566–2574. ( 10.1002/dvdy.21676) [DOI] [PubMed] [Google Scholar]
- 10.Ardeleanu C, Arsene D, Hinescu M, Andrei F, Gutu D, Luca L, Popescu LM. 2009. Pancreatic expression of DOG1: a novel gastrointestinal stromal tumor (GIST) biomarker. Appl. Immunohistochem. Mol. Morphol. 17, 413–418. ( 10.1097/PAI.0b013e31819e4dc5) [DOI] [PubMed] [Google Scholar]
- 11.Carles A, et al. 2006. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene 25, 1821–1831. ( 10.1038/sj.onc.1209203) [DOI] [PubMed] [Google Scholar]
- 12.Espinosa I, et al. 2008. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 32, 210–218. ( 10.1097/PAS.0b013e3181238cec) [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Pinilla PJ, et al. 2009. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370–G1381. ( 10.1152/ajpgi.00074.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. 2009. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl Acad. Sci. USA 106, 21 413–21 418. ( 10.1073/pnas.0900176106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SJ, et al. 2009. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 587, 4887–4904. ( 10.1113/jphysiol.2009.176198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanich JE, Gibbons SJ, Eisenman ST, Bardsley MR, Rock JR, Harfe BD, Ordog T, Farrugia G. 2011. Ano1 as a regulator of proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G1044–G1051. ( 10.1152/ajpgi.00196.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. 2009. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 16, 66–93. ( 10.2174/092986709787002835) [DOI] [PubMed] [Google Scholar]
- 18.Shen MR, Chou CY, Chiu WT. 2003. Streptomycin and its analogues are potent inhibitors of the hypotonicity-induced Ca2+ entry and Cl− channel activity. FEBS Lett. 554, 494–500. ( 10.1016/S0014-5793(03)01231-6) [DOI] [PubMed] [Google Scholar]
- 19.Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, Mihatsch MJ, Kallioniemi OP, Sauter G. 1999. Tissue microarrays for gene amplification surveys in many different tumor types. Clin. Cancer Res. 5, 1966–1975. [PubMed] [Google Scholar]
- 20.Huang X, Godfrey TE, Gooding WE, McCarty KS, Jr, Gollin SM. 2006. Comprehensive genome and transcriptome analysis of the 11q13 amplicon in human oral cancer and synteny to the 7F5 amplicon in murine oral carcinoma. Genes Chromosomes. Cancer 45, 1058–1069. ( 10.1002/gcc.20371) [DOI] [PubMed] [Google Scholar]
- 21.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. 2003. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 78, 323–335. ( 10.1023/A:1023033708204) [DOI] [PubMed] [Google Scholar]
- 22.Shiu KK, Natrajan R, Geyer FC, Ashworth A, Reis-Filho JS. 2010. DNA amplifications in breast cancer: genotypic-phenotypic correlations. Future. Oncol. 6, 967–984. ( 10.2217/fon.10.56) [DOI] [PubMed] [Google Scholar]
- 23.Duvvuri U, et al. 2012. TMEM16A, induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 72, 3270–3281. ( 10.1158/0008-5472.CAN-12-0475-T) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Lu M, Liu B, Huang Y, Wang K. 2012. Inhibition of Ca2+-activated Cl− channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 326, 41–51. ( 10.1016/j.canlet.2012.07.015) [DOI] [PubMed] [Google Scholar]
- 25.Mazzone A, Eisenman ST, Strege PR, Yao Z, Ordog T, Gibbons SJ, Farrugia G. 2012. Inhibition of cell proliferation by a selective inhibitor of the Ca(2+)-activated Cl(-) channel, Ano1. Biochem. Biophys. Res. Commun. 427, 248–253. ( 10.1016/j.bbrc.2012.09.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, et al. 2012. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension through promoting basilar smooth muscle cell proliferation. Circulation 125, 697–707. ( 10.1161/CIRCULATIONAHA.111.041806) [DOI] [PubMed] [Google Scholar]
