Abstract
Hyalomma scupense (syn. Hyalomma detritum) is a two-host domestic endophilic tick of cattle and secondarily other ungulates in the Maghreb region (Africa). This species transmits several pathogens, among which two are major livestock diseases: Theileria annulata and Theileria equi. Various other pathogens are also transmitted by this tick species, such as Anaplasma phagocytophilum and Ehrlichia bovis. Hyalomma scupense is common in sub-humid and semi-arid areas of several regions in the world, mainly in the Maghreb region. In this region, adults attach to animals during the summer season; larvae and nymphs attach to their hosts during autumn, but there is a regional difference in H. scupense phenology. There is an overlap between immature and adult ticks, leading in some contexts to a dramatic modification of the epidemiology of tick-borne diseases. This tick species attaches preferentially to the posterior udder quarters and thighs. Tick burdens can reach 130 ticks per animal, with a mean of 60 ticks. Calves are 70 times less infested than adult cattle. The control can be implemented through six options: (i) rehabilitation of the farm buildings by roughcasting and smoothing the outer and inner surfaces of the enclosures and walls. This control option should be recommended to