Abstract
Cancer must be viewed as a ‘tissue’, constituted of both transformed cells and a heterogeneous microenvironment, the ‘tumour microenvironment’ (TME). The TME undergoes a complex remodelling during the course of multistep tumourigenesis, hence strongly contributing to tumour progression. Ion channels and transporters (ICTs), being expressed on both tumour cells and in the different cellular components of the TME, are in a strategic position to sense and mediate signals arising from the TME. Often, this transmission is mediated by integrin adhesion receptors, which are the main cellular receptors capable of mediating cell-to-cell and cell-to-matrix bidirectional signalling. Integrins can often operate in conjunction with ICT because they can behave as functional partners of ICT proteins. The role of integrin receptors in the crosstalk between tumour cells and the TME is particularly relevant in the context of pancreatic cancer (PC), characterized by an overwhelming TME which actively contributes to therapy resistance. We discuss the possibility that this occurs through integrins and ICTs, which could be exploited as targets to overcome chemoresistance in PC.
Keywords: tumour microenvironment, ion channels, integrins, cell signalling, pancreatic cancer, desmoplastic reaction
1. Introduction
Tumour biology can be understood only taking into account both the individual transformed cells and the ‘tumour microenvironment’ (TME). The TME is a complex array of cells and extracellular matrix (ECM) proteins that tumour cells construct during the course of multistep tumourigenesis [1] and strongly influences the behaviour and malignancy of the transformed cells. Moreover, the TME may change during tumour progression, hence it may differ (structurally and functionally) from the primary tumour to its metastases [2,3]. The TME greatly varies among cancers of different histogenesis. For example, in leukaemias, it is mainly represented by the bone marrow, with the complex array of stromal and vascular cells which constitute the bone marrow nike, where leukaemia stem cells reside [4]. In carcinomas, a clear distinction is made between the neoplastic cells, named as the ‘parenchyma’, and the TME, indicated as the ‘tumour stroma’. An active and overwhelming tumour stroma (in this case, addressed as ‘desmoplastic reaction’) characterizes some specific carcinomas, such as breast, prostate or pancreatic cancer (PC) [5]. In particular, the desmoplastic reaction is one of the histopathological and functional hallmarks of PC: histopathological analysis reveals the presence of dense collagen (types I and III) bundles associated with fibroblasts, with loss of basement integrity and invasion of malignant cells into the interstitial matrix with exposure of collagens. The ECM in PC also contains fibronectins, tenascin-C, laminins, mainly secreted by pancreatic stellate cells (PSCs), a cellular phenotype peculiar of PC [6]. The desmoplastic reaction in PC is associated with an abnormal vasculature with numerous circuitous small leaky blood vessels and capillaries [7]. On the whole, the desmoplastic reaction is one of the major contributors to PC malignancy (see below).
Taking into account the relevance of the tumour stroma, antineoplastic therapeutic strategies must be tuned to target the ‘cancer tissue’, e.g. not only tumour cells, but also the cellular constituents of the TME [8,9]. In this context, deciphering the role of ion channel and transporter (ICT) proteins in the crosstalk between the tumour cells and the various constituents of the TME merits particular attention, also from a therapeutic viewpoint.
In this review, we briefly describe the TME as well as ICTs present in the different cells of the TME. For molecular and functional description of ICTs, we refer to other papers [10]. Then, we focus on adhesion receptors of the integrin family, and on their functional interaction with ICT. Because most of these data have been reported elsewhere [11], in this review, we mainly focus on PC, where the TME drives tumour progression and resistance to therapy.
2. The tumour microenvironment and its ion channels and transporters profile
The TME comprises both cells (endothelial cells and their precursors, fibroblasts and specialized mesenchymal cells as well as cells of the innate and specific immunity) and the proteins of the ECM. The main ICTs expressed in the cells of the TME have been detailed in [11] and are summarized in table 1. We must remember the prevalent role of Ca2+-permeable channels (both voltage-dependent and non-selective channels of the TRP family) in endothelial cells (ECs; reviewed in [18,19]). The electrochemical driving force for Ca2+ entry is provided by Ca2+-dependent K+ channels (KCa), and the cooperation between Ca2+-permeable channels and KCa can serve to sustain the Ca2+-dependent secretion of growth and vasodilating factors by ECs. Because many recent studies are revealing distinctive gene expression profiles and cell-surface markers of tumour-associated versus normal ECs [20], it is possible that ICT can contribute to determine such difference. Owing to the relevant role of ECs and tumour angiogenesis in tumour progression, ICTs could be exploited to develop novel anti-angiogenesis therapies to selectively target the ECs inside the cancer tissue.
Table 1.
Examples of ion channels expressed by different cell types participating to the TME complexity.
| TME cells | ion channel type | evidence of ion channel expression |
|---|---|---|
| human mesenchymal cells | ||
| endothelial cells | TRPC1, TRPC4, TRPC6, TRPM7, TRPV1, and TRPV4 | [12] |
| endothelial cells | Orai1ans Stim1 (CRAC channels) | [12] |
| endothelial cells | KCa | [11] |
| bone marrow mesenchymal cells | KATP | [13] |
| bone marrow–umbilical cord vein mesenchymal cells | KV1.1, KV4.2, KV1.4, Kir2.1, heag1, MaxiK, hNE-Na, and TWIK-1 | [14] |
| mesenchymal cells | nicotinic and muscarinic receptors | [14] |
| innate immune cells | ||
| neutrophils | TRP, KCa, Cl | [11] |
| macrophages | KIR, ligand-gated cation channel P2X7 | [11] |
| specific immune cells | ||
| lymphocytes | Kv1.3, KCa3.1, TRP | [15–17] |
Inside the TME, both ‘cancer-associated fibroblasts’ [21] and specialized mesenchymal cells, such as myofibroblasts or the PSCs [22], are present. Although cancer-associated fibroblasts and specialized mesenchymal cells are not malignant, in that they do not bear cancerogenic mutations, they can exhibit epigenetic changes, which affect their behaviour [23]. Moreover, they are actively secreting ECM proteins, behaving as the main determinant of the desmoplastic reaction. The latter, directly or through the creation of a hypoxic environment, regulates tumour progression and dictates therapy resistance [24].