- 27.Ruiz C, et al. 2012. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS ONE 7, e43265 ( 10.1371/journal.pone.0043265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzner M, Martins JR, Barro Soria R, Ousingsawat J, Scheidt K, Schreiber R, Kunzelmann K. 2008. Eag1 and bestrophin 1 are upregulated in fast growing colonic cancer cells. J. Biol. Chem. 283, 7421–7428. ( 10.1074/jbc.M703758200) [DOI] [PubMed] [Google Scholar]
- 29.Ayoub C, et al. 2010. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br. J. Cancer 103, 715–726. ( 10.1038/sj.bjc.6605823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies AR, Belsey MJ, Kozlowski RZ. 2004. Volume-sensitive organic osmolyte/anion channels in cancer: novel approaches to studying channel modulation employing proteomics technologies. Ann. NY Acad. Sci. 1028, 38–55. ( 10.1196/annals.1322.004) [DOI] [PubMed] [Google Scholar]
- 31.Kunzelmann K. 2005. Ion channels and cancer. J. Membr. Biol. 205, 159–173. ( 10.1007/s00232-005-0781-4) [DOI] [PubMed] [Google Scholar]
- 32.Schwab A, Fabian A, Hanley PJ, Stock C. 2012. Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913. ( 10.1152/physrev.00018.2011) [DOI] [PubMed] [Google Scholar]
- 33.Sontheimer H. 2008. An unexpected role for ion channels in brain tumor metastasis. Exp. Biol. Med. (Maywood). 233, 779–791. ( 10.3181/0711-MR-308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunzelmann K, et al. 2011. Anoctamins. Pflugers Arch. 462, 195–208. ( 10.1007/s00424-011-0975-9) [DOI] [PubMed] [Google Scholar]
- 35.Cuddapah VA, Sontheimer H. 2011. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 301, C541–C549. ( 10.1152/ajpcell.00102.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer LP. 2010. Forming the cell rear first: breaking cell symmetry to trigger directed cell migration. Nat. Cell Biol. 12, 628–632. ( 10.1038/ncb0710-628) [DOI] [PubMed] [Google Scholar]
- 37.Le Clainche C, Carlier MF. 2008. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513. ( 10.1152/physrev.00021.2007) [DOI] [PubMed] [Google Scholar]
- 38.Tian Y, Kongsuphol P, Hug MJ, Ousingsawat J, Witzgall R, Schreiber R, Kunzelmann K. 2011. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 25, 1058–1068. ( 10.1096/fj.10-166884) [DOI] [PubMed] [Google Scholar]
- 39.Perez-Cornejo P, Gokhale A, Duran C, Cui Y, Xiao Q, Hartzell HC, Faundez V. 2012. Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc. Natl Acad. Sci. USA 109, 10 376–10 381. ( 10.1073/pnas.1200174109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger SL. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12, 142–148. ( 10.1016/S0959-437X(02)00279-4) [DOI] [PubMed] [Google Scholar]
- 41.Jenuwein T, Allis CD. 2001. Translating the histone code. Science 293, 1074–1080. ( 10.1126/science.1063127) [DOI] [PubMed] [Google Scholar]
- 42.Erlich RB, et al. 2012. Preclinical evaluation of dual PI3K-mTOR inhibitors and histone deacetylase inhibitors in head and neck squamous cell carcinoma. Br. J. Cancer 106, 107–115. ( 10.1038/bjc.2011.495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gammoh N, Lam D, Puente C, Ganley I, Marks PA, Jiang X. 2012. Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death. Proc. Natl Acad. Sci. USA 109, 6561–6565. ( 10.1073/pnas.1204429109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rock JR, Lopez MC, Baker HV, Harfe BD. 2007. Identification of genes expressed in the mouse limb using a novel ZPA microarray approach. Gene Expr. Patterns 8, 19–26. ( 10.1016/j.modgep.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 45.Chung CH, et al. 2011. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003). J. Clin. Oncol. 29, 1326–1334. ( 10.1200/JCO.2010.32.3295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelczar P, et al. 2013. Inactivation of patched1 in mice leads to development of gastrointestinal stromal-like tumors that express pdgfrα but not kit. Gastroenterology 144, 134–144. ( 10.1053/j.gastro.2012.09.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman DM, et al. 2003. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425, 846–851. ( 10.1038/nature01972) [DOI] [PubMed] [Google Scholar]
- 48.Qualtrough D, Buda A, Gaffield W, Williams AC, Paraskeva C. 2004. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. Int. J. Cancer 110, 831–837. ( 10.1002/ijc.20227) [DOI] [PubMed] [Google Scholar]
- 49.Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR. 2012. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod. Pathol. 25, 919–929. ( 10.1038/modpathol.2012.57) [DOI] [PubMed] [Google Scholar]
- 50.Rose DP, Davis TE. 1981. Plasma thyronine levels in carcinoma of the breast and colon. Arch. Intern. Med. 141, 1161–1164. ( 10.1001/archinte.1981.00340090057014) [DOI] [PubMed] [Google Scholar]
- 51.Koehl GE, Spitzner M, Ousingsawat J, Schreiber R, Geissler EK, Kunzelmann K. 2010. rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APCMin/+ mice. Oncogene 29, 1553–1560. ( 10.1038/onc.2009.435) [DOI] [PubMed] [Google Scholar]
- 52.Bera TK, Das S, Maeda H, Beers R, Wolfgang CD, Kumar V, Hahn Y, Lee B, Pastan I. 2004. NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc. Natl Acad. Sci. USA 101, 3059–3064. ( 10.1073/pnas.0308746101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das S, Hahn Y, Nagata S, Willingham MC, Bera TK, Lee B, Pastan I. 2007. NGEP, a prostate-specific plasma membrane protein that promotes the association of LNCaP cells. Cancer Res. 67, 1594–1601. ( 10.1158/0008-5472.CAN-06-2673) [DOI] [PubMed] [Google Scholar]
- 54.Dutertre M, et al. 2010. Exon-based clustering of murine breast tumor transcriptomes reveals alternative exons whose expression is associated with metastasis. Cancer Res. 70, 896–905. ( 10.1158/0008-5472.CAN-09-2703) [DOI] [PubMed] [Google Scholar]
- 55.Kmit A, van Kruchten R, Ousingsawat J, Mattheij NJ, Senden-Gijsbers B, Heemskerk JW, Bevers EM, Kunzelmann K. 2013. Calcium-activated and apoptotic phospholipid scrambling induced by Ano6 can occur independently of Ano6 ion currents. Cell Death Dis. 4, e611 ( 10.1038/cddis.2013.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K. 2011. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc. Natl Acad. Sci. USA 108, 18 168–18 172. ( 10.1073/pnas.1016733108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK. 2010. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am. J. Physiol. Cell Physiol. 298, C14–C25. ( 10.1152/ajpcell.00654.2008) [DOI] [PubMed] [Google Scholar]
- 58.Suzuki J, Umeda M, Sims PJ, Nagata S. 2010. Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838. ( 10.1038/nature09583) [DOI] [PubMed] [Google Scholar]
- 59.Yang H, et al. 2012. TMEM16F forms a Ca(2+)-activated cation channel required for lipid scrambling in platelets during blood coagulation. 151, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. 2013. Calcium-dependent phospholipid scramblase activity of TMEM16 family members. J. Biol. Chem. 288, 13 305–13 316. ( 10.1074/jbc.M113.457937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dauner K, Mobus C, Frings S, Mohrlen F. 2013. Targeted expression of anoctamin calcium-activated chloride channels in rod photoreceptor terminals of the rodent retina. Invest. Ophthalmol. Vis. Sci. 54, 3126–3136. ( 10.1167/iovs.13-11711) [DOI] [PubMed] [Google Scholar]
- 62.Tian Y, Schreiber R, Kunzelmann K. 2012. Anoctamins are a family of Ca2+ activated Cl− channels. J. Cell Sci. 125, 4991–4998. ( 10.1242/jcs.109553) [DOI] [PubMed] [Google Scholar]
- 63.Szteyn K, Schmid E, Nurbaeva MK, Yang W, Münzer P, Kunzelmann K, Lang F, Shumilina E. 2012. Expression and functional significance of the Ca2+activated Cl− channel ANO6 in dendritic cells. Cell Physiol. Biochem. 30, 1319–1332. ( 10.1159/000343321) [DOI] [PubMed] [Google Scholar]