Abstract
Vascularization is crucial for solid tumour growth and invasion, providing metabolic support and sustaining metastatic dissemination. It is now accepted that ion channels and transporters play a significant role in driving the cancer growth at all stages. They may represent novel therapeutic, diagnostic and prognostic targets for anti-cancer therapies. On the other hand, although the expression and role of ion channels and transporters in the vascular endothelium is well recognized and subject of recent reviews, only recently has their involvement in tumour vascularization been recognized. Here, we review the current literature on ion channels and transporters directly involved in the angiogenic process. Particular interest will be focused on tumour angiogenesis in vivo as well as in the different steps that drive this process in vitro, such as endothelial cell proliferation, migration, adhesion and tubulogenesis. Moreover, we compare the ‘transportome’ system of tumour vascular network with the physiological one.
Keywords: tumour vascularization, endothelial cells, ion channels, aquaporins, transporters
1. Introduction
Endothelium is a multifaceted and dynamic interface between blood components and tissues. Several diseases are due to the altered function of endothelial cells (ECs), which mediate the control of metabolism, water supply, inflammation and immune response. The importance of vascularization in tumour progression sparked hopes that manipulating this process could offer therapeutic opportunities [1,2]. Nowadays, hundreds of thousands of patients benefit of antiangiogenic therapies, approved by the U.S. Food and Drug Administration, that focus on vascular endothelium growth factor (VEGF) as a major drug target. The anti-VEGF antibody (bevacizumab (Avastin)) and multi-targeted pan-VEGF receptor tyrosine kinase inhibitors are used in combination with chemotherapy, cytokine therapy or radiotherapy for several advanced metastatic cancers [3]. On the other hand, despite promising results, emerging data indicate that responses to vascular targeting therapy (VTT) are short-lived, and resistance develops in the majority of patients.
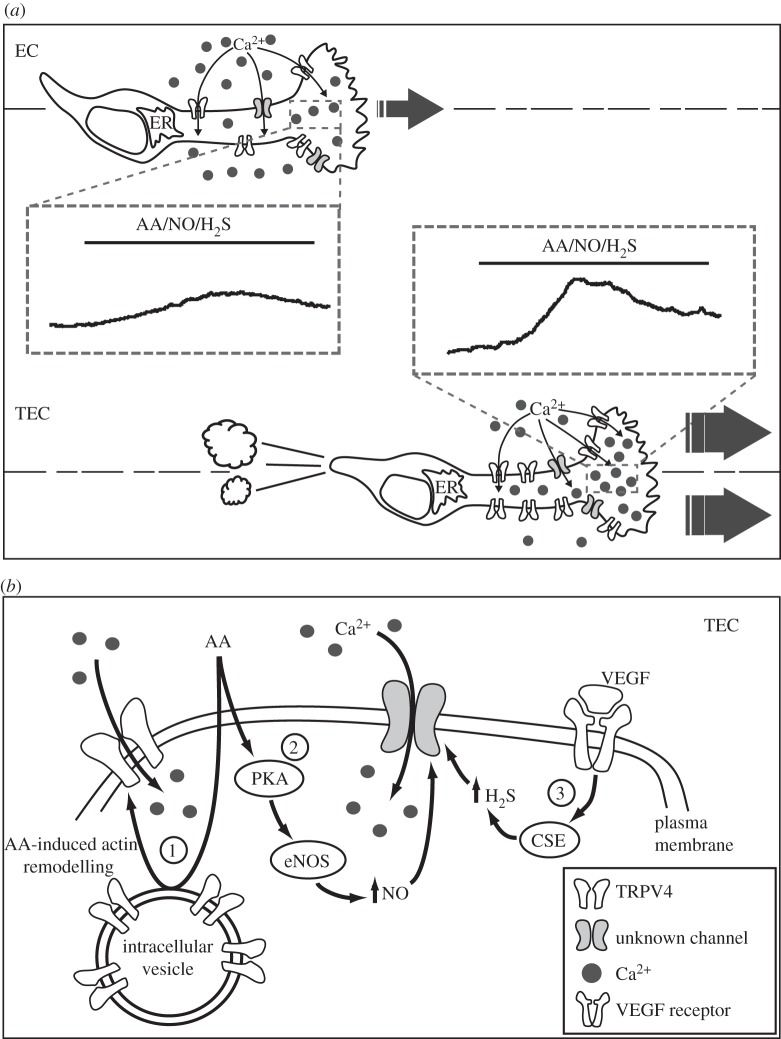
A possible reason for this partial failure may be the high instability of EC within the tumour. It is now well established that normal and altered EC are highly heterogeneous in structure and function, owing to genetic modifications and the variability of the local microenvironment [4,5]. The basic properties of EC obtained from different human tumours (tumour-derived EC, TEC) have been investigated only recently by a limited number of groups [6–9]. TEC isolated and cultured from human kidney (RTEC) and breast carcinomas (BTEC) on the basis of membrane markers exhibit altered genotype, gene expression, phenotype and function. They are often aneuploid, display chromosomal instability avoid senescence, a process typical of normal EC, and display enhanced proliferation, motility and tubulogenic potential [6,9–12]. Moreover, TEC-mediated intracellular signalling is quite different from that observed in normal human microvascular EC (figure 1). Interestingly, proangiogenic Ca2+ signals and their related pathways (mediated by AA, NO, H2S) are significantly altered in TEC compared with normal EC [13–15]. As an example, both AA- and H2S-mediated Ca2+ signals are involved in promigratory effects in TEC, but not in normal EC (figure 1).
Figure 1.
(a) Schematic representation of the differences between normal endothelial cells (ECs) and tumour-derived endothelial cells (TECs) in terms of Ca2+-related intracellular signalling pathways. Arachidonic acid (AA), nitric oxide (NO) and hydrogen sulfide (H2S)-promoted Ca2+i signals are significantly upregulated in TEC compared with EC. These differences are at least in part due to TRPV4 overexpression and consequent TEC migration. (b) Scheme of the signal transduction pathway involved in proangiogenic Ca2+ signals in TEC: (1) AA-mediated actin-remodelling promotes TRPV4 vesicles to traffic and insert in the plasma membrane; as a consequence, more functional channels allow Ca2+ entry required for TEC migration. (2) Activation of endothelial NO synthase (eNOS) mediated by AA-mediated protein kinase A (PKA) promotes NO release and consequent Ca2+ entry via unknown channels. (3) VEGF promotes promigratory Ca2+ signals mediated by H2S via cystathionine γ-lyase (CSE).
Being involved in nearly all of the ‘hallmarks of cancer’ as defined by Hanahan & Weinberg [16], there is an increasing consensus on the idea that ion channels and transporters could play a significant role in driving cancer progression at all stages. They may be seen as potential novel therapeutic, diagnostic and prognostic targets for anti-cancer therapies.
Nonetheless, although the expression and role of ionic channels and transporters (collectively indicated as ‘trasportome’) in the vascular endothelium is well recognized and subject of a number of recent reviews [17–20], ‘trasportome’ entered only recently as a major player in tumour vascularization [21,22].
Here, we discuss current literature focused on trasportome and angiogenesis. Moreover, starting from a critical review of the experimental data obtained so far in vitro and in vivo, we try to define the most promising checkpoints at which tumour vascular transportome differs from the physiological one.
2. Voltage-gated channels
Although ECs are generally described as non-excitable cells, a number of experimental evidences suggest a role for voltage-dependent channels (VOCs) in both cultured and freshly isolated EC [22]. On the other hand, the role of VOCs in tumour progression has been largely described and different data point to Na+, K+ and Ca2+ channels as key players, suitable to be specifically potential target in clinical treatments [23].
K+ channels (KV) attracted most of the work in oncology since the early discovery unveiling their role in the control of cell proliferation [24,25]. Ether-á-go-go-1 (EAG1, KCNH1, KV10.1) is a CNS-localized voltage-gated K+ channel that is found ectopically expressed in many solid tumours [25]. Monoclonal antibodies against human EAG1, developed by Stuhmer's and Pardo's groups, might represent a suitable tool in cancer therapy [26]. KV10.1 expression might offer an advantage to tumours through increased vascularization and resistance to hypoxia: indeed, EAG1 regulates cellular oxygen homeostasis, increasing HIF-1 activity, and thereby VEGF secretion and tumour vascularization [27]; accordingly, EAG1 silencing inhibits tumour growth and angiogenesis in osteosarcoma in vivo [28] (table 1 and figure 2).
Table 1.
Ion channels and carriers involved in the different phases of angiogenesis. HMEC, human microvascular EC; HPAEC, human pulmonary artery EC; HUVEC, human umbilical vein EC; EA.hy926, EC line derived from HUVEC fused with human lung adenocarcinoma cell line A549; PAEC, porcine aortic endothelial cells; BTEC, tumour-derived EC from breast carcinoma; H5VEC, heart endothelioma (H5V) EC; MAEC, mouse aortic EC; EPC, endothelial progenitor cells; RCC-EPC, EPC isolated from renal carcinoma patients; Numbers in parenthesis indicate the respective reference number.
| channels/transporters | TRPC1 | TRPC6 | TRPC3,4,5 | TRPV4 | TRPM2 | TRPM7 | Orai1/Stim1 | Nav | Cav | Kv | VRAC | NHE1 | NCX | AQP1 | nAChR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| migration | RCC-EPC [29] EPC [30] |
HMEC [31] | BTEC [32] | RCC-EPC [29] HUVEC [33–35] |
HUVEC [36] | HUVEC [37] | HUVEC [38] | HUVEC [36,39] | MAEC from KO mice [40] | HMEC [41,42] EPC [43] |
|||||
| survival and proliferation | RCC-EPC [29] EPC [30] |
HMEC [31] HUVEC [44] |
PAEC [45] | H5V EC [46] | HUVEC, HMEC [47–49] | RCC-EPC [29] | HUVEC [36] | Kv 1.3-HUVEC [50] | HUVEC [38] | HUVEC [36,39] | human hepatic sinusoidal ECs [51] | human pulmonary artery ECs, human retinal microvascular ECs, HUVEC, HMEC [41] EPC [43] |
|||
| in vitro tube formation | RCC-EPC [29] HUVEC, EA.hy926 [52] |
HMEC [31,53] | EA.hy926 [52] | RCC-EPC [29] HUVEC, EPC [34,52] |
HUVEC [36] | microvascular ECs from the rat adrenal medulla (RAMECs), HMEC [54] | HUVEC [38] | HUVEC [36,39] | MAEC from KO mice [40] | HUVEC, HMEC [41,55] EPC [43] |
|||||
| permeability | HMEC,HUVEC [56–58] | HPAEC [59] Frog mesenteric microvessels [60] |
H5V EC [46] | HUVEC [61] | |||||||||||
| in vivo angiogenesis | zebrafish [62] | CAM [44] | collateral growth [45] | HERG-1-Retinoblastoma [63] EAG1-Xenograft in SCID mice and human osteosarcoma [27,28] |
CAM [54] | xenograft in nude mice [38] Rabbit cornea [64] |
human mammary carcinoma, glioblastoma [65,66] AQP1 KO mice and C57BL/6 mice [40,67,68] Bone marrow angiogenesis in patients with active multiple myeloma [51] |
disc angiogenesis system, hind limb ischemia [55] Breast, colon and lung tumour cells implanted in CAM [69] |
Figure 2.
Schematic of channels/transporters role in the different key steps of tumour vascularization. The mechanisms are presented in representative EC, SMC, EPC and tumours without any tissue specification. EC, endothelial cells; EPC, endothelial progenitor cells; VSM, vascular smooth muscle cells; MAPK, mitogen-activated protein kinase; PI3K, Phosphatidylinositide 3-kinases; AKT, protein kinase B; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; bFGF, basic fibriblast growth factor; VEGF, vascular endothelium growth factor; VEGFR, VEGF receptor; NFAT, nuclear factor of activated T-cells; PAR, protease-activated receptors; PTEN, phosphatase and tensin homolog; PKC, protein kinase C.
A promising issue is related to other K+ channels, such as human ether-a-gogo related gene-1 (hERG1)-Kv11 [25,70,71]; Pillozzi et al. [72] showed that hERG1 channels regulate vegf-a expression and VEGF-A secretion in cancer cells potentially promoting angiogenesis. Moreover, the levels of VEGF-A, hERG1, microvessel density and proliferation-related parameters are cross-linked in two cases of bilateral retinoblastoma patients [63]. Besides the role of KV10 and KV11, KV 1.3 channels are involved in VEGF-mediated human umbilical vein EC (HUVEC) proliferation: VEGF-mediated hyperpolarization via margatoxin (MTX)-sensitive KV channels causes Ca2+ entry, increase in NO synthesis, finally resulting in EC growth enhancement [50] (table 1 and figure 2). All together, the data point out an important role for K+ channels in the crosstalk between cancer cells and tumour endothelium by VEGF release enhancement. This particular function of K+ channels makes them clinically interesting as potential targets to promote vascular ‘normalization’ during a critical window of the antiangiogenic treatments (see also §8).
Voltage-gated Na+ channels (VGSCs) are also expressed in non-excitable cells and functionally upregulated in metastatic tumour cells [73–75]. Recently, a clear relationship between functional expression and biological role of VGSCs in EC has been described [36]. The main functional VGSC isoforms in HUVEC are Nav1.5 and Nav1.7. VGSC activity potentiates VEGF-induced ERK1/2 activation by attenuating membrane depolarization, altering [Ca2+]i kinetics and PKC activity and thus increasing cellular proliferation, chemotaxis and tubulogenesis [36] (table 1 and figure 2). Moreover, Ca2+ inflow through reverse mode sodium–calcium exchanger (NCX) is required for PKC activation and targeting to the plasma membrane, as well as for VEGF-induced ERK1/2 phosphorylation and downstream EC functions in angiogenesis [39] (see also §7). The data unveil an intriguing mechanism for the control of Vm in non-excitable cells by VGSCs in response to physiological stimuli in vitro.
Regarding voltage-gated Ca2+ channels (VGCCs), most of the studies have been conducted on human breast carcinoma cell lines, which actually express VGCCs, mainly of the T-type [76–78]. Nevertheless, the role and expression of VGCCs in endothelium are still debated [3,7,23]. Conflicting data could arise from the use of different EC lines and their variable behaviour. In HUVEC, angiotensin II stimulates Ca2+ influx via CaV and promotes cell migration [37]. On the other hand, CaV expressed by VSMCs could play an antiangiogenic role through indirect effects on EC: nifedipine, an inhibitor of L-type calcium channels, stimulates VEGF production from human coronary SMCs, an effect abolished by PKC inhibitors and a bradykinin B2 receptor antagonist [79] (table 1 and figure 2).
3. Transient receptor potential proteins and STIM1-ORAI1 complex
Transient receptor potential (TRP) channels trigger Ca2+ signals that control the initiation and progression of cancer. It is not therefore surprising that the expression and function of some TRP proteins are altered during carcinogenesis [21,80].
TRPs are widely expressed in endothelium and their activity is related to normal and tumour vascularization [17,18]. TRP-mediated Ca2+ influx can be triggered by the release from intracellular Ca2+ stores, giving rise to store-operated Ca2+ entry (SOCE), or alternatively by the store-independent Ca2+ entry (NSOCE) [81].
VEGF mediates NSOCE through TRPC6 in human microvascular EC [60,82]. Dominant negative TRPC6 significantly reduces EC number, migration, tubulogenesis and sprouting [31,44]. Phosphatase and tensin homologue (PTEN) regulates cell surface expression of TRPC6, and consequently Ca2+ entry, endothelial permeability and angiogenesis in human pulmonary EC [59] (table 1 and figure 2). TRPC6 can also exert its proangiogenic role indirectly through its activity on cancer cells. In glioma cells from glioblastoma multiforme, inhibition of hypoxia enhanced TRPC6 expression and NFAT activation, markedly reducing the number of branch points in EC grown in conditioned medium harvested from glioma cells [53] (table 1 and figure 2).
Other groups reported a role of VEGF-mediated SOCE owing to TRPC1 in the enhancement of HMVEC and HUVEC permeability [56–58]. Remarkably, TRPC1 is proangiogenic in vivo: knockdown of zebrafish TRPC1 by morpholinos caused severe angiogenic defects in intersegmental vessel sprouting, presumably owing to impaired filopodia extension during EC migration [62] (table 1 and figure 2).
This ability of VEGF to activate different channels could simply depend on tissue variability, especially between small capillaries and large vessels. Accordingly, the pattern of TRPCs expressed in HMVEC and HUVEC is different, TRPC4 being undetectable in HMVEC [31].
Besides TRPC1 and TRPC6, also Orai1 and STIM1, components of the so-called calcium release activated currents (CRAC) channels, concur to the VEGF-mediated SOCE in HUVEC [33,34]. VEGF stimulation promotes STIM1 clustering and Orai1 activation [34]. Moreover, knockdown of Orai1 inhibits VEGF-mediated HUVEC migration, proliferation and tubulogenesis [33–35]. On the other hand, thrombin-induced decrease in EC permeability requires STIM1, but is unrelated to Orai1 and Ca2+ entry [61] (table 1 and figure 2).
Interestingly, STIM1, as well as TRPC1 and TRPC4 knockdown, inhibits tube formation in both HUVEC and EA.hy926 cells [52].
Because VEGF regulates several activities in EC, the discovery of a specific role for each channel in selected cell functions, such as migration, proliferation and permeability, could let us to overcome the use of the broad VEGFR inhibitors (see also §8).
TRPV4 is another emerging player in angiogenesis. The availability of highly selective antagonists for this channel makes it a promising molecular target for antiangiogenic treatments [83]. TRPV4 is widely expressed in the vascular endothelium where it acts as a mechanosensor during changes in cell morphology, cell swelling and shear stress [83–86]. Both shear stress and agonist-activation of TRPV4 enhance EC proliferation as well as collateral growth after arterial occlusion [45]. We recently provided strong evidences about the role of TRPV4 in AA-mediated TEC migration: it is upregulated in BTEC and RTEC compared with dermal HMEC and normal kidney glomerular EC [32]. AA-activated TRPV4 is essential for BTEC migration: its loss results in decreased Ca2+ responses to the TRPV4-specific agonist 4α-phorbol 12,13-didecanoate and in a complete inhibition of AA-induced migration. The mechanism by which AA regulates TRPV4 was also revealed in BTEC. AA remodels actin and triggers TRPV4 recruitment in the plasma membrane, finally leading to BTEC migration [32] (table 1; figures 1 and 2).
However, as previously stated, TRPV4 is ubiquitous in healthy vascular endothelium and plays a physiological role both in large arteries and microvessels: these relevant activities require careful consideration of its therapeutic potential. On the other hand, an overexpression of TEC could be exploited for a tumour-targeted therapy based on lower inhibiting doses of TRPV4 antagonists which could selectively affect TEC and not normal EC.
A number of cellular stress factors, including hypoxia, nutrient deprivation and reactive oxygen species, are important stimuli for angiogenic signalling [87]. TRPM2 promotes macrovascular pulmonary EC permeability in an H2O2-dependent manner. TRPM2 knockdown or overexpression of the TRPM2 short isoform (a dominant negative for TRPM2 long isoform) significantly reduces the H2O2-/Ca2+-mediated increase of paracellular permeability and cell death in H5V EC [46,88] (table 1 and figure 2). These data open the exciting possibility of targeting TRPM2 for endothelial protection against ROS-induced cell damage [89].
Finally, TRPM7, a Ca2+- and Mg2+-permeable channel that regulates Mg2+ homeostasis, is involved in a number of vascular disorders such as hypertension and dysfunction of endothelial and smooth muscle cells [90]. A notable feature of TRPM7 is the presence of a kinase domain at its C-terminus, making TRPM7 unique among ion channels, and allowing its involvement both in cellular Mg2+ homeostasis and broad signalling [91]. TRPM7 acts negatively on HUVEC proliferation and migration, whereas its functions on HMEC seem to be different [47–49] (table 1 and figure 2).
In addition to canonical angiogenesis, tumour vascularization may be supported by bone marrow (BM)-derived endothelial progenitor cells (EPCs) incorporating within sprouting neovessels. This feature hinted at EPC inhibition as a novel therapeutic target to pursue along with antiangiogenic treatments [1,87]. Suppression of Orai1 in EPC prevents SOCE and tubule formation [34,92]. Moreover, EPCs isolated from RCC patients (RCC-EPCs) display an increased SOCE, which correlates with Orai1, Stim1 and TRPC1 overexpression when compared with EPCs from healthy patients: genetic suppression of Stim1, Orai1 and TRPC1 affects SOCE in RCC-EPCs [29]. TRPC1 regulates proliferation and migration of EPCs isolated from rats BM [30] (see also table 1 and figure 2).
4. Nicotinic receptors
nAChR are homo- or hetero-pentameric ion channels activated by endogenous acetylcholine or exogenous agonists such as nicotine [93]. ECs express most of the known mammalian nAChR subunits [41,55,94]. In particular, α7 nAChR mediates the main effects of nicotine on EC, such as proliferation, survival, migration, tube formation and intracellular signalling. Interestingly, α9 and α7 nAChRs exert opposing effects on nicotine-induced cell proliferation and survival [41,42,69].
Exposure to nicotine upregulates α7-nAChR and pharmacological inhibition of α7-nAChR by mecamylamine or a-bungarotoxin significantly and reversibly reduces EC tubulogenesis in vitro. Even more importantly, pharmacological inhibitors or genetic disruption of α7-nAChR significantly suppress neo-angiogenesis in inflammation, ischaemia and neoplasia in several models. The angiogenic effect of nAChR is exerted through MAPK, PI3K/Akt and NF-kB pathway; however, because nAChR-mediated angiogenesis is partially inhibited only in α7-nAChR-deficient mouse, other nAChR isoforms are presumably involved [41]. Nicotine triggers neo-angiogenesis in breast, colon and lung tumour cells implanted in chick chorioallantoic membranes and promotes b-FGF release through the recruitment of nicotinic receptor, v3 integrin and MAPK pathway [94–96]. The ability of nicotine to promote late EPCs proliferation, migration, adhesion and tubulogenesis strongly suggests that its role is not restricted to mature EC [43] (table 1 and figure 2)
5. Volume-regulated anion channels
Resting normal EC expresses volume-regulated anion channels (VRACs), mainly permeable to chloride ions and activated by osmotic cell swelling and shear stress. Endothelial VRACs are open in resting conditions and contribute to the maintenance of the resting potential in non-stimulated cells, in addition to K+ channels [22].
VRAC blockers (Mibefradil, NPPB, tamoxifen and Clomiphene) inhibit tube formation of rat and human microvascular EC and are strongly antiangiogenic in vivo [54] (table 1). Although the mechanism of VRACs involvement in angiogenesis has not been clarified yet, one possible explanation is that endothelial VRACs are partially activated under isosmotic conditions and provide a background Cl– conductance that contributes to the setting of the resting membrane potential in association with K+ currents. Depending on the membrane potential and equilibrium potential for Cl−, VRAC activation can cause either depolarization or hyperpolarization, affecting the driving force for calcium and thus indirectly regulating calcium signalling shape [97].
6. Water channels
Aquaporins (AQPs) allow passive water flow in response to local osmotic gradients. They contribute to epithelial secretion and absorption, and cell volume regulation. Ectopic AQP expression is associated with several human cancers [24,98]. In particular, AQP1 is involved in cell motility and tumour vascularization [65,99,100]: its expression in tumour cells and vessels is variable being dependent not only on the origin of the tumour, but also on its location in the host animal. This observation strengthens the inductive role of the microenvironment on tumour features.
Interestingly, AQP1 is upregulated in human brain tumours: little or no AQP1 expression is found in normal human brain microvessel endothelium, consistently with its general low permeability. On the other hand, vascular AQP1 expression increases with the progression from normal brain to low-grade to high-grade astrocytoma [66].
Verkman and co-workers [67] studied AQP1 role in angiogenesis in vivo by implanting melanoma cells in AQP1 null mice and syngenic mice lacking AQP1. In both cases, the authors observed a markedly lower density of microvessels and the presence of islands of viable tumour cells surrounded by necrotic tissue compared with control mice. Functional analyses on mouse aortic EC isolated from AQP1 null mice and wild-type mice revealed an impaired migration, invasiveness and capability to form capillary-like structures in matrigel [67]. On the other hand, intratumoural injections of AQP1 siRNAs in a mouse model of melanoma suggest that AQP1 inhibition can hamper tumour growth significantly lowering microvessel density [40]. Vascular AQP1 is also overexpressed in both human and rodent chronic liver disease. AQP1 promotes angiogenesis, fibrosis and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs, as revealed on human and mouse hepatic EC [68]. Finally, microvessel overexpression of AQP1 is associated with BM angiogenesis in patients with active multiple myeloma [51] (table 1 and figure 2).
7. Carriers
Besides the role of ion channels, extensive evidence points out the involvement of carriers and transporters in tumour progression [101,102].
(a). Sodium–proton exchanger
It is well recognized that pathological elevations of pHi can concur to some features of malignant cells [103]. All tumours share an altered regulation of hydrogen ion dynamics and tumour progression correlates with the peculiar acid–base balance in cancer cells: an extracellular acid microenvironment (pHe, 6.2–6.9 versus 7.3–7.4 of normal cells) linked to an alkaline intracellular pH (pHi, 7.12–7.7 versus 6.99–7.05 of normal cells). Because NHE is a universal and conserved regulator of cellular proton balance, it received great attention. Through its action, the inwardly directed Na+ gradient can drive the uphill extrusion of protons that drives pHi alkalinization and pHe acidification [103].
The highly hypoxic tumour microenvironment hyperactivates NHE1 and, because specific NHE1 inhibitors (cariporide) are available, some authors propose them for innovative combination trials with antiangiogenic drugs. Low concentrations of cariporide can lead to a decrease in pHi and downregulation of VEGF. Moreover, exposure to cariporide inhibits HUVEC proliferation and migration promoted by conditional medium from K562 leukaemia cells. In vivo experiments directly confirmed that inhibition of NHE1 by cariporide could affect tumour growth and angiogenesis [104]. Blocking NHE1 reduces VEGF release from the tumour cells suggesting that, in addition to being stimulated by hypoxia, VEGF production and angiogenesis are linked to acidic pHe and to the NHE1-dependent changes in pH [38]. Systemic amiloride perfusion also reduced neovascularization experimentally induced in an animal model, probably through inhibition of NHE1 [105].
(b). Sodium–calcium exchanger
Sodium influx mediated by non-selective cation channels can lead to its accumulation beneath the plasma membrane. This event may increase [Ca2+]i by locally inverting (3Na+ out: 1 Ca2+ in) the operation mode of NCX [64].
As already described above (paragraph on VCSCs), an intriguing example has been described in HUVEC, in which a coupling between NCX and VGSCs occurs [39].
8. Conclusion
Since the seminal hypothesis proposed by Judas Folkman in the 1970s, interference with tumour vascularization has been considered a key therapeutic opportunity in cancer treatment [2].
Unfortunately, despite promising results, VTT appear short-lived and resistance develops in the majority of patients [106]. The relative inefficacy of VTT may be due to several reasons.
More suitable preclinical cancer models are required in oncological practice. As previously stated, vessels in cancer significantly differ from normal vasculature, and the instability of EC within the tumour is a relevant feature. To this purpose, the use of TEC seems a more appropriate model compared with the normal EC. We expect that more detailed studies on the ‘transportome’ in tumour vascularization using the aforementioned models (besides the EC models already in use) will give new input in unveiling the differences in signalling, transcriptome profiles and vascular ‘ZIP codes’ and will likely prove to be important for understanding the conversion of normal ECs into tumour-associated ECs. As a preliminary example, overexpression of TRPV4 in TEC [32] could be useful for selectively targeted therapy using lower doses of channel antagonists which affect TEC reducing secondary undesired effects on normal EC.
Another high priority challenge is the research of novel molecular anti-vascular targets (related or unrelated to VEGF signalling). The evaluation of their clinical potential, in particular as combination therapy with current VEGF (receptor) inhibitors, is likely to expand the antiangiogenic armamentarium. In particular, it could be useful to narrow the field of action for VEGF-mediated targeted therapy. In this context, the recent interest in human ‘transportome’ involvement in tumour vascularization is a promising field, because several members are activated downstream of the recruitment of VEGF receptors. For example, whereas the interference with the bulk VEGF signalling alters the activity of a multitude of different cells and functions, targeting TRPC6 or Orai1 may affect only EC migration and proliferation [31,34,44,53,92], whereas TRPC1 and STIM1 may selectively influence vascular permeability [56–58,61].
It is worth noting that channels and transporters are widely distributed and ubiquitous. This feature has to be carefully taken in account when considering them as clinical targets. This problem could be overcome by directed targeted therapies taking advantage from nano-biomedicine: for example, nanoparticle functionalization with peptide cyclic RGD for angiogenesis-specific targeting [107] together with a specific channel modulator could be successfully used.
On the other hand, the ubiquitous expression of the channels could be used as a positive feature, owing to the redundancy of the signalling pathways which regulates the different hallmarks of cancer: in other words, the use of specific channels to selectively co-target different key steps of carcinogenesis besides tumour vascularization, could result in more effective and long-lasting therapies. For example, TRPC6 channels targeting could affect VEGF release from tumour cells as well as EC migration and tumour vascularization [31,44,53].
Another important issue is the therapeutic potential of sustained vessel normalization to suppress metastasis and enhance chemotherapy. Indeed, several preclinical studies have revealed that the high levels of VEGF in tumours induce vessel abnormalities. It is reasonable to postulate that these vessel abnormalities could be decreased by lowering VEGF signalling. VEGF-targeted therapy induces characteristic features of vessel normalization, including reduced number and size of immature tumour vessels and increased pericyte coverage, together with decreased permeability, oedema and interstitial fluid pressure [108]. Interfering with K+ channels, such as EAG1 and hERG1, TRPC6 channels or NHE exchanger on tumour cells could be useful to promote vascular ‘normalization’ by interfering with VEGF signalling during a critical window of the antiangiogenic treatments.
Finally, even if big efforts have been produced in the past years in order to characterize and study the involvement of transportome in cancer cell biology, and in particular in tumour vascularization, the field is relatively novel. The scientific interest on this topic is largely increasing as pointed out by a PubMed search. The research on transportome and cancer is expected to expand even more in the next decade, and we believe that the oncogenic roles of channels, as well as the molecular mechanisms responsible for their regulation, will be largely unveiled.
Acknowledgements
We thank Daniele Avanzato (PhD student in Complex Systems in Life Sciences, University of Torino) for art graphics.
References
- 1.Carmeliet P. 2005. Angiogenesis in life, disease and medicine. Nature 438, 932–936. ( 10.1038/nature04478) [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. 1971. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186. ( 10.1056/NEJM197111182852108) [DOI] [PubMed] [Google Scholar]
- 3.Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887. ( 10.1016/j.cell.2011.08.039) [DOI] [PubMed] [Google Scholar]
- 4.Regan ER, Aird WC. 2012. Dynamical systems approach to endothelial heterogeneity. Circ. Res. 111, 110–130. ( 10.1161/CIRCRESAHA.111.261701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi J-T, et al. 2003. Endothelial cell diversity revealed by global expression profiling. Proc. Natl Acad. Sci. USA 100, 10 623–10 628. ( 10.1073/pnas.1434429100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussolati B, Grange C, Camussi G. 2011. Tumor exploits alternative strategies to achieve vascularization. FASEB J. 25, 2874–2882. ( 10.1096/fj.10-180323) [DOI] [PubMed] [Google Scholar]
- 7.Ghilardi C, Chiorino G, Dossi R, Nagy Z, Giavazzi R, Bani M. 2008. Identification of novel vascular markers through gene expression profiling of tumor-derived endothelium. BMC Genomics 9, 201 ( 10.1186/1471-2164-9-201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhati R, et al. 2008. Molecular characterization of human breast tumor vascular cells. Am. J. Pathol. 172, 1381–1390. ( 10.2353/ajpath.2008.070988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohga N, et al. 2012. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 180, 1294–1307. ( 10.1016/j.ajpath.2011.11.035) [DOI] [PubMed] [Google Scholar]
- 10.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. 2003. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 17, 1159–1161. [DOI] [PubMed] [Google Scholar]
- 11.Grange C, Bussolati B, Bruno S, Fonsato V, Sapino A, Camussi G. 2006. Isolation and characterization of human breast tumor-derived endothelial cells. Oncol. Rep. 15, 381–386. [PubMed] [Google Scholar]
- 12.Park M-T, et al. 2012. The radiosensitivity of endothelial cells isolated from human breast cancer and normal tissue in vitro. Microvasc. Res. 84, 140–148. ( 10.1016/j.mvr.2012.06.002) [DOI] [PubMed] [Google Scholar]
- 13.Fiorio Pla A, Grange C, Antoniotti S, Tomatis C, Merlino A, Bussolati B, Munaron L. 2008. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol. Cancer Res. 6, 535–545. ( 10.1158/1541-7786.MCR-07-0271) [DOI] [PubMed] [Google Scholar]
- 14.Fiorio Pla A, Genova T, Pupo E, Tomatis C, Genazzani A, Zaninetti R, Munaron L. 2010. Multiple roles of protein kinase a in arachidonic acid-mediated Ca2+ entry and tumor-derived human endothelial cell migration. Mol. Cancer Res. 8, 1466–1476. ( 10.1158/1541-7786.MCR-10-0002) [DOI] [PubMed] [Google Scholar]
- 15.Pupo E, Pla AF, Avanzato D, Moccia F, Cruz JE, Tanzi F, Merlino A, Mancardi D, Munaron L. 2011. Hydrogen sulfide promotes calcium signals and migration in tumor-derived endothelial cells. Free Radic. Biol. Med. 51, 1765–1773. ( 10.1016/j.freeradbiomed.2011.08.007) [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 17.Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. 2012. Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am. J. Physiol. Cell Physiol. 302, C9–C15. ( 10.1152/ajpcell.00280.2011) [DOI] [PubMed] [Google Scholar]
- 18.Munaron L, Genova T, Avanzato D, Antoniotti S, Fiorio Pla A. 2013. Targeting calcium channels to block tumor vascularization. Recent Patents Anti-cancer Drug Discov. 8, 27–37. [DOI] [PubMed] [Google Scholar]
- 19.Yao X, Garland CJ. 2005. Recent developments in vascular endothelial cell transient receptor potential channels. Circ. Res. 97, 853–863. ( 10.1161/01.RES.0000187473.85419.3e) [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. 2008. TRP channel and cardiovascular disease. Pharmacol. Ther. 118, 337–351. ( 10.1016/j.pharmthera.2008.03.008) [DOI] [PubMed] [Google Scholar]
- 21.Nilius B, Owsianik G, Voets T, Peters JA. 2007. Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217. ( 10.1152/physrev.00021.2006) [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Droogmans G. 2001. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 81, 1415–1459. [DOI] [PubMed] [Google Scholar]
- 23.Becchetti A. 2011. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am. J. Physiol. Cell Physiol. 301, C255–C265. ( 10.1152/ajpcell.00047.2011) [DOI] [PubMed] [Google Scholar]
- 24.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. 2009. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr. Med. Chem. 16, 66–93. ( 10.2174/092986709787002835) [DOI] [PubMed] [Google Scholar]
- 25.Wulff H, Castle NA, Pardo LA. 2009. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 8, 982–1001. ( 10.1038/nrd2983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gómez-Varela D, et al. 2007. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 67, 7343–7349. ( 10.1158/0008-5472.CAN-07-0107) [DOI] [PubMed] [Google Scholar]
- 27.Downie BR, Sánchez A, Knötgen H, Contreras-Jurado C, Gymnopoulos M, Weber C, Stühmer W, Pardo LA. 2008. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J. Biol. Chem. 283, 36 234–36 240. ( 10.1074/jbc.M801830200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Wu X, Zhong D, Zhai W, Ding Z, Zhou Y. 2012. Short hairpin RNA (shRNA) Ether à go-go 1 (Eag1) inhibition of human osteosarcoma angiogenesis via VEGF/PI3K/AKT signaling. Int. J. Mol. Sci. 13, 12 573–12 583. ( 10.3390/ijms131012573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodola F, et al. 2012. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE 7, e42541 ( 10.1371/journal.pone.0042541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuang C, Yu Y, Wang K, Qian D, Den M, Huang L. 2012. Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cells Dev. 21, 487–496. ( 10.1089/scd.2011.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO. 2008. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 15, 605–614. ( 10.1080/10739680802220323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorio Pla A, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, Paria B, Munaron L, Ambudkar IS. 2012. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene 31, 200–212. ( 10.1038/onc.2011.231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. 2008. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 103, 1289–1299. ( 10.1161/01.RES.0000338496.95579.56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, et al. 2011. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ. Res. 108, 1190–1198. ( 10.1161/CIRCRESAHA.111.243352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beech DJ. 2012. Orai1 calcium channels in the vasculature. Pflügers Arch. 463, 635–647. ( 10.1007/s00424-012-1090-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrikopoulos P, et al. 2011. Angiogenic functions of voltage-gated Na+ Channels in human endothelial cells: modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 286, 16 846–16 860. ( 10.1074/jbc.M110.187559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martini A, Bruno R, Mazzulla S, Nocita A, Martino G. 2010. Angiotensin II regulates endothelial cell migration through calcium influx via T-type calcium channel in human umbilical vein endothelial cells. Acta Physiol. 198, 449–455. ( 10.1111/j.1748-1716.2009.02070.x) [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Fukumura D, Jain RK. 2002. Acidic extracellular pH induces vascular endothelial growth factor (VEGF) in human glioblastoma cells via ERK1/2 MAPK signaling pathway: mechanism of low pH-induced VEGF. J. Biol. Chem. 277, 11 368–11 374. ( 10.1074/jbc.M108347200) [DOI] [PubMed] [Google Scholar]
- 39.Andrikopoulos P, Baba A, Matsuda T, Djamgoz MBA, Yaqoob MM, Eccles SA. 2011. Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J. Biol. Chem. 286, 37 919–37 931. ( 10.1074/jbc.M111.251777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicchia GP, Stigliano C, Sparaneo A, Rossi A, Frigeri A, Svelto M. 2013. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 91, 613–623. ( 10.1007/s00109-012-0977-x) [DOI] [PubMed] [Google Scholar]
- 41.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. 2002. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Invest. 110, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu JCF, Chruscinski A, De Jesus Perez VA, Singh H, Pitsiouni M, Rabinovitch M, Utz PJ, Cooke JP. 2009. Cholinergic modulation of angiogenesis: role of the 7 nicotinic acetylcholine receptor. J. Cell. Biochem. 108, 433–446. ( 10.1002/jcb.22270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M, Liu Q, Sun J, Yi K, Wu L, Tan X. 2011. Nicotine improves the functional activity of late endothelial progenitor cells via nicotinic acetylcholine receptors. Biochem. Cell Biol. 89, 405–410. ( 10.1139/o11-032) [DOI] [PubMed] [Google Scholar]
- 44.Ge R, Tai Y, Sun Y, Zhou K, Yang S, Cheng T, Zou Q, Shen F, Wang Y. 2009. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 283, 43–51. ( 10.1016/j.canlet.2009.03.023) [DOI] [PubMed] [Google Scholar]
- 45.Troidl C, et al. 2009. Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J. Cell. Mol. Med. 13, 2613–2621. ( 10.1111/j.1582-4934.2008.00579.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. 2008. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 102, 347–355. ( 10.1161/CIRCRESAHA.107.160176) [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Xiong Z-G. 2009. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc. Res. 83, 547–557. ( 10.1093/cvr/cvp153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldoli E, Maier JAM. 2012. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis 15, 47–57. ( 10.1007/s10456-011-9242-0) [DOI] [PubMed] [Google Scholar]
- 49.Baldoli E, Castiglioni S, Maier JAM. 2013. Regulation and function of TRPM7 in human endothelial cells: TRPM7 as a potential novel regulator of endothelial function. PLoS ONE 8, e59891 ( 10.1371/journal.pone.0059891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erdogan A, et al. 2005. Margatoxin inhibits VEGF-induced hyperpolarization, proliferation and nitric oxide production of human endothelial cells. J. Vasc. Res. 42, 368–376. ( 10.1159/000087159) [DOI] [PubMed] [Google Scholar]
- 51.Vacca A, Frigeri A, Ribatti D, Nicchia GP, Nico B, Ria R, Svelto M, Dammacco F. 2001. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br. J. Haematol. 113, 415–421. ( 10.1046/j.1365-2141.2001.02738.x) [DOI] [PubMed] [Google Scholar]
- 52.Antigny F, Girardin N, Frieden M. 2012. Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J. Biol. Chem. 287, 5917–5927. ( 10.1074/jbc.M111.295733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chigurupati S, et al. 2010. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 70, 418–427. ( 10.1158/0008-5472.CAN-09-2654) [DOI] [PubMed] [Google Scholar]
- 54.Manolopoulos VG, Liekens S, Koolwijk P, Voets T, Peters E, Droogmans G, Lelkes PI, De Clercq E, Nilius B. 2000. Inhibition of angiogenesis by blockers of volume-regulated anion channels. Gen. Pharmacol. 34, 107–116. ( 10.1016/S0306-3623(00)00052-5) [DOI] [PubMed] [Google Scholar]
- 55.Egleton RD, Brown KC, Dasgupta P. 2009. Angiogenic activity of nicotinic acetylcholine receptors: Implications in tobacco-related vascular diseases. Pharmacol. Ther. 121, 205–223. ( 10.1016/j.pharmthera.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 56.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. 2003. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J. Biol. Chem. 278, 33 492–33 500. ( 10.1074/jbc.M302401200) [DOI] [PubMed] [Google Scholar]
- 57.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. 2004. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L1303–L1313. ( 10.1152/ajplung.00240.2004) [DOI] [PubMed] [Google Scholar]
- 58.Jho D, Mehta D, Ahmmed G, Gao X-P, Tiruppathi C, Broman M, Malik AB. 2005. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2+ influx. Circ. Res. 96, 1282–1290. ( 10.1161/01.RES.0000171894.03801.03) [DOI] [PubMed] [Google Scholar]
- 59.Kini V, Chavez A, Mehta D. 2010. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J. Biol. Chem. 285, 33 082–33 091. ( 10.1074/jbc.M110.142034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pocock TM, Foster RR, Bates DO. 2004. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am. J. Physiol. Heart Circ. Physiol. 286, H1015–H1026. ( 10.1152/ajpheart.00826.2003) [DOI] [PubMed] [Google Scholar]
- 61.Shinde AV, et al. 2013. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci. Signal. 6, ra18 ( 10.1126/scisignal.2003425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu P, Gu S, Bu J, Du J. 2010. TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ. Res. 106, 1221–1232. ( 10.1161/CIRCRESAHA.109.207670) [DOI] [PubMed] [Google Scholar]
- 63.Fortunato P, Pillozzi S, Tamburini A, Pollazzi L, Franchi A, La Torre A, Arcangeli A. 2010. Irresponsiveness of two retinoblastoma cases to conservative therapy correlates with up- regulation of hERG1 channels and of the VEGF-A pathway. BMC Cancer 10, 504 ( 10.1186/1471-2407-10-504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blaustein MP, Lederer WJ. 1999. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854. [DOI] [PubMed] [Google Scholar]
- 65.Endo M, Jain RK, Witwer B, Brown D. 1999. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 58, 89–98. ( 10.1006/mvre.1999.2158) [DOI] [PubMed] [Google Scholar]
- 66.Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. 2002. Increased aquaporin 1 water channel expression in human brain tumours. Br. J. Cancer 87, 621–623. ( 10.1038/sj.bjc.6600512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. 2005. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434, 786–792. ( 10.1038/nature03460) [DOI] [PubMed] [Google Scholar]
- 68.Huebert RC, Jagavelu K, Hendrickson HI, Vasdev MM, Arab JP, Splinter PL, Trussoni CE, Larusso NF, Shah VH. 2011. Aquaporin-1 promotes angiogenesis, fibrosis, and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs. Am. J. Pathol. 179, 1851–1860. ( 10.1016/j.ajpath.2011.06.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng MKC, Wu J, Chang E, Wang B, Katzenberg-Clark R, Ishii-Watabe A, Cooke JP. 2007. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 27, 106–112. ( 10.1161/01.ATV.0000251517.98396.4a) [DOI] [PubMed] [Google Scholar]
- 70.Munaron L, Arcangeli A. 2013. Editorial: ion fluxes and cancer. Recent Patents Anti-Cancer Drug Discov. 8, 1–3. ( 10.2174/1574892811308010001) [DOI] [PubMed] [Google Scholar]
- 71.D'Amico M, Gasparoli L, Arcangeli A. 2013. Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Patents Anti-Cancer Drug Discov. 8, 53–65. [DOI] [PubMed] [Google Scholar]
- 72.Pillozzi S, et al. 2007. VEGFR-1 (FLT-1), β1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 110, 1238–1250. ( 10.1182/blood-2006-02-003772) [DOI] [PubMed] [Google Scholar]
- 73.Yildirim S, Altun S, Gumushan H, Patel A, Djamgoz MBA. 2012. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 323, 58–61. ( 10.1016/j.canlet.2012.03.036) [DOI] [PubMed] [Google Scholar]
- 74.Djamgoz MBA, Onkal R. 2013. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Patents Anti-Cancer Drug Discov. 8, 66–84. [DOI] [PubMed] [Google Scholar]
- 75.House CD, et al. 2010. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 70, 6957–6967. ( 10.1158/0008-5472.CAN-10-1169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bertolesi GE, Shi C, Elbaum L, Jollimore C, Rozenberg G, Barnes S, Kelly MEM. 2002. The Ca2+ channel antagonists mibefradil and pimozide inhibit cell growth via different cytotoxic mechanisms. Mol. Pharmacol. 62, 210–219. ( 10.1124/mol.62.2.210) [DOI] [PubMed] [Google Scholar]
- 77.Asaga S, Ueda M, Jinno H, Kikuchi K, Itano O, Ikeda T, Kitajima M. In press Identification of a new breast cancer-related gene by restriction landmark genomic scanning. Anticancer Res. 26, 35–42. [PubMed] [Google Scholar]
- 78.Panner A, Wurster RD. 2006. T-type calcium channels and tumor proliferation. Cell Calcium 40, 253–259. ( 10.1016/j.ceca.2006.04.029) [DOI] [PubMed] [Google Scholar]
- 79.Miura S-I, Fujino M, Matsuo Y, Tanigawa H, Saku K. 2005. Nifedipine-induced vascular endothelial growth factor secretion from coronary smooth muscle cells promotes endothelial tube formation via the kinase insert domain-containing receptor/fetal liver kinase-1/NO pathway. Hypertens. Res. 28, 147–153. ( 10.1291/hypres.28.147) [DOI] [PubMed] [Google Scholar]
- 80.Gkika D, Prevarskaya N. 2011. TRP channels in prostate cancer: the good, the bad and the ugly? Asian J. Androl. 13, 673–676. ( 10.1038/aja.2011.18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ambudkar IS, Ong HL. 2007. Organization and function of TRPC channelosomes. Pflügers Arch. 455, 187–200. ( 10.1007/s00424-007-0252-0) [DOI] [PubMed] [Google Scholar]
- 82.Cheng H-W, James AF, Foster RR, Hancox JC, Bates DO. 2006. VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 1768–1776. ( 10.1161/01.ATV.0000231518.86795.0f) [DOI] [PubMed] [Google Scholar]
- 83.Everaerts W, Nilius B, Owsianik G. 2010. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog. Biophys. Mol. Biol. 103, 2–17. ( 10.1016/j.pbiomolbio.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 84.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. 2004. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl Acad. Sci. USA 101, 396–401. ( 10.1073/pnas.0303329101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartmannsgruber V, Heyken W-T, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. 2007. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2, e827 ( 10.1371/journal.pone.0000827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. 2009. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104, 1123–1130. ( 10.1161/CIRCRESAHA.108.192930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.North S, Moenner M, Bikfalvi A. 2005. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 218, 1–14. ( 10.1016/j.canlet.2004.08.007) [DOI] [PubMed] [Google Scholar]
- 88.Sun L, Yau H-Y, Wong W-Y, Li RA, Huang Y, Yao X. 2012. Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. PLoS ONE 7, e43186 ( 10.1371/journal.pone.0043186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orfanelli U, Wenke A-K, Doglioni C, Russo V, Bosserhoff AK, Lavorgna G. 2008. Identification of novel sense and antisense transcription at the TRPM2 locus in cancer. Cell Res. 18, 1128–1140. ( 10.1038/cr.2008.296) [DOI] [PubMed] [Google Scholar]
- 90.Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. 2011. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ. J. 75, 237–245. ( 10.1253/circj.CJ-10-1021) [DOI] [PubMed] [Google Scholar]
- 91.Paravicini TM, Chubanov V, Gudermann T. 2012. TRPM7: a unique channel involved in magnesium homeostasis. Int. J. Biochem. Cell Biol. 44, 1381–1384. ( 10.1016/j.biocel.2012.05.010) [DOI] [PubMed] [Google Scholar]
- 92.Dragoni S, et al. 2011. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells 29, 1898–1907. ( 10.1002/stem.734) [DOI] [PubMed] [Google Scholar]
- 93.Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P. 2009. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. 1–18. Nat. Rev. Drug Discov. 8 (9): 733–750. ( 10.1038/nrd2927) [DOI] [PubMed] [Google Scholar]
- 94.Cardinale A, Nastrucci C, Cesario A, Russo P. 2012. Nicotine: specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol. 42, 68–89. ( 10.3109/10408444.2011.623150) [DOI] [PubMed] [Google Scholar]
- 95.Mousa S, Mousa SA. 2006. Cellular and molecular mechanisms of nicotine's pro-angiogenesis activity and its potential impact on cancer. J. Cell. Biochem. 97, 1370–1378. ( 10.1002/jcb.20741) [DOI] [PubMed] [Google Scholar]
- 96.Arias HR, Richards VE, Ng D, Ghafoori ME, Le V, Mousa SA. 2009. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int. J. Biochem. Cell Biol. 41, 1441–1451. ( 10.1016/j.biocel.2009.01.013) [DOI] [PubMed] [Google Scholar]
- 97.Nilius B, Droogmans G. 2003. Amazing chloride channels: an overview. Acta Physiol. Scand. 177, 119–147. ( 10.1046/j.1365-201X.2003.01060.x) [DOI] [PubMed] [Google Scholar]
- 98.Monzani E, Shtil AA, La Porta CAM. 2007. The water channels, new druggable targets to combat cancer cell survival, invasiveness and metastasis. Curr. Drug Targets 8, 1132–1137. ( 10.2174/138945007782151342) [DOI] [PubMed] [Google Scholar]
- 99.Verkman AS. 2012. Aquaporins in clinical medicine. Annu. Rev. Med. 63, 303–316. ( 10.1146/annurev-med-043010-193843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alleva K, Chara O, Amodeo G. 2012. Aquaporins: another piece in the osmotic puzzle. FEBS Lett. 586, 2991–2999. ( 10.1016/j.febslet.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 101.Cardone RA, Casavola V, Reshkin SJ. 2005. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786–795. ( 10.1038/nrc1713) [DOI] [PubMed] [Google Scholar]
- 102.Monteith GR, Davis FM, Roberts-Thomson SJ. 2012. Calcium channels and pumps in cancer: changes and consequences. J. Biol. Chem. 287, 31 666–31 673. ( 10.1074/jbc.R112.343061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reshkin SJ, Cardone RA, Harguindey S. 2013. Na+-H+ exchanger, pH regulation and cancer. Recent Patents Anti-Cancer Drug Discov. 8, 85–99. [DOI] [PubMed] [Google Scholar]
- 104.Gao W, et al. 2011. Inhibition of K562 leukemia angiogenesis and growth by selective Na+/H+ exchanger inhibitor cariporide through down-regulation of pro-angiogenesis factor VEGF. Leukemia Res. 35, 1506–1511. ( 10.1016/j.leukres.2011.07.001) [DOI] [PubMed] [Google Scholar]
- 105.Avery RL, Connor TB, Farazdaghi M. 1990. Systemic amiloride inhibits experimentally induced neovascularization. Arch. Ophthalmol. 108, 1474–1476. ( 10.1001/archopht.1990.01070120122041) [DOI] [PubMed] [Google Scholar]
- 106.Ebos JML, Kerbel RS. 2011. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8, 210–221. ( 10.1038/nrclinonc.2011.21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng J, Gu Y-J, Wang Y, Cheng SH, Wong W-T. 2011. Nanotherapeutics in angiogenesis: synthesis and in vivo assessment of drug efficacy and biocompatibility in zebrafish embryos. Int. J. Nanomed. 6, 2007–2021. ( 10.2147/IJN.S20145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carmeliet P, Jain RK. 2011. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427. ( 10.1038/nrd3455) [DOI] [PubMed] [Google Scholar]