Abstract
The change of a normal, healthy cell to a transformed cell is the first step in the evolutionary arc of a cancer. While the role of oncogenes in this ‘passage’ is well known, the role of ion transporters in this critical step is less known and is fundamental to our understanding the early physiological processes of carcinogenesis. Cancer cells and tissues have an aberrant regulation of hydrogen ion dynamics leading to a reversal of the normal tissue intracellular to extracellular pH gradient (ΔpHi to ΔpHe). When this perturbation in pH dynamics occurs during carcinogenesis is less clear. Very early studies using the introduction of different oncogene proteins into cells observed a concordance between neoplastic transformation and a cytoplasmic alkalinization occurring concomitantly with a shift towards glycolysis in the presence of oxygen, i.e. ‘Warburg metabolism’. These processes may instigate a vicious cycle that drives later progression towards fully developed cancer where the reversed pH gradient becomes ever more pronounced. This review presents our understanding of the role of pH and the NHE1 in driving transformation, in determining the first appearance of the cancer ‘hallmark’ characteristics and how the use of pharmacological approaches targeting pH/NHE1 may open up new avenues for efficient treatments even during the first steps of cancer development.
Keywords: pH and cancer, NHE1, angiogenesis, invasion, growth factors, tumour microenvironment
1. Introduction
For the last 30 years, a gene-centric approach has predominated cancer biology creating a perception of cancer as a complex collection of diseases unrelated among themselves and has led to the idea of a tailored therapy for each patient based on the tumours' gene expression pattern. A major paradigm shift is now occuring towards the search for the fundamental underlying principles that could form a unified theory of transformation, progression and metastasis. This reductionist ‘recasting’ of cancer as a single disease could correspondingly permit the development of more general therapeutic strategies that exploit common underlying forces. This approach to cancer at the level of its metabolic character and constraints has led to the unifying paradigms that tumours depend on angiogenesis (endothelial-centric paradigm) and on aerobic glycolytic metabolism (metabolic-centric paradigm). Importantly, these two processes can interact through a novel ‘pH-centric paradigm’, which helps to develop the tumour metabolic microenvironment (TMM) and further drive metastatic progression.
In this regard, both ion transport and cytoplasmic pH play crucial roles in multiple cell functions including control of cell growth and proliferation, growth factor activity, cell membrane potential, mitochondrial activity, cell volume, enzyme activity, DNA synthesis, differentiation, oncogenesis and oncogene action [1–3]. A great deal of accumulating evidence over the last years has amply demonstrated that practically all tumours have in common a pivotal characteristic: the aberrant regulation of hydrogen ion dynamics [1–5]. Cancer cells have an acid–base balance that is completely different from that observed in normal tissues and that increases with the increasing neoplastic state: an extracellular acid microenvironment (pHe) linked to a ‘malignant’ alkaline intracellular pH (pHi). Indeed, tumour cells have the alkaline pHi values of 7.12–7.7 versus 6.99–7.05 in normal cells while producing acidic pHe values of 6.2–6.9 versus 7.3–7.4 in normal cells. This creates a reversed pH gradient (ΔpHi to ΔpHe) across the cell membrane that is markedly displaced from the electrochemical equilibrium for protons, which increases as the tumour progresses. This specific and pathological reversal of the pH gradient in cancer cells and tissues compared with normal tissue completely alters their thermodynamic molecular energetics, regardless of their pathology and genetic origins and can now be considered to be a defining characteristic of tumour cells [4,6,7]. Indeed, the induction and/or maintenance of intracellular alkalinization and its subsquent extracellular acidosis [2,4,6,7] have been repeatedly implicated as playing a pivotal role in the maintenance and active progression of the neoplastic process [1,2,4].
The development and maintenance of this reversed pH gradient is directly owing to the proton (H+) secretory ability of the tumour cells and increases with increasing tumour aggressiveness [2] and local tumour hypoxia [8]. This proton secretion depends on the buffering capacity of the cell and is driven by a series of transporters and enzymes, including carbonic anhydrases (CAs), vacuolar H+-ATPases, the H+/Cl− symporter, the monocarboxylate transporter (MCT, mainly MCT1; also known as the lactate-proton symporter), the Na+-dependent Cl−/HCO3− exchangers and ATP synthase (for reviews, see [1,2,4,9]).
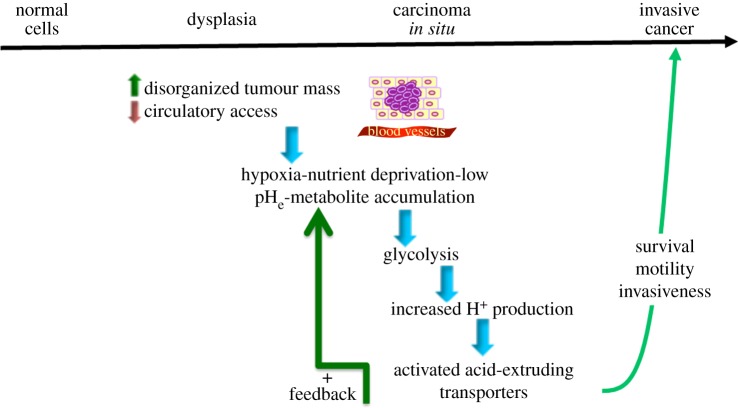
The prevailing hypothesis most often considers the formation of the reversed pH gradient to be a characteristic of advanced, hypoxic tumours where the classical hypoxia-induced glycolytic metabolism is turned on creating high intracellular lactate and proton levels with a consequent upregulation of proton and lactate extruders to compensate such that the cytosol is alkalinized [8]. The inefficient removal of protons and lactic acid from the extracellular space, owing to the poorly perfused dense tumour tissue, creates the acidic extracellular microenvironment and the reversed pH gradient (figure 1, adapted from [8]).
Figure 1.
The tumour microenvironment selects for increased acid extrusion. The basic steps of the classical scheme of oncogenic evolution are shown above the upper line. The advance of the tumour along this line is usually thought to be driven by mutations that activate neoplastic oncogenes or inactivate tumour suppressors. Recently, there has been an increase in our understanding concerning the mechanisms by which hypoxia acidifies the extracellular tumour microenvironment by increasing glycolytic metabolism concomitantly with the upregulation of a series of proton transporters that result in the well-described reversed pH gradient that may well be the most specific tumour hallmark of all and that drives many of the other tumour characteristic behaviours. Many of these tumour-specific pH-regulatory proteins are transcriptionally regulated by the hypoxia-inducible factor-1 (HIF-1α) the stability of which is increased by hypoxia in the poorly vascularized and insufficiently perfused tumour environment. The changes in pH-regulating proteins shown in the figure confer advantages to the cancer cells and play an important role in tumour metastasis and in resistance to therapy. (Adapted from [8].) (Online version in colour.)