Finally, the TME is densely infiltrated by cells of both the innate and adaptive arms of the immune system, whose exact role in controlling tumour progression is still debated [25,26]. Neutrophil ion channels (mainly TRPs, KCa and Cl– channels; reviewed in [27]) are exploited to accomplish the antimicrobial activity which characterizes these cells of innate immunity. Macrophages express KIR channels, which are involved in cell adhesion, and in turn affect the Ca2+-dependent macrophage activation [28]. Macrophages also express P2X7 receptors, which mediate the release of lysosomal cathepsin [29]. This fact could contribute to ECM remodelling, with a strong impact on malignant progression. The complex array of ion channels which contribute to T lymphocyte activation has been thoroughly described [15,16]: a coordinated influx of Ca2+ is indeed essential to trigger T lymphocyte activation, and an unique contingent of ion channels (including Kv1.3 and KCa3.1 K+ channels) orchestrate the duration and intensity of the Ca2+ signals. Moreover, the balance of these channel types constitutes a specific functional marker of activated lymphocytes, thus providing a possible novel therapeutic target [11].
The ECM of the TME comprises proper matrix proteins, a multitude of growth factors and cytokines that, more or less directly, affect malignant cells [30]. Type I collagen, fibronectin and thrombospondins are the ECM proteins which characterize the TME, with collagen I being the main determinant of the desmoplastic reaction. ECM proteins can affect tumour progression by controlling cell motility as well as by dictating tumour cell differentiation programmes [30]. The ECM of the TME is produced by both tumour and mesenchymal cells, which also modulate their ECM by secreting proteases [31]. TME remodelling can lead to the release of molecules sequestered in the ECM, such as the vascular endothelial growth factors [32], and many cleavage products of ECM proteins [2], which further control tumour angiogenesis and metastasis formation.
The crosstalk between the ECM and tumour cells, as well as of the cellular components of the TME, is mainly mediated through the intervention of adhesion receptors of the integrin family.
3. Cell adhesion molecules: the integrin family
(a). Integrin structure and function
Integrin receptors are transmembrane proteins formed by non-covalent association of α- and β-subunits. To date, 18 α- and eight β-subunits are known in mammals. All subunits are type I transmembrane glycoproteins with a short cytoplasmic tail (20–70 amino acids), a membrane-spanning helix and a large multidomain extracellular portion [33]. The β4-subunit is an exception because its cytoplasmic domain contains around 1000 amino acids [34]. Integrin subunits can combine to form at least 24 functional heterodimers, each of which binds a specific array of ECM proteins, or cell adhesion molecules (CAMs), that act as ‘counterreceptors’.
Integrins are more than CAMs: they can transmit bidirectional signals across the plasma membrane. On the cell surface, integrins are normally in the low affinity state, and they can be activated through an ‘inside-out’ signalling pathway. During this process, two cellular activators, talin and kindlin, play an essential role, and the binding of talin to the β integrin cytoplasmic tail is proposed to be the final step in integrin activation [35]. Conversely, the binding of the integrin extracellular ligands transmits signals inward, a process called ‘outside-in’ signalling. Outside-in signalling of integrin exerts significant influences on cell mobility, proliferation, differentiation, etc. [36]. Because the list of papers describing the many facets of integrin-mediated signalling pathways is immense, we refer to those [33,37,38], and only limit to summarize that integrins seem to be linked to almost all of the known signalling pathways, including induction of cytosolic kinases, stimulation of the phosphoinositide metabolism, activation of Ras/MAPK and protein kinase C (PKC) pathways and regulation of small GTPases. Moreover, integrin signalling often overlaps with that triggered by growth factor or cytokine receptors [38]. The overlap and proper integration of differently arising signals, makes physiological sense, because cells must integrate multiple stimuli from the ECM, growth factors, hormones and mechanical stress, to organize appropriate responses. The same integration occurs and determines the fate of even more in tumour cells inside the cancer tissue.
(b). Integrin relationships with ion channels
The relationships between integrins and ion channels in the cell-to-cell and cell-to-matrix interactions have been long described [39]. The earliest indications came from studies on neuronal and leukaemic cells, in which many cellular processes elicited by the engagement of adhesive proteins, such as differentiation, migration and neurite extension, turned out to depend on ion channel activation [40–43]. When associated with integrins, ion channel function becomes bidirectional itself: it is regulated by extracellular signals (through integrins) and in turn controls integrin activation and/or expression [39]. Interestingly, the same kind of complex bidirectional signalling has been observed for some ion transporters, in particular those mediating proton fluxes [44–46], which are so relevant in the establishment of a reversed H+ gradient which characterizes neoplastic malignancy [47].
The bidirectional crosstalk between integrins and ion channels occurs through different mechanisms: it may rely on cytoplasmic messengers, such as Ca2+ or protein kinases, commuting between the two proteins (reviewed in [39]). For example, T lymphocyte activation in which β1 integrins are involved is underlied by a coordinated influx of Ca2+, which is controlled by and, conversely, regulates K+ channels [16]. The transmission of mechanical forces at focal adhesion sites is triggered by integrins and mediated by calcium [48], but also involves the activation of signalling molecules, such as FAK and c-Src [49].
Another aspect of integrin/ion channel interaction is the fact that integrins and ion channels can interact directly at the plasma membrane level. In other words, the two proteins co-assemble on the plasma membrane and give rise to supramolecular complexes, which constitute platforms for triggering and orchestrating downstream intracellular signals. The first evidence was obtained in immune cells by Levite et al. [50], who found that the β1 integrin subunit associated with Kv1.3 channels in T lymphocytes. Shortly afterwards, a physical link between Kv1.3 channels and β1 integrins was described in melanoma cells [51]. Our group found that the β1 integrin subunit associates with another K+ channel, Kv 11.1 or hERG1, on the plasma membrane of tumour cells, either leukaemias or solid cancers [52–56]. This complex can also involve growth factor or chemokine receptors and, once assembled, recruits cytosolic signalling proteins, which in turn activate intracellular signalling in an integrin- and ion channel-dependent manner. This has a clear negative impact on the leukaemia disease [54], can trigger chemoresistance [55] or control angiogenesis and tumour progression [56].
Another mechanism involving the interaction between integrins and ion channels contributes to determine integrin recycling [57]. In particular, CLIC3 chloride channels colocalize with active α5β1 integrins in late endosomes/lysosomes, allowing the integrin to be retrogradely transported and recycled to the plasma membrane at the cell rear. This mechanism also involves Rab25 and has a clear impact on cancer behaviour. In fact, in PC, active integrins and CLIC3 are necessary for cancer cell invasion [57].
(c). Integrins and ion channels: role in cell migration
A most interesting aspect regards the comprehension of several mechanisms by which integrins and different channel types interact in controlling cell migration. Besides being a fundamental component of embryogenesis and tissue remodelling in the adult, these processes are relevant in tumour cell invasiveness and metastatic spread. As typical mediators of cell interaction with the environment, it is not surprising that integrins play major roles in eukaryotic cell migration. Moreover, we are nowadays aware that several types of Ca2+-activated and voltage-dependent K+ channels are also implicated in the cell migration machinery. This rapidly growing field has been reviewed recently [58,59] and will not be discussed in detail here. We limit our discussion to the fact that K+ channels can form complexes, and thereby modulate several proteins involved in cell movement, such as FAK [60,61], cortactin [62,63] and integrins themselves. Interesting speculations can derive from studies on α9β1 integrins, which can regulate cell movement by activating inward rectifier K+ channels (IRK) [64]. IRK channels, along with the integrin, are physically linked to spermidine/xspermine N1-acetyltransferase, the key enzyme in the pathway that acetylates spermine and spermidine to putrescine, thus controlling the intracellular concentration of polyamines. Polyamines are critical regulators of neoplastic growth and also the main intracellular messengers controlling IRK activity. A functional network may hence be figured out, where an adequate intracellular concentration of polyamines converges to trigger a proper α9β1-dependent cell movement, through the modulation of IRK channels [65].
(d). Integrins and ion channels in the cells of the tumour microenvironment
Integrins and ion channels also interact at the level of the TME. One example involves cells of the innate immune system: neutrophils release Cl– to accomplish their antimicrobial activity; Cl– release occurs through the activation of Cl– channels which is, at least in part, dependent on β2 integrin-mediated adherence to fibronectin [66]. Macrophages express KIR channels, whose activity is modulated by VLA4 (α4β1) integrin receptors and hence by cell adhesion, which in turn affects the Ca2+-dependent macrophage activation [28]. Another example is represented by ECs and their Cl channels of the CLCA protein family. In ECs, CLCA2 behaves as a vascular addressin for metastatic, blood-borne, cancer cells, facilitating vascular arrest of cancer cells via adhesion to β4 integrins, and hence promoting metastatic spread. In addition, the β4-integrin–CLCA complex stimulates Src-dependent cell signalling through FAK and extracellular signal-regulated kinase (ERK), leading to increased proliferation of metastatic foci [67].
A list of ion channels physically or functionally linked to integrins in the cells of TME is reported in table 2.
Table 2.
Examples of interactions between ion channels and integrin subunits in cells of TME.
| cell type | ion channel type | integrin subunit type | evidence of ion channel expression |
|---|---|---|---|
| endothelial cells | Ca2+ channels (BKCa–increased cytosolic Ca2+) | β1, β3 | [39,68] |
| endothelial cells | TRPV4 | β1 | [11] |
| vascular smooth muscle cells | L.type Cav1.2 | α5β1 | [39] |
| neutrophils | Cl– | β2 | [45] |
| lymphocytes | Kv 1.3, KCa | β1 | [16,50] |
4. The role of the tumour microenvironment in the progression of pancreatic cancer
PC, mainly represented by the histological form of pancreatic ductal adenocarcinoma, is one of the most lethal gastrointestinal malignancies, representing the second leading cause of death among them. The overall 5-year survival rate is less than 6%, in the most recent American Database (http://seer.cancer.gov/csr/1975_2006), and a median survival of 18 months from diagnosis for those operated-on patients with no evidence of residual disease. Similar disappointing figures are available from European surveys [69]. The malignant nature of PC is mainly due to its aggressive growth and rapid development of distant metastases, thus making treatment extremely difficult. Additionally, PC is locally invasive, surrounded by a dense desmoplastic reaction (see §1) which can involve adjacent vital structures, limiting the chance for complete resection. Indeed, less than 20% of patients are candidates for surgical resection at the time of diagnosis, while almost one half have metastatic disease. Moreover, of the few patients who undergo surgery with radical intent (R0), most will develop a recurrence within 15 months. Although surgery remains the cornerstone of cure, the addition of adjuvant treatments is required [70]. Chemotherapy (in Europe) or radiochemotherapy (in North America) has been used, either as adjuvant to surgery or as definitive treatment for unresectable disease, with conflicting results [71,72]. Failure of traditional therapeutic approaches for this devastating disease had led to many efforts towards the study of molecular biology and targeted therapies, in order to create a multimodal therapeutic strategy [73]. Among target therapy drugs, only erlotinib that targets the EGF-R, has been shown to improve survival when used in combination with gemcitabine, compared with gemcitabine alone. Nevertheless, the clinical response rate remains modest, mainly owing to the intrinsic chemoresistance of PC cells [74]. Investigating mechanisms mediating chemoresistance is therefore of clinical interest in drug development of new agents. As stated in §1 one of the major contributors to PC malignancy and therapy resistance is represented by the desmoplastic reaction. Further understanding of how the TME facilitates PC cell malignancy will identify unique targets that may finally improve the treatment of patients with PC [6]. Some insights are briefly reported below.
(a). Interaction between tumour and stromal cells in pancreatic cancer: the role of pancreatic stellate cells
Normal development of glandular structures requires interactions between stromal cells and the epithelial cells that will eventually line the surface of the gland. In the pancreas, mesenchymal cells stimulate adjacent pluripotent cells to form acini, while inducing other remote cells to complete the endocrine pathway. These interactions in normal development require specific proteins within the ECM, notably laminin. Crosstalk between mesenchymal and epithelial compartments occurs through soluble messengers acting on a paracrine (or autocrine) manner, cell-surface receptor activation through direct cell-to-cell contact, and specific ECM proteins secreted by mesenchymal cells [75,76]. Perturbations of the normal mesenchymal–epithelial interactions can lead to unregulated cell growth, as occurs in cancer.
PSCs are the predominant mesenchymal cells within the PC stroma and the main determinant of the desmoplastic reaction [77]. PSCs originate from bone-marrow-derived mesenchymal stem cells, and are similar to myofibroblasts found in other tumour stroma (breast, prostate cancer). PSCs have the ability to transdifferentiate from a ‘quiescent’, retinoid/lipid storing phenotype in the normal pancreas to an ‘activated’, α-smooth muscle actin producing myofibroblastic phenotype. Activators of PSCs in vivo include cytokines (IL1, IL6, IL8 and TNF-α) growth factors (PDGF and TGF-β) and reactive oxygen species released by damaged inflammatory cells recruited in response to injury to the pancreas. Activated PSCs, in turn, can produce autocrine factors, such as PDGF, TGF-β, cytokines and proinflammatory molecules which may potentiate an activated phenotype [77–79]. PSCs, besides being implicated in the genesis of chronic pancreatitis, are critical for the development of the desmoplastic reaction in PC, being the main source of ECM proteins [78]. Moreover, because PSCs are activated by PC cells in vitro, a synergistic relationship between the two types of cells occurs, that favours the development and progression of PC.
(b). Integrins in pancreatic cancer
In PC, it is well established that ECM proteins, such as collagen, fibronectin and tenascin-C, interact with cell-surface integrins to provide intracellular signals to both PSCs and PC cells [80]. For example, in PSCs, β1 integrins are important not only to modulate cell adhesion to the basement membrane, but also to determine the proper formation and differentiation of acini during normal development [81]. PC cells express at least two major classes of integrins: β1-containing integrins (mainly α2β1 and α5β1), which mediate cell adhesion to fibronectin, laminin and collagen (type I and IV), and αvβ5, which is mainly involved in PC cell adhesion to vitronectin [82,83]. Integrin engagement has been reported to determine the malignant phenotype of PC cells by regulating cell proliferation [82], invasion [84] as well as by cytokine secretion [85]. For example, tenascin-C can affect PC cell growth and migration through the activation of β1 integrin intracellular signalling pathways [86]. Some recent evidence indicates that intergrin involvement in PC cell invasion can also occur through a complex interaction with EGF receptor (EGF-R) and the modulation of Src-centred intracellular signalling pathways [84].
On the whole, integrins, mainly those containing the β1 subunit, being expressed both in PC cells and in PSCs, and regulating the production of ECM proteins by the latter, are pivotal in regulating tumour–stroma interaction in PC. β1 integrins can hence drive different aspects of tumour cell behaviour, including chemo- and radio-resistance (see below).
(c). Ion channels and transporters in pancreatic cancer
In the past few years, different ICT proteins have been characterized in PC cells: TRP cationic channels of the ‘melastatin-related’ type (TRPM) have been reported to be overexpressed in PC cells. In particular, both TRPM8 [87] and TRPM7 [88] are overexpressed in pancreatic ductal adenocarcinoma cells, where they regulate either cell proliferation or migration. TRPM7 activation triggers a Mg2+-sensitive ‘suppressor of cytokine signalling 3a’ (Socs3a) pathway, which regulates exocrine pancreatic epithelial cell proliferation, both in development and cancer [89]. Moreover, Dong et al. [90] reported that TGF-β induces Ca2+ entry in PC cells via TRPC1 channels and the Na+/Ca2+ exchanger NCX1, thus raising intracellular Ca2+ concentration. Ca2+ increase is in turn essential for PKCα activation and subsequent tumour cell invasion [90]. As described above [57], CLIC3 channels regulate integrin recycling and behave as independent prognostic indicators in PC. This highlights the importance of active integrin trafficking as well as Cl– channels as potential drivers to cancer progression. Among voltage-gated K+ channels, Kv 11.1 (hERG1) is expressed in PC and identifies a patient group with worse prognosis [91]. Because hERG1 physically and functionally interacts with β1 integrins in different tumour cells (see above) including PC [91], it is tempting to speculate that it could drive PC malignancy through the modulation of integrin-mediated signalling. Finally, the sodium hydrogen exchanger 1 (HNE1) is expressed in PC cells [92,93] and is activated by growth factors such as neurotensin (NT). The NT/HNE1 pathway may be implicated in the early progression of PC by localized acidification and induction of an aerobic glycolytic phenotype with higher metastatic potential.
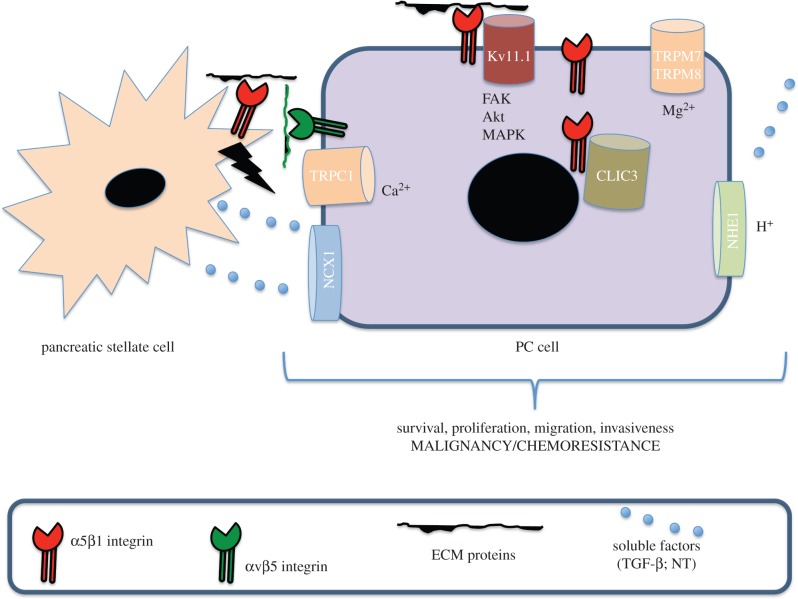
A model illustrating tumour–stroma interaction in PC, as well as the involvement of integrins, is reported in figure 1. Those ICTs which interact with integrins to modulate the crosstalk between PC cells and the TME are also illustrated.
Figure 1.
A model illustrating tumour–stroma interaction in PC as well as the involvement of integrins.
(d). The tumour microenvironment drives therapy resistance in pancreatic cancer
The altered crosstalk between stromal ECM proteins and integrins expressed on both tumour and TME cells is also implicated in mechanisms of acquired resistance to chemo- and radiotherapy in PC. An in vitro study using different PC cell lines cultured in the presence of collagen, fibronectin or laminin, showed different induction of chemosensitivity, depending on the type of substrate [94]. Indeed, inhibiting ECM-integrin function in combination with chemotherapy may be a potential therapeutic intervention that could specifically target the desmoplastic reaction. One example could be the monoclonal antibody targeting α5β1 or αvβ3 integrin (Vitaxin). Moreover, PSCs are known to promote radioprotection of PC cells: this effect is dependent on a signalling pathway triggered by β1 integrin and converging on FAK [95].
Treatment failure in PC may be due, at least in part, to our limited understanding of how the fibrotic tissue and the stromal cells present within this tumour can facilitate the rapid progression of this cancer type. Therefore, a better understanding of the mechanisms which regulate PC cell interactions with the TME, with particular attention to the crosstalk between integrins and ICTs, could open a new perspective to effectively treat PC.
Acknowledgements
The author's research has been lately supported by the Italian Association for Cancer Research (AIRC), the Associazione Noi per Voi (Firenze), the Istituto Toscano Tumori (ITT), the Association for International Cancer Research (AICR), the Italian Ministry of University and Scientific Research (PRIN 2003, 2005, 2008), the Ente Cassa di Risparmio di Firenze.
References
- 1.Comoglio PM, Trusolino L. 2005. Cancer: the matrix is now in control. Nat. Med. 11, 1156–1159. ( 10.1038/nm1105-1156) [DOI] [PubMed] [Google Scholar]
- 2.Girieca L, Ruegg C. 2008. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem. Cell Biol. 130, 1091–1103. ( 10.1007/s00418-008-0530-8) [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA, Pollard JW. 2009. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252. ( 10.1038/nrc2618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nwajei F, Konopleva M. 2013. The bone marrow microenvironment as niche retreats for hematopoietic and leukemic stem cells. Adv. Hematol. 2013, 8 ( 10.1155/2013/953982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahadevan D, Von Hoff DD. 2007. Tumor–stroma interactions in pancreatic ductal denocarcinoma. Mol. Cancer Ther. 6, 1186–1197. ( 10.1158/1535-7163.MCT-06-0686) [DOI] [PubMed] [Google Scholar]
- 6.Li X, Ma Q, Xu Q, Duan W, Lei J, Wu E. 2012. Targeting the cancer–stroma interaction: a potential approach for pancreatic cancer treatment. Curr. Pharm. Des. 18, 2404–2415. ( 10.2174/13816128112092404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruickshank AH. 1986. Pathology of the pancreas. Berlin and Heidelberg, Germany: Springer-Verlag. [Google Scholar]
- 8.Hanahan D, Weimberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 9.Ruoslahti E, Bhatia SN, Sailor MJ. 2010. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 188, 759–768. ( 10.1083/jcb.200910104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. 2009. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 16, 66–93. ( 10.2174/092986709787002835) [DOI] [PubMed] [Google Scholar]
- 11.Arcangeli A. 2011. Ion channels and transporters in cancer. 3. Ion channels in the tumor cell-microenvironment cross talk. Am. J. Physiol. Cell. Physiol. 301, C762–C771. ( 10.1152/ajpcell.00113.2011) [DOI] [PubMed] [Google Scholar]
- 12.Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. 2012. Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am. J. Physiol. Cell Physiol. 302, C9–C15. ( 10.1152/ajpcell.00280.2011) [DOI] [PubMed] [Google Scholar]
- 13.Diehlmann A, Bork S, Saffrich R, Veh RW, Wagner W, Derst C. 2011. KATP channels in mesenchymal stromal stem cells: strong up-regulation of Kir6.2 subunits upon osteogenic differentiation. Tissue Cell 43, 331–336. ( 10.1016/j.tice.2011.06.004) [DOI] [PubMed] [Google Scholar]
- 14.Pillozzi S, Becchetti A. 2012. Ion channels in hematopoietic and mesenchymal stem cells. Stem Cells Int. 2012, 9 ( 10.1155/2012/217910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Coursey TE, Chandy KG, Gupta S, Cahalan MD. 1984. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature 307, 465.–. ( 10.1038/307465a0) [DOI] [PubMed] [Google Scholar]
- 16.Cahalan MD, Chandy KG. 2009. The functional network of ion channels in T lymphocytes. Immunol. Rev. 231, 59–87. ( 10.1111/j.1600-065X.2009.00816.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feske S, Skolnik EY, Prakriya M. 2012. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 12, 532–547. ( 10.1038/nri3233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevarskaya N, Skryma R, Shuba Y. 2010. Ion channels and the hallmarks of cancer. Trends Mol. Med. 16, 107–121. ( 10.1016/j.molmed.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 19.Beech DJ. 2012. Orai1 calcium channels in the vasculature. Pflugers Arch. 463, 635–647. ( 10.1007/s00424-012-1090-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. 2010. Heterogeneity of the tumor vasculature. Semin. Thromb. Hemost. 36, 321–331. ( 10.1055/s-0030-1253454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalluri R, Zeisberg M. 2006. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401. ( 10.1038/nrc1877) [DOI] [PubMed] [Google Scholar]
- 22.Farrow B, Rowley D, Dang T, Berger DH. 2009. Characterization of tumor derived pancreatic stellate cells. J. Surg. Res. 157, 96–102. ( 10.1016/j.jss.2009.03.064) [DOI] [PubMed] [Google Scholar]
- 23.Sato N, Maehara N, Goggins M. 2004. Gene expression profiling of tumor stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 64, 6950–6956. ( 10.1158/0008-5472.CAN-04-0677) [DOI] [PubMed] [Google Scholar]
- 24.Bhowmick NA, Moses HL. 2005. Tumor–stroma interactions. Curr. Opin. Genet. Dev. 15, 97–101. ( 10.1016/j.gde.2004.12.00) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindea G, Mlecnik B, Fridman WH, Page's F, Galon J. 2010. Natural immunity to cancer in humans. Curr. Opin. Immunol. 22, 215–222. ( 10.1016/j.coi.2010.02.006) [DOI] [PubMed] [Google Scholar]
- 26.Johansson M, Denardo DG, Coussens LM. 2008. Polarized immune responses differentially regulate cancer development. Immunol. Rev. 222, 145–154. ( 10.1111/j.1600-065X.2008.00600.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becchetti A, Pillozzi S, Morini R, Nesti E, Arcangeli A. 2010. New insights into the regulation of ion channels by integrins. Int. Rev. Cell. Mol. Biol. 279, 135–190. ( 10.1016/S1937-6448(10)79005-5) [DOI] [PubMed] [Google Scholar]
- 28.Colden-Stanfield M. 2010. Adhesion-dependent modulation of macrophage K+ channels. Adv. Exp. Med. Biol. 674, 81–94. ( 10.1007/978-1-4419-6066-5_8) [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. 2010. P2X7 Receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J. Immunol. 185, 2611–2619. ( 10.4049/jimmunol.1000436) [DOI] [PubMed] [Google Scholar]
- 30.Barkan D, Green JE, Chambers AF. 2010. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 46, 1181–1188. ( 10.1016/j.ejca.2010.02.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessenbrock K, Plaks V, Werb Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67. ( 10.1016/j.cell.2010.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J. 2003. The biology of VEGF and its receptors. Nat. Med. 9, 669–676. ( 10.1038/nm0603-669) [DOI] [PubMed] [Google Scholar]
- 33.Hynes RO. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. ( 10.1016/S0092-8674(02)00971-6) [DOI] [PubMed] [Google Scholar]
- 34.de Pereda JM, Wiche G, Liddington RC. 1999. Crystal structure of a tandem pair of fibronectin type III domains from the cytoplasmic tail of integrin alpha6beta4. EMBO J. 18, 4087–4095. ( 10.1093/emboj/18.15.4087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science 302, 103–106. ( 10.1126/science.1086652) [DOI] [PubMed] [Google Scholar]
- 36.Ginsberg MH, Partridge A, Shattil SJ. 2005. Integrin regulation. Curr. Opin. Cell Biol. 17, 509–516. ( 10.1016/j.ceb.2005.08.010) [DOI] [PubMed] [Google Scholar]
- 37.Miranti CK, Brugge JS. 2002. Sensing the environment. A historical perspective of integrin signal transduction. Nat. Cell Biol. 4, E83–E90. ( 10.1038/ncb0402-e83) [DOI] [PubMed] [Google Scholar]
- 38.Cabodi S, et al. 2010. Integrins and signal transduction. Adv. Exp. Med. Biol. 674, 43–54. ( 10.1007/978-1-4419-6066-5_5) [DOI] [PubMed] [Google Scholar]
- 39.Arcangeli A, Becchetti A. 2006. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16, 631–639. ( 10.1016/j.tcb.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 40.Arcangeli A, et al. 1993. Integrin mediated neurite outgrowth in neuroblastoma cells depends on the activation of potassium channels. J. Cell Biol. 122, 1131–1143. ( 10.1083/jcb.122.5.1131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becchetti A, Arcangeli A, Del Bene MR, Olivotto M, Wanke E. 1992. Response to fibronectin-integrin interaction in leukaemia cells: delayed enhancing of a K+ current. Proc. R. Soc. Lond. B 248, 235–240. ( 10.1098/rspb.1992.0067) [DOI] [PubMed] [Google Scholar]
- 42.Doherty P, Ashton SV, Moore SE, Walsh FS. 1991. Morphoregulatory activities of NCAM and N- cadherin can be accounted for by G protein dependent activation of L- and N-type neuronal Ca2+ channels. Cell 67, 21–33. ( 10.1016/0092-8674(91)90569-K) [DOI] [PubMed] [Google Scholar]
- 43.Hofmann G, et al. 2001. HERG K+ channels activation during β1 integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3 integrin in the preosteoclastic leukemia cell line FLG 29.1. J. Biol. Chem. 276, 4923–4931. ( 10.1074/jbc.M005682200) [DOI] [PubMed] [Google Scholar]
- 44.Belusa R, Aizman O, Andersson RM, Aperia A. 2002. Changes in Na+-K+-ATPase activity influence cell attachment to fibronectin. Am. J. Physiol. Cell Physiol. 282, C302–C309. ( 10.1152/ajpcell.00117.2001) [DOI] [PubMed] [Google Scholar]
- 45.Menegazzi R, Busetto S, Cramer R, Dri P, Patriarca P. 2000. Role of intracellular chloride in the reversible activation of neutrophil b2 integrins: a lesson from TNF stimulation. J. Immunol. 165, 4606–4614. ( 10.1016/j.bcmd.2009.01.006) [DOI] [PubMed] [Google Scholar]
- 46.Tominaga T, Barber DL. 1998. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol. Biol. Cell 9, 2287–2303. ( 10.1091/mbc.9.8.2287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardone RA, Casavola V, Reshkin SJ. 2005. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795. ( 10.1038/nrc1713) [DOI] [PubMed] [Google Scholar]
- 48.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. 2002. Regulation of ion channels by integrins. Cell Biochem. Biophys. 36, 41–66. ( 10.1385/CBB:36:1:41) [DOI] [PubMed] [Google Scholar]
- 49.Katsumi A, Orr AW, Tzima E, Schwartz MA. 2004. Integrins in mechanotransduction. J. Biol. Chem. 279, 12 001–12 004. ( 10.1074/jbc.R300038200) [DOI] [PubMed] [Google Scholar]
- 50.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. 2000. Extracellular K+ and opening of voltage gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and β1 integrins. J. Exp. Med. 191, 1167–1176. ( 10.1084/jem.191.7.1167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artym VV, Petty HR. 2002. Molecular proximity of Kv1.3 voltage-gated potassium channels and β1-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 120, 29–37. ( 10.1085/jgp.20028607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherubini A, et al. 2002. HERG K+ channels and β1 integrins interact through the assembly of a macromolecular complex. Ann. NY Acad. Sci. 973, 559–561. ( 10.1111/j.1749-6632.2002.tb04701.x) [DOI] [PubMed] [Google Scholar]
- 53.Cherubini A, et al. 2005. hERG1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signalling. Mol. Biol. Cell 16, 2972–2983. ( 10.1091/mbc.E04-10-0940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pillozzi S, et al. 2007. VEGFR-1 (FLT-1), β1 integrin and hERG K+ channel form a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 110, 1238–1250. ( 10.1182/blood-2006-02-003772) [DOI] [PubMed] [Google Scholar]
- 55.Pillozzi S, et al. 2011. Chemotherapy resistance in acute lymphoblastic leukemia requires hERG1 channels and is overcome by hERG1 blockers. Blood 117, 902–914. ( 10.1182/blood-2010-01-262691) [DOI] [PubMed] [Google Scholar]
- 56.Crociani O, et al. 2013. hERG1 channels modulate integrin signaling to trigger angiogenesis and tumor progression in colorectal cancer. Sci. Rep. ( 10.1038/srep03188) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Dozynkiewicz MA, et al. 2012. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 22, 131–145. ( 10.1016/j.devcel.2011.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C. 2007. Cells move when ions and water flow. Pflugers Arch. 453, 421–432. ( 10.1007/s00424-006-0138-6) [DOI] [PubMed] [Google Scholar]
- 59.Nechyporuk-Zloy V, Dieterich P, Oberleithner H, Stock C, Schwab A. 2008. Dynamics of single potassium channel proteins in the plasma membrane of migrating cells. Am. J. Physiol. Cell Physiol. 294, C1096–1102. ( 10.1152/ajpcell.00252.2007) [DOI] [PubMed] [Google Scholar]
- 60.Rezzonico R, et al. 2003. Focal adhesion kinase pp125FAK interacts with the large conductance calcium-activated hSlo potassium channel in human osteoblasts: potential role in mechanotransduction. J. Bone Miner. Res. 18, 1863–1871. ( 10.1359/jbmr.2003.18.10.1863) [DOI] [PubMed] [Google Scholar]
- 61.Wei JF, et al. 2008. Formation of Kv2.1-FAK complex as a mechanism of FAK activation, cell polarization and enhanced motility. J. Cell Physiol. 217, 544–557. ( 10.1002/jcp.21530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. 2006. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J. 20, 2588–2590. ( 10.1096/fj.06-6152fje) [DOI] [PubMed] [Google Scholar]
- 63.Williams MR, Markey JC, Doczi MA, Morielli AD. 2007. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc. Natl Acad. Sci. USA 104, 17 412–17 417. ( 10.1073/pnas.0703865104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Hart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. 2008. The α9β1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc. Natl Acad. Sci. USA 105, 7188–7193. ( 10.1073/pnas.0708044105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandenberg CA. 2008. Integrins step up the pace of cell migration through polyamines and potassium channels. Proc. Natl Acad. Sci. USA 105, 7109–7110. ( 10.1073/pnas.0803231105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menegazzi R, Busetto S, Decleva E, Cramer R, Dri P, Patriarca P. 1999. Triggering of chloride ion efflux from human neutrophils as a novel function of leukocyte β2 integrins: relationship with spreading and activation of the respiratory burst. J. Immunol. 162, 423–434. [PubMed] [Google Scholar]
- 67.Abdel-Ghany M, Cheng H, Elble RC, Lin H, DiBiasio J, Pauli BU. 2003. The interacting binding domains of the β4 integrin and calcium activated chloride channels (CLCAs) in metastasis. J. Biol. Chem. 278, 49 406–49 416. ( 10.1074/jbc.M309086200) [DOI] [PubMed] [Google Scholar]
- 68.Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. 1993. Integrin β-1 and β-3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J. Cell Biol. 121, 163–170. ( 10.1083/jcb.121.1.163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenner H, et al. 2009. Long-term survival expectations of cancer patients in Europe in 2000–2002. Eur. J. Cancer 45, 1028–1041. ( 10.1016/j.ejca.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 70.Yang GY, Wagner TD, Fuss M, Thomas CR. 2005. Multimodality approaches for pancreatic cancer. Cancer J. Clin. 55, 352–367. ( 10.3322/canjclin.55.6.352) [DOI] [PubMed] [Google Scholar]
- 71.Hochster HS, Haller DG, de Gramont A, Berlin JD, Philip PA, Moore MJ, Ajani JA. 2006. Consensus report of the international society of gastrointestinal oncology on therapeutic prograss in advanced pancreatic cancer. Cancer 107, 676–685. ( 10.1002/cncr.22036) [DOI] [PubMed] [Google Scholar]
- 72.Zuckerman DS, Ryan DP. 2008. Adjuvant therapy for pancreatic cancer. Cancer 112, 243–249. ( 10.1002/cncr.23174) [DOI] [PubMed] [Google Scholar]
- 73.Rivera F, López-Tarruella S, Vega-Villegas ME, Salcedo M. 2009. Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy. Cancer Treat. Rev. 35, 335–339. ( 10.1016/j.ctrv.2008.11.007) [DOI] [PubMed] [Google Scholar]
- 74.El Maalouf G, Le Tourneau C, Batty GN, Faivre S, Raymond E. 2009. Markers involved in resistance to cytotoxics and targeted therapeutics in pancreatic cancer. Cancer Treat. Rev. 35, 167–174. ( 10.1016/j.ctrv.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 75.Cunha GR. 1994. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 74, 1030–1044. () [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Manna P, Kobayashi H, Spilde T, Bhatia A, Preuett B, Prasadan K, Hembree M, Gittes GK. 2004. Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev. Biol. 269, 252–263. ( 10.1016/j.ydbio.2004.01.043) [DOI] [PubMed] [Google Scholar]
- 77.Apte MV, et al. 2004. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29, 179–187. ( 10.1097/00006676-200410000-00002) [DOI] [PubMed] [Google Scholar]
- 78.Aoki H, et al. 2006. Existence of autocrine loop between interleukin-6 and transforming growth factorbeta1 in activated rat pancreatic stellate cells. J. Cell. Biochem. 99, 221–228. ( 10.1002/jcb.20906) [DOI] [PubMed] [Google Scholar]
- 79.Bachem MG, et al. 2005. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128, 907–921. ( 10.1053/j.gastro.2004.12.036) [DOI] [PubMed] [Google Scholar]
- 80.Grzesiak JJ, Ho JC, Moossa AR, Bouvet M. 2007. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas 35, 293–301. ( 10.1097/mpa.0b013e31811f4526) [DOI] [PubMed] [Google Scholar]
- 81.Riopel MM, Li J, Liu S, Leask A, Wang R. 2013. β1 integrin-extracellular matrix interactions are essential for maintaining exocrine pancreas architecture and function. Lab. Invest. 93, 31–40. ( 10.1038/labinvest.2012) [DOI] [PubMed] [Google Scholar]
- 82.Grzesiak JJ, Bouvet M. 2007. Determination of the ligand binding specificities of α2β1 and α1β1 integrins in a novel three-dimensional in vitro model of pancreatic cancer. Pancreas 34, 220–228. ( 10.1097/01.mpa.0000250129.64650.f6) [DOI] [PubMed] [Google Scholar]
- 83.Grzesiak JJ, Bouvet M. 2006. The α2β1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br. J. Cancer 94, 1311–1319. ( 10.1038/sj.bjc.6603088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA. 2009. Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res. 69, 1383–1391. ( 10.1158/0008-5472.CAN-08-3612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lowrie AG, Salter DM, Ross JA. 2004. Latent effects of fibronectin, α5β1 integrin, and the cytoskeleton regulate pancreatic carcinoma cell IL8 secretion. Br. J. Cancer 91, 1327–1334. ( 10.1038/sj.bjc.6602132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paron I, Berchtold S, Vörös J, Shamarla M, Erkan M, Höfler H, Esposito I. 2011. Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS ONE 6, e21684 ( 10.1371/journal.pone.0021684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yee NS, Zhou W, Lee M. 2010. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 297, 49–55. ( 10.1016/j.canlet.2010.04.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rybarczyk P, et al. 2012. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 131, E851–E861. ( 10.1002/ijc.27487) [DOI] [PubMed] [Google Scholar]
- 89.Yee NS, Zhou W, Liang IC. 2011. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Model. Mech. 4, 240–254. ( 10.1242/dmm.004564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong H, et al. 2010. Molecular mechanisms underlying Ca2+-mediated motility of human pancreatic duct cells. Am. J. Physiol. Cell Physiol. 299, C1493–C1503. ( 10.1152/ajpcell.00242.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arcangeli A, et al. 2013. Prognostic significance and functional relevance of hERG1 potassium channel expression in pancreatic ductal adenocarcinoma. 47th Pancreas Club Meeting- 17–18 May P016.
- 92.Olszewski U, Hlozek M, Hamilton G. 2010. Activation of Na+/H+ exchanger 1 by neurotensin signaling in pancreatic cancer cell lines. Biochem. Biophys. Res. Commun. 393, 414–419. ( 10.1016/j.bbrc.2010.02.009) [DOI] [PubMed] [Google Scholar]
- 93.Provost JJ, Wallert MA. 2013. Inside out: targeting NHE1 as an intracellular and extracellular regulator of cancer progression. Chem. Biol. Drug Des. 81, 85–101. ( 10.1111/cbdd.12035) [DOI] [PubMed] [Google Scholar]
- 94.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. 2004. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas 28, 38–44. ( 10.1097/00006676-200401000-00006) [DOI] [PubMed] [Google Scholar]
- 95.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. 2011. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 71, 3453–3458. ( 10.1158/0008-5472.CAN-10-1633) [DOI] [PMC free article] [PubMed] [Google Scholar]