However, when and how this characteristic cytosolic alkalinization of tumour cells first takes place is less clear. There are data demonstrating that the first steps of this pH gradient reversal take place at the earliest phases of neoplastic transformation and is strongly associated with the first appearance of glycolysis in the presence of oxygen; the so-called Warburg effect.
2. The first appearance of the reversed proton gradient in oncogene-driven neoplastic transformation and the role of NHE1
This cancer cell-specific increased proton secretion with the resultant initiation of the reversed proton gradient appears during the very first steps of neoplastic transformation. Oncogene-dependent transformation results in a rapid cytoplasmic alkalinization as an elevated pHi together with increased NHE1 activity was very early on implicated as a crucial factor in neoplastic transformation driven by the ras and v-mos oncogenes [10,11]. These studies also observed that these oncogene-dependent transformations resulted in an increased glycolysis and it was not clear at the time whether the driving factor for increased pHi was the stimulated NHE1 or the increased glycolysis. This question was resolved in a study using the inducible expression of an oncogene (HPV16 E7) to dissect the time-dependence of the appearance of the the hallmarks demonstrated that the first step in oncogene-dependent transformation of normal cells is the activation of the NHE1 with the subsequent cytosolic alkalinization [12]. A kinetic analysis of the activation of the NHE1 demonstrated that the oncogene-driven neoplastic transformation constitutively activates NHE1 by increasing the affinity of the allosteric proton regulatory site increasing the sensitivity of the NHE1 to the intracellular protons and increasing its activity with a resultant intracellular alkalinization and extracellular acidification. This alkalinization drove the subsequent development of a series of transformation/cancer hallmarks, such as increased growth rate, substrate-independent growth, growth factor independence, glycolysis in aerobic conditions and tumour growth in nude mice [12]. Altogether, these data demonstrate that oncogenes use NHE1-induced alkalinization to produce very early the unique cancer-specific altered pH regulation with the resulting pH-profile and the hallmark phenotypes characteristic of cancer cells [13].
3. Role of pH in developing and maintaining Warburg metabolism
Another unique hallmark of cancer cells that is receiving ever increasing attention is their shift to glycolytic metabolism relative to oxidative phosphorylation (OxyPhos), even under aerobic conditions. This was first described by Warburg [14], hence known as the Warburg effect. As stated above, early experiments of the oncogene activation showed the first appearance of glycolytic metabolism to be an early effect/consequence of oncogene-driven transformation of normal cells [10,11].
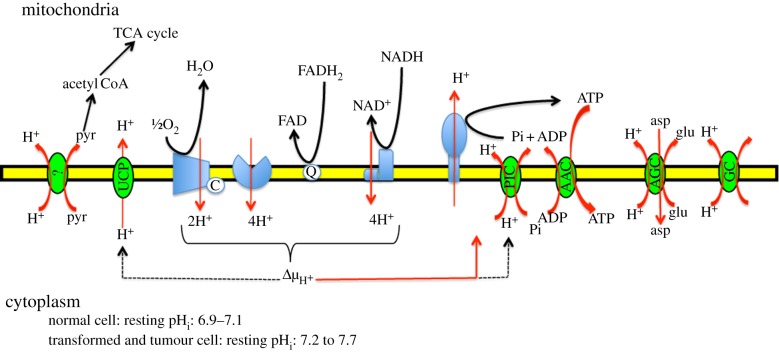
There is further evidence that both pHi and pHe are important in driving this ever increasing dependence on glycolysis and decreasing dependence on OxyPhos as the tumour cell progresses (reviewed in [6,7]). Briefly, as both the processes of OxyPhos and gycolysis are exquisitely but oppositely pH sensitive, a rapid shift of cell metabolic patterns follows alkalinization probably through the modulation of multiple proteins in unison to control this process. Indeed, on the one hand, alkaline pHi even slightly above steady-state levels stimulates the activity of glycolytic enzymes, such as phosphofructokinase-1 (PFK-1) and lactate dehydrogenase while inhibiting gluconeogenesis [15–19]. These changes in enzyme function probably occur through dynamic changes in protein conformation driven by post-translational modifications via the rapid and reversible change in the charge of amino acid side chains by protons [20,21]. As this type of post-translational modification does not require an enzyme, it permits the sensitive adaptation of the system to small and rapid shifts in cytosolic pH. On the other hand, the proper functioning of numerous mitocondrial proton transporters and proton-driven transporters that are involved in regulating OxyPhos metabolism has a strong dependence on a relatively high cytosolic proton concentration [6]. In all, at least 10 transporters regulating mitocondrial activity depend on a high, constant, regulated cytosol–mitocondrial proton gradient (figure 2). Interestingly, high levels of ATP inhibit glycolysis at two points (phosphofructokinase-1 and pyruvate kinase-1) and, therefore, the reduction of ATP produced as a result of the lowered trans-inner mitocondrial membrane proton gradient would relax this inhibition and thus further stimulate the glycolytic chain. Altogether, this reciprocal metabolic shift may well be the most sensitive cellular pHi sensor of all.
Figure 2.
Model of pH-dependent mitochondrial processes. Simplified schematic view of the H+-dependent mitochondrial processes occuring across the mitochondrial inner membrane, including the OxyPhos machinery and various phosphate and nutrient transporters. Complexes I (NADH dehydrogenase) and II (succinate dehydrogenase) receive electrons from either NADH or FADH2. Electrons are then carried between complexes by the carrier molecules coenzyme Q/ubiquinone (UQ) and cytochrome c (CYC). The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. ATP synthesis by Complex V (ATP synthase) is driven by the proton gradient and occurs in the mitochondrial matrix. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor. A total of 10 protons are ejected from the mitochondrial matrix per two electrons transferred from NADH to oxygen via the respiratory chain. Therefore, when the cytosol of the transformed cell becomes alkaline, the much larger volume of the cytosol together with the very high permeability of the outer membrane to protons means that the protons pumped out of the matrix are dispersed onto the cytosol and the whole system runs-down. In addition, the F1F0 ATP synthase is a reversible enzyme, and so a reduction in proton concentration in the intermembrane space could even result in a consumption of ATP. Further, this altered proton concentration will also reduce the inner membrane potential that is fundamental for driving the adenine nucleotide translocase (ADP/ATP carrier), an antiporter that catalyses exchange of ADP for ATP across the inner mitochondrial membrane. At normal cellular pH, ATP has four negative charges, while ADP has three negative charges. ADP3-/ATP4- exchange is driven by, and uses up, the membrane potential generated by respiration (one charge per ATP). Furthermore, phosphate then re-enters the mitochondrial matrix with H+, by an electroneutral symport mechanism. Pi entry is driven by and uses up the pH gradient (equivalent to one mole of H+ per mole of ATP). (Online version in colour.)
Altogether, this evidence supports the hypothesis that it is the alkaline pHi that is the driver of this metabolic shift and this pHi-dependent shift is one of the ‘cornerstones’ in the altered metabolism that the pH perturbation creates. Indeed, a recent paper added further weight to this conclusion observing, using a novel NHE1 inhibitor, that the Warburg effect may be explained completely through the elevation of pHi in cancer cells [22]. An added depth and complexity to this field comes from the demonstration that lower pHe (in both the presence and absence of extracellular lactate) has profound effects on tumour cell gene expression, including genes involved in glycolysis [23] and that inhibition of the NHE1 results in changes in expression patterns of a number of genes including many that regulate metabolism [24].
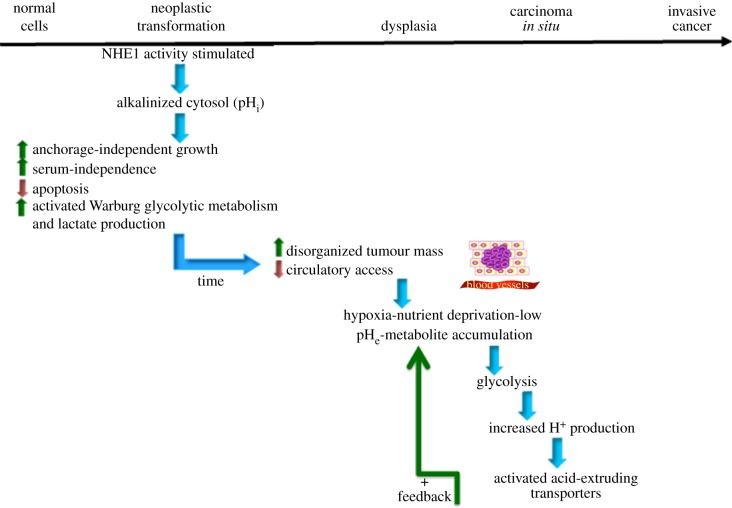
These complex dynamics of the pH–metabolism interaction engages a vicious cycle from very early on: the oncogene-driven alkalinization increases glycolysis and proliferation, generating a need for a high energy consumption which maintains a high proton production that, through stimulated proton efflux transport systems, further alkalinizes the cell that even further reduces OxyPhos and increases glycolysis. Figure 3 shows how this very early alteration of pH dynamics and consequent metabolic disruption sets the stage for the conditions necessary for the later stages of metastatic progression and illustrates the pH-centric paradigm for carcinogenesis and metastatic progression mentioned in the Introduction. Interestingly, neoplastic progression is the result of clonal selection of increasingly more aggressive cells. However, the accumulation of genetic defects resulting in malignant cells is faster than that theoretically predicted. Recently, this contradiction has been resolved with the observation that the tumour microenvironment drives the selection of aggressive cells within a tumour by contributing to tumour genetic instability [25–27]. Thus, cancer is an example of a synergistic, positive feedback interaction of genotype and phenotype in which the resulting phenotype from the initial genotypic alteration sets the stage for further genotypic alterations.
Figure 3.
Unified carcinogenic evolutionary scheme from pH centric viewpoint. On the basis of the studies discussed in this review, we can now see that the alkalinazation of the cytosol occurs even in the very first steps of oncogene-driven neoplastic transformation and is probably the fundamental physiological alteration used by the oncogene to transform a normal cell (left side of scheme). Most importantly, the alkalinization is a key event for the establishment and maintenance of oncogenic transformation. Upon expression of an oncoprotein, the cells develop a series of phenotypes characteristic of neoplastic transformation: increased growth rate, anchorage-independent growth, serum-independent growth, increased Warburg glycolysis and in vivo tumour development in nude mice. The development of these tumour hallmarks during transformation are inhibited by blocking the alkalinization via the clamping of pHi at neutral values or by inhibiting NHE1 confirming that activation of the NHE1 and the resulting cellular alkalinization is a key mechanism in oncogenic transformation and is essential for the development and maintenance of the transformed phenotype. Interestingly, deoxyglucose treatment can decrease the glycolysis to the levels of transformed cells treated with the NHE1 inhibitor, DMA, but has little effect on cellular growth in comparison with the DMA treatment [12]. Therefore, glycolysis appears not to play an important role in the increase in growth rate observed upon transformation. Altogether, this suggests that targeting either the tumour pH or NHE1 directly could be important therapeutic strategies even in the early stages of cancer. (Adapted from [8].) (Online version in colour.)
This fundamental role of the NHE1 in modulating cellular metabolism is perhaps not surprising considering the postulated role of ancient NHEs together with the ancient ATP synthase in the origin of chemiosmotic coupling and membrane bioenergetics [28]. Indeed, ancient NHEs are thought to have played a key role from the time of proto-cells to permit the development of ever less permeable membranes and ever higher levels of carbon and energy fluxes, (i.e. energy production and harnessing) by permitting cells dependent on the proton gradients supplied from the environment also to pump sodium ions.
4. The first steps in the development of the tumour microenvironment
As stated above, this increase in pHi of the transformed cell drives obligate tumour DNA synthesis, cell cycle progression and both substrate-independent and serum-independent growth, resulting in a pathological and disorganized increase in cell number and density [4,29]. A consequence of increased tumour cell density is a corresponding decrease in access to circulation that creates an hypoxic condition reducing the cells ability to run their mitochondrial oxidative respiratory chain and increasing the need to fulfil their energy demand through glycolytic metabolism and increased glucose consumption.
Glycolysis is much less efficient than oxidative metabolism in producing ATP (two molecules of ATP per molecule of glucose, compared to up to 38 ATP per glucose in a full cycle of glycolysis–Krebs cycle–OxyPhos). More importantly, each round of glycolysis produces two protons, challenging the tumour cell with an ever increasing acid load [30] and pHi would rapidly decline which could be lethal if not compensated for by increased proton extrusion which results in additional pHe acidification [29]. The alkaline shift in the pHi dependence of the NHE1 observed upon transformation greatly increases the acid extrusion ability of the transformed cell and, thus, may comprize the first transformation alteration that can drive the subsequent development of the microenvironment characteristic of neoplastic cells (figure 3).
Therefore, an adaptative feature of cancer cells, and especially of highly aggressive cancer cells, is the overexpression and the increased activity of multiple pH-regulating transporters and enzymes such as V-ATPase [4,30], CAs [31,32], the proton-linked MCTs [33,34] and Cl−/HCO3− exchangers. As an example, NHE1 is overexpressed in cervical cancer [35] and hepatocellular carcinoma [36] and is correlated with clinical outcome, while its activity is upregulated in glioma [37] and breast cancer cells [12,38].
The increasing hypoxia of the tumour also necessitates a new blood supply that is achieved through neoangiogenesis, whereby new blood vessels are formed from pre-existing ones [29]. However, neoplastic vascularization occurs uncoordinatedly, resulting in a chaotic, functionally poor vasculature incapable of meeting tumoural demands of oxygen and serum and causing an efficient washout of metabolic products (i.e. carbonic acid) which even further exacerbates the low pHe. The physiological environment, tumour metabolism, angiogenesis and vasularization are, therefore, inextricably linked. Altogether, these processes give rise to the tumour-specific metabolic microenvironment defined as extracellular areas within tumours characterized by dynamic, interacting areas of (i) hypoxia, (ii) low serum nutrients and (iii) acidic pHe. Studies have demonstrated a pathogenic role of both low nutrients and the acidic interstitial pHe of tumours by giving a selective advantage for tumour progression and metastasis. Low pHe together with low nutrients [39] or low pHe alone have been shown to drive large changes in gene expression independently of hypoxia [23,40,41] and have also been associated with tumour progression by impacting multiple processes including increased invasion [23,41–43] and metastasis [39,44,45]. In this context, low nutrient concentrations [46,47] or low pHe [48] have been shown to preferentially stimulate NHE1 activity in tumour cells but not in normal cells which further drives the vicious positive feedback cycle. Accordingly, emphasis is shifting toward elucidating the unique responses of cancer cells to their own microenvironment and determining how this contributes to metastasis.
5. Mechanism underlying increased NHE1 activity and alkalinization during transformation
NHE1 is a member of a family of integral membrane secondary active acid extruders that mediate the electoneutral 1 : 1 exchange of extracelluar sodium for intracellular protons across the cell membrane with a Km for extracellular sodium ranging from 10 to 50 mM. Through its action, the inwardly directed sodium gradient can drive the uphill extrusion of protons that alkalinizes pHi and acidifies pHe. For a detailed review of the structure and biophysical characteristics of NHE1 please refer to a recent review [8].
One of NHE1’s fundamental characteristics is the exquisite sensitivity to pHi through an internal allosteric proton-binding regulatory site such that when pHi drops below a threshold level it is activated and, in this way, intracellular protons are an important allosteric regulator of NHE1 activity independently of their function as a substrate for the exchange with external sodium [49]. This pHi sensitivity determines its activity set-point, i.e. the pHi at which it first starts to be activated and, in normal cells, the set-point is at their physiological, resting pHi such that the NHE1 is quiescent. It becomes activated only when the cell is acidified and functions to return the cell to neutral pHi and this activation results in a sigmoid regulatory dependence of NHE1 activity on the intracellular proton concentration. This same process is used to increase NHE1 activity in growth factor-induced cells and another study to determine the mechanism of tumour cell activation demonstrated that serum removal stimulated NHE1 activity specifically in tumour cells through a PI3K-dependent increase of the affinity of this allosteric site [46]. Oncogene-driven neoplastic transformation was found to constitutively activate NHE1 and raise pHi by increasing the affinity of this allosteric proton regulatory site which mimicks the lowering of cytosolic pH [12]. It was recently demonstrated that a PKA-RhoA-induced inhibition of p38alpha MAPK is involved in this mechanism [50].
There are two additional regulatory mechanisms for the activity of NHE1 that might play a secondary role in transformation. The cytoplasmic tail contains numerous ser/thr residues, some of which are constitutively phosphorylated in quiescent cells [51] and are further phosphorylated in response to extracellular stimuli [52]. Additionally, the cytoplasmic tail also contains numerous binding sites for multiple protein partners, such that the NHE1 is also able to act as a scaffolding protein [53–55]. These partner proteins include the 14-3-3 adaptor protein, calcineurin homologous protein, CA II, calmodulin, ERM proteins (ezrin, radixin and moesin), heat shock protein 70 (HSP70) and PI(4,5)P2 [8,55,56]. Recently, a direct binding with B-Raf that activates NHE1 was described [57]. Additionally, through its binding to the actin-binding protein ezrin, NHE1 can directly regulate cytoskeleton dynamics independently of its ion transporting capabilities [58]. Together with transport, these three activities make the NHE1 a very important membrane-bound integrator for many signalling networks and cellular processes and these aspects of the role of NHE1 in the regulation of neoplastic transformation has yet to be studied.
There is now ample evidence that in addition to these above stimuli tumour cell NHE1 is further activated by the components of the TMM previously described [29]: low serum [47,47], acidic pHe [48] and hypoxia [8,59–61], which links these components into a dynamic, reciprocal system that drives further microenvironmental acidification and malignant progression starting from the first moments of neoplastic transformation. Altogether, these data lead to the recognition of a synergistic, positive feedback interaction between the tumour cell and both the metabolic and stromal microenvironments in tumours and suggests that NHE1 may have an important role in integrating these interactions.
6. Other pH-regulating systems that could be secondarly involved in driving and maintaining the altered pH dynamics in transformed cells and in the development of the tumour metabolic microenvironment
As stated above, there is increasing evidence that oncogenic transformation modifies the metabolic programme of cells and that a common alteration is the upregulation of glycolysis with the subsequent production of lactate in the cytosol. The MCTs are a family of symporters involved in the transmembrane transport of lactate, pyruvate and ketone bodies together with protons [62]. Thus, MCTs would not only remove lactate from the cytosol but bring about the expulsion of hydrogen ions and in the attempt to increase pHi would decrease pHe. MCTs are known to have important roles in cancer as studies have shown that inhibition of MCT1, both in vitro [63–65] and in vivo [64], decreased pHi and retarded tumour growth. This specificity to glycolytic tumours has suggested that its presence might be used to specifically supply therapeutic substances [66]. The upregulation of MCTs would also allow the continuous conversion of glucose to lactate. Therefore, while there is currently no data to demonstrate that one or more of the MCT isoforms are upregulated, the fact that lactate is starting to be produced in larger levels during transformation could mean that they could have a secondary role in the further development of the alkalinized phenotype. Indeed, it is possible that the positive feedback aspect shown in figure 3 activated by the increased lactate and proton load could secondarily activate one of the MCT isoforms.
CA activity has been found to be important in maintaining uniformly alkaline pHi in small tumour spheroids [67], and CAIX was recently found to be broadly localized in the interior of rat brain C6 tumour [68]. CAIX is one of the most important upregulated proteins by HIF-1alpha in response to hypoxia both in normal and cancerous tissues [69]. Interestingly, the activity of NHE1 has also been shown to be enhanced via its direct binding to CAII [70,71], although the relevance of this interaction in tumour cells has yet to be determined. As another consequence of the transformation/pHi-driven upregulation of glycolysis would also be the subsequent over-production of CO2 in the cytosol, it is possible that even in the absence of hypoxia the transformed cell upregulates the expression and/or activity of a CA isoform.
7. Implications for therapy: targeting NHE1 in the first stages of oncogenesis
(a). Emerging implications of NHE1 disregulated activity in early diagnosis and therapy
The idea of an acid–base approach to the treatment of cancer dates back from the early 1930s [72]. Owing to the importance of NHE1 in numerous physiological and pathophysiological processes, a number of inhibitors have been developed. The most part belong to two groups of modifications of the structure of the the first compounds found to have inhibitory activity: the K+-sparing diuretic, amiloride. Amiloride, however, also inhibits the epithelial Na+ channel ENaC, the Na+/Ca2+ exchanger (NCX) and the acid-sensing cation channel-1 which is part of the ENaC family. Furthermore, while NHE1 is the isoform most sensitive to amiloride, NHE2 is also inhibited and to a lesser extent NHE5 [73]. While not being a specific inhibitor of NHE1, amiloride has been used as a cancer therapeutic in both animal models and clinically where its use had clear anti-neoplastic effects with few side effects [74]. A series of more specific NHE1 inhibitory drugs based on the chemical scaffold of amiloride that were designed using double substitutions of the nitrogen of the 5-amino pyrazine derivatives at the R5 and R5′ groups and had a slightly higher inhibitory activity and specificity for NHE1 and very low activity towards NCX and ENaC [75]. Some of the best known and most studied of these pyrazines are dimethylamiloride (DMA; R5:-CH3 and R′5: -CH3), N-ethylisopropylamiloride (EIPA; R5:-C2H5 and R′5: -CH(CH3)2) and HMA (-(CH2)6-).
Two additional sets of alterations have given rise to a new series of inhibitors where the pyrazine moiety of amiloride was substituted with a phenyl ring or a heterocycle pyridine to produce benzoylguanidines of which two have been passed phase trials: the simultaneous substitution of the 6-chloro by a sulfomethyl with the deletion of the 2-amino or its replacement by a methyl group gave rise to the benzoylguanidine group of inhibitors cariporide (HOE-642; R2: -H and R5: -CH(CH3)2 [76] and eniporide (EMD85131; R2: -CH3 and R5: -N ring [77]. These compounds no longer inhibited the ENaC and the Na+/Ca+ exchanger and became much more selective towards NHE1. Inhibitors of this series have been shown effective in retarding tumour development in mice [12] or in rendering chemiotherapy more effective [78,79].
Besides amiloride, the only compounds with NHE1 inhibitory activity that have undergone clinical trials are cariporide and eniporide, however these trials were not in the field of cancer but for ischaemic-reperfusion injury. Importantly, the potency of cariporide and some other NHE inhibitors is related to the ionization state of the guanidine residues that depends on the pH to be positively charged: e.g. zoniporide (pKa = 7.2), TY-12533 (pKa = 6.93) and, especially, cariporide (pKa = 6.28) [75,80–82], making these compounds more efficient at inhibiting NHE1. Indeed, cariporide will be even more active at very low pHe (i.e. IC50 = 22 versus 120 nM at pHe 6.2 and 6.7, respectively [83]. Therefore, the acidic tumour microenvironment, which can be as low as 6.2, could turn out to be an advantage in terms of dose-dependent side effects as these compounds, especially cariporide, would be more efficient at inhibiting NHE1.
Clearly, a clinically reasonable approach would try to minimize the systemic dose of the drug in order to dissociate the adverse effects, and probably off-target effects, from the beneficial effects. This could probably already be the case due to the increase in efficacy at low pHe for cariporide described above and is also the idea considered in using therapeutic strategies combining NHE1 inhibitors with chemiotherapeutic agents and/or ‘biologic’ targets such as growth factor receptor inhibitors, anti-angiogenic therapies and/or hyperthermia. As cariporide, eniporide and/or amiloride have passed all clinical phases, a potential future direction could be a combinatorial therapy of NHE1 inhibitors with inhibitors of one or both of these individual therapies (for recent reviews, see [3,8,55,56,74,84,85]). Interestingly, in this context, rats having a lifelong treatment with cariporide had a greatly extended lifespan and this was interpreted as being due to a reduced occurrence of cancer [76].
An interesting additional strategy targeting the low extracellular pH of tumours comes from recent technology addressing the development of a novel class of pH responsive luminescent gold nanoparticles (that importantly are renal clearable) that selectively bind to cells in an acidic environment to improve the delivery of known chemotherapeutic agents specifically to the tumour [86]. An advantage of these novel pH responsive nanoparticles is that they can be used to specifically target the acidic microenvironment of tumour cells irrespective of tumour type and can be used as imaging probes for early diagnosis of tumours, thus combining early cancer detection and treatment methods.
Funding statement
This work was supported by grant no. 11348 of the Italian Association for Cancer Research (AIRC) and PRIN grant 2009 N.1341. The S.J.R. laboratory is part of the Italian network ‘Istituto Nazionale Biostrutture e Biosistemi’ (INBB), the ‘Centro di Eccellenza di Genomica in Campo Biomedico ed Agrario’ of the University of Bari and the project ‘BioBoP’ of the Region Puglia. There are no potential conflicts of interest.
References
- 1.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. 2009. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 29, 2127–2136. [PubMed] [Google Scholar]
- 2.Harguindey S, Orive G, Luis Pedraz J, Paradiso A, Reshkin SJ. 2005. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer: two faces of the same coin—one single nature. Biochim. Biophys. Acta 1756, 1–24. [DOI] [PubMed] [Google Scholar]
- 3.Provost JJ, Wallert MA. 2013. Inside out: targeting NHE1 as an intracellular and extracellular regulator of cancer progression. Chem. Biol. Drug Des. 81, 85–101. ( 10.1111/cbdd.12035) [DOI] [PubMed] [Google Scholar]
- 4.Cardone RA, Casavola V, Reshkin SJ. 2005. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795. ( 10.1038/nrc1713) [DOI] [PubMed] [Google Scholar]
- 5.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. 2011. Imaging pH and metastasis. NMR Biomed. 24, 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderon-Montano J, Burgos-Moron E, Perez-Guerrero C, Salvador J, Robles A, Lopez-Lazaro M. 2011. Role of the Intracellular pH in the metabolic switch between oxidative phosphorylation and aerobic glycolysis: relevance to cancer. WebmedCentral CANCER 2, WMC001716. [Google Scholar]
- 7.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. 2011. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol. 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boedtkjer E, Bunch L, Pedersen SF. 2012. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr. Pharm. Des. 18, 1345–1371. ( 10.2174/138161212799504830) [DOI] [PubMed] [Google Scholar]
- 9.Parks SK, Chiche J, Pouyssegur J. 2011. pH control mechanisms of tumor survival and growth. J. Cell Physiol. 226, 299–308. ( 10.1002/jcp.22400) [DOI] [PubMed] [Google Scholar]
- 10.Doppler W, Jaggi R, Groner B. 1987. Induction of v-mos and activated Ha-ras oncogene expression in quiescent NIH 3T3 cells causes intracellular alkalinisation and cell-cycle progression. Gene 54, 147–153. ( 10.1016/0378-1119(87)90357-X) [DOI] [PubMed] [Google Scholar]
- 11.Hagag N, Lacal JC, Graber M, Aaronson S, Viola MV. 1987. Microinjection of ras p21 induces a rapid rise in intracellular pH. Mol. Cell Biol. 7, 1984–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. 2000. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 14, 2185–2197. ( 10.1096/fj.00-0029com) [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 14.Warburg O. 1956. On respiratory impairment in cancer cells. Science 124, 269–270. [PubMed] [Google Scholar]
- 15.Kuwata F, Suzuki N, Otsuka K, Taguchi M, Sasai Y, Wakino H, Ito M, Ebihara S, Suzuki K. 1991. Enzymatic regulation of glycolysis and gluconeogenesis in rabbit periodontal ligament under various physiological pH conditions. J. Nihon Univ. Sch. Dent. 33, 81–90. ( 10.2334/josnusd1959.33.81) [DOI] [PubMed] [Google Scholar]
- 16.Peak M, al-Habori M, Agius L. 1992. Regulation of glycogen synthesis and glycolysis by insulin, pH and cell volume: interactions between swelling and alkalinization in mediating the effects of insulin. Biochem. J. 282, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietl K, et al. 2010. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 184, 1200–1209. ( 10.4049/jimmunol.0902584) [DOI] [PubMed] [Google Scholar]
- 18.Chiche J, Brahimi-Horn MC, Pouyssegur J. 2010. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J. Cell Mol. Med. 14, 771–794. ( 10.1111/j.1582-4934.2009.00994.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santo-Domingo J, Demaurex N. 2012. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the renaissance of mitochondrial pH. J. Gen. Physiol. 139, 415–423. ( 10.1085/jgp.201110767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb BA, Chimenti M, Jacobson MP, Barber DL. 2011. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677. ( 10.1038/nrc3110) [DOI] [PubMed] [Google Scholar]
- 21.Schönichen A, Webb BA, Jacobson MP, Barber DL. 2013. Considering protonation as a posttranslational modification regulating protein structure and function. Annu. Rev. Biophys. 42, 289–314. ( 10.1146/annurev-biophys-050511-102349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata H, Che XF, Miyazawa K, Tomoda A, Konishi M, Ubukata H, Tabuchi T. 2011. Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK45 and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncol. Rep. 25, 341–346. [DOI] [PubMed] [Google Scholar]
- 23.Chen JL, et al. 2010. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS Genet. 6, e1001093 ( 10.1371/journal.pgen.1001093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putney LK, Barber DL. 2004. Expression profile of genes regulated by activity of the Na–H exchanger NHE1. BMC Genomics 5, 46 ( 10.1186/1471-2164-5-46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RK, Tsan R, Radinsky R. 1997. Influence of the host microenvironment on the clonal selection of human colon carcinoma cells during primary tumor growth and metastasis. Clin. Exp. Metastasis 15, 140–150. ( 10.1023/A:1018400826845) [DOI] [PubMed] [Google Scholar]
- 26.Reynolds TY, Rockwell S, Glazer PM. 1996. Genetic instability induced by the tumor microenvironment. Cancer Res. 56, 5754–5757. [PubMed] [Google Scholar]
- 27.Radinsky R. 1995. Modulation of tumor cell gene expression and phenotype by the organ-specific metastatic environment. Cancer Metastasis Rev. 14, 323–338. ( 10.1007/BF00690601) [DOI] [PubMed] [Google Scholar]
- 28.Lane N, Martin WF. 2012. The origin of membrane bioenergetics. Cell 151, 1406–1416. ( 10.1016/j.cell.2012.11.050) [DOI] [PubMed] [Google Scholar]
- 29.Vaupel P, Okunieff P, Neuringer LJ. 1989. Blood flow, tissue oxygenation, pH distribution, and energy metabolism of murine mammary adenocarcinomas during growth. Adv. Exp. Med. Biol. 248, 835–845. ( 10.1007/978-1-4684-5643-1_95) [DOI] [PubMed] [Google Scholar]
- 30.Griffiths JR. 1991. Are cancer cells acidic? Br. J. Cancer 64, 425–427. ( 10.1038/bjc.1991.326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. 2009. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368. ( 10.1158/0008-5472.CAN-08-2470) [DOI] [PubMed] [Google Scholar]
- 32.Supuran CT. 2008. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181. ( 10.1038/nrd2467) [DOI] [PubMed] [Google Scholar]
- 33.Chiche J, Le Fur Y, Vilmen C, Frassineti F, Daniel L, Halestrap AP, Cozzone PJ, Pouysségur J, Lutz NW. 2012. In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int. J. Cancer 130, 1511–1520. ( 10.1002/ijc.26125) [DOI] [PubMed] [Google Scholar]
- 34.Pouyssegur J, Dayan F, Mazure NM. 2006. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443. ( 10.1038/nature04871) [DOI] [PubMed] [Google Scholar]
- 35.Chiang AC, Massague J. 2008. Molecular basis of metastasis. N. Engl. J. Med. 359, 2814–2823. ( 10.1056/NEJMra0805239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Wang D, Dong W, Song Z, Dou K. 2010. Over-expression of Na+/H+ exchanger 1 and its clinicopathologic significance in hepatocellular carcinoma. Med. Oncol. 27, 1109–1113. ( 10.1007/s12032-009-9343-4) [DOI] [PubMed] [Google Scholar]
- 37.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. 2000. Malignant gliomas display altered pH regulation by NHE1 compared with nontransformed astrocytes. Am. J. Physiol. Cell Physiol. 278, C676–C688. [DOI] [PubMed] [Google Scholar]
- 38.Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec J-Y, Roger S, Gore J. 2011. Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H+ efflux in caveolae. Oncogene 30, 2070–2076. ( 10.1038/onc.2010.574) [DOI] [PubMed] [Google Scholar]
- 39.Schlappack OK, Zimmermann A, Hill RP. 1991. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br. J. Cancer 64, 663–670. ( 10.1038/bjc.1991.378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rofstad EK. 2000. Microenvironment-induced cancer metastasis. Int. J. Radiat. Biol. 76, 589–605. ( 10.1080/095530000138259) [DOI] [PubMed] [Google Scholar]
- 41.Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, Gatenby RA, Gillies RJ. 2008. Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis 25, 411–425. ( 10.1007/s10585-008-9145-7) [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Zaguilan R, Seftor EA, Seftor REB, Chu Y-W, Gillies RJ, Hendrix MJC. 1996. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 14, 176–186. ( 10.1007/BF00121214) [DOI] [PubMed] [Google Scholar]
- 43.Giusti I, D'Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, Pavan A, Dolo V. 2008. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia 10, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. 2006. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 66, 6699–6707. ( 10.1158/0008-5472.CAN-06-0983) [DOI] [PubMed] [Google Scholar]
- 45.Martin NK, Gaffney EA, Gatenby RA, Maini PK. 2010. Tumour–stromal interactions in acid-mediated invasion: a mathematical model. J. Theor. Biol. 267, 461–470. ( 10.1016/j.jtbi.2010.08.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V. 2000. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na+/H+ exchange, motility, and invasion induced by serum deprivation. J. Biol. Chem. 275, 5361–5369. ( 10.1074/jbc.275.8.5361) [DOI] [PubMed] [Google Scholar]
- 47.Cardone RA, et al. 2007. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol. Biol. Cell 18, 1768–1780. ( 10.1091/mbc.E06-07-0617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busco G, et al. 2010. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 24, 3903–3915. ( 10.1096/fj.09-149518) [DOI] [PubMed] [Google Scholar]
- 49.Aronson PS, Nee J, Suhm MA. 1982. Modifier role of internal H+ in activating the Na+–H+ exchanger in renal microvillus membrane vesicles. Nature 299, 161–163. ( 10.1038/299161a0) [DOI] [PubMed] [Google Scholar]
- 50.Cardone RA, et al. 2008. HPV16 E7-dependent transformation activates NHE1 through a PKA-RhoA-induced inhibition of p38alpha. PLoS ONE 3, e3529 ( 10.1371/journal.pone.0003529) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Sardet C, Franchi A, Pouyssegur J. 1989. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56, 271–280. ( 10.1016/0092-8674(89)90901-X) [DOI] [PubMed] [Google Scholar]
- 52.Meima ME, Webb BA, Witkowska HE, Barber DL. 2009. The sodium–hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J. Biol. Chem. 284, 26 666–26 675. ( 10.1074/jbc.M109.019448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgartner M, Patel H, Barber DL. 2004. Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am. J. Physiol. Cell Physiol. 287, C844–C850. ( 10.1152/ajpcell.00094.2004) [DOI] [PubMed] [Google Scholar]
- 54.Meima ME, Mackley JR, Barber DL. 2007. Beyond ion translocation: structural functions of the sodium–hydrogen exchanger isoform-1. Curr. Opin. Nephrol. Hypertens. 16, 365–372. ( 10.1097/MNH.0b013e3281bd888d) [DOI] [PubMed] [Google Scholar]
- 55.Amith SR, Fliegel L. 2013. Regulation of the Na+/H+ exchanger (NHE1) in breast cancer metastasis. Cancer Res. 73, 1259–1264. ( 10.1158/0008-5472.CAN-12-4031) [DOI] [PubMed] [Google Scholar]
- 56.Stock C, Ludwig FT, Schwab A. 2012. Is the multifunctional Na+/H+ exchanger isoform 1 a potential therapeutic target in cancer? Curr. Med. Chem. 19, 647–660. ( 10.2174/092986712798992101) [DOI] [PubMed] [Google Scholar]
- 57.Karki P, Li X, Schrama D, Fliegel L. 2011. B-Raf associates with and activates the NHE1 isoform of the Na+/H+ exchanger. J. Biol. Chem. 286, 13 096–13 105. ( 10.1074/jbc.M110.165134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Putney LK, Denker SP, Barber DL. 2002. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 42, 527–552. ( 10.1146/annurev.pharmtox.42.092001.143801) [DOI] [PubMed] [Google Scholar]
- 59.Rios EJ, Fallon M, Wang J, Shimoda LA. 2005. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L867–L874. ( 10.1152/ajplung.00455.2004) [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Wang D, Dong W, Song Z, Dou K. 2010. Inhibition of Na+/H+ exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett. 295, 198–204. ( 10.1016/j.canlet.2010.03.001) [DOI] [PubMed] [Google Scholar]
- 61.Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM. 2011. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS ONE 6, e28851 ( 10.1371/journal.pone.0028851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. 2012. Role of monocarboxylate transporters in human cancers: state of the art. J. Bioenerg. Biomembr. 44, 127–139. ( 10.1007/s10863-012-9428-1) [DOI] [PubMed] [Google Scholar]
- 63.Fang J, Quinones QJ, Holman TL, Morowitz MJ, Wang Q, Zhao H, Sivo F, Maris JM, Wahl ML. 2006. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol. Pharmacol. 70, 2108–2115. ( 10.1124/mol.106.026245) [DOI] [PubMed] [Google Scholar]
- 64.Sonveaux P, et al. 2008. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahl ML, Owen JA, Burd R, Herlands RA, Nogami SS, Rodeck U, Berd D, Leeper DB, Owen CS. 2002. Regulation of intracellular pH in human melanoma: potential therapeutic implications. Mol. Cancer Ther. 1, 617–628. [PubMed] [Google Scholar]
- 66.Birsoy K, et al. 2013. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 45, 104–108. ( 10.1038/ng.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hulikova A, Vaughan-Jones RD, Swietach P. 2011. Dual role of CO2/HCO3− formula buffer in the regulation of intracellular pH of three-dimensional tumor growths. J. Biol. Chem. 286, 13 815–13 826. ( 10.1074/jbc.M111.219899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grillon E, Farion R, Fablet K, De Waard M, Tse CM, Donowitz M, Rémy C, Coles JA. 2011. The spatial organization of proton and lactate transport in a rat brain tumor. PLoS ONE 6, e17416 ( 10.1371/journal.pone.0017416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald PC, Winum JY, Supuran CT, Dedhar S. 2012. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 3, 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. 2006. A novel carbonic anhydrase II binding site regulates NHE1 activity. Biochemistry 45, 2414–2424. ( 10.1021/bi051132d) [DOI] [PubMed] [Google Scholar]
- 71.Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. 2002. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 277, 36 085–36 091. ( 10.1074/jbc.M111952200) [DOI] [PubMed] [Google Scholar]
- 72.Goldfeder A. 1953. Some recent developments in the cancer field. J. Am. Med. Womens Assoc. 8, 124–130. [PubMed] [Google Scholar]
- 73.Chambrey R, Achard JM, St John PL, Abrahamson DR, Warnock DG. 1997. Evidence for an amiloride-insensitive Na+/H+ exchanger in rat renal cortical tubules. Am. J. Physiol. 273, C1064–C1074. [DOI] [PubMed] [Google Scholar]
- 74.Matthews H, Ranson M, Kelso MJ. 2011. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int. J. Cancer 129, 2051–2061. ( 10.1002/ijc.26156) [DOI] [PubMed] [Google Scholar]
- 75.Masereel B, Pochet L, Laeckmann D. 2003. An overview of inhibitors of Na+/H+ exchanger. Eur. J. Med. Chem. 38, 547–554. ( 10.1016/S0223-5234(03)00100-4) [DOI] [PubMed] [Google Scholar]
- 76.Scholz W, Albus U, Counillon L, Gögelein H, Lang HJ, Linz W, Weichert A, Schölkens BA. 1995. Protective effects of HOE642, a selective sodium–hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc. Res. 29, 260–268. [PubMed] [Google Scholar]
- 77.Baumgarth M, Beier N, Gericke R. 1997. (2-Methyl-5-(methylsulfonyl)benzoyl)guanidine Na+/H+ antiporter inhibitors. J. Med. Chem. 40, 2017–2034. ( 10.1021/jm960768n) [DOI] [PubMed] [Google Scholar]
- 78.Miraglia E, Viarisio D, Riganti C, Costamagna C, Ghigo D, Bosia A. 2005. Na+/H+ exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int. J. Cancer 115, 924–929. ( 10.1002/ijc.20959) [DOI] [PubMed] [Google Scholar]
- 79.Lauritzen G, Jensen MBF, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF. 2010. NBCn1 and NHE1 expression and activity in δNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp. Cell Res. 316, 2538–2553. ( 10.1016/j.yexcr.2010.06.005) [DOI] [PubMed] [Google Scholar]
- 80.Guzman-Perez A, et al. 2001. Discovery of zoniporide: a potent and selective sodium-hydrogen exchanger type 1 (NHE-1) inhibitor with high aqueous solubility. Bioorg. Med. Chem. Lett. 11, 803–807. ( 10.1016/S0960-894X(01)00059-2) [DOI] [PubMed] [Google Scholar]
- 81.Aihara K, et al. 2000. Cardioprotective effect of TY-12533, a novel Na+/H+ exchange inhibitor, on ischemia/reperfusion injury. Eur. J. Pharmacol. 404, 221–229. ( 10.1016/S0014-2999(00)00613-0) [DOI] [PubMed] [Google Scholar]
- 82.Fukumoto S, Imamiya E, Kusumoto K, Fujiwara S, Watanabe T, Shiraishi M. 2002. Novel, non-acylguanidine-type Na+/H+ exchanger inhibitors: synthesis and pharmacology of 5-tetrahydroquinolinylidene aminoguanidine derivatives. J. Med. Chem. 45, 3009–3021. ( 10.1021/jm0104567) [DOI] [PubMed] [Google Scholar]
- 83.Xue J, Haddad GG. 2010. The Na+/H+ exchanger: a target for therapeutic intervention in cerebral ischemia. In New strategies in stroke intervention. Ionic transporters, pumps, and new channels (ed. Lucio A.), pp. 113–128. Totowa, NJ: Humana Press. [Google Scholar]
- 84.Loo SY, Chang MK, Chua CS, Kumar AP, Pervaiz S, Clement MV. 2012. NHE-1: a promising target for novel anti-cancer therapeutics. Curr. Pharm. Des. 18, 1372–1382. ( 10.2174/138161212799504885) [DOI] [PubMed] [Google Scholar]
- 85.Reshkin SJ, Cardone RA, Harguindey S. 2013. Na+–H+ exchanger, pH regulation and cancer. Recent Pat. Anticancer Drug Discov. 8, 85–99. [DOI] [PubMed] [Google Scholar]
- 86.Mengxiao Y, Zhou C, Liu J, Hankins JD, Zheng J. 2011. Luminescent gold nanoparticles with pH-dependent membrane adsorption. J. Am. Chem. Soc. 133, 11 014–11 017. ( 10.1021/ja201930p) [DOI] [PMC free article] [PubMed] [Google Scholar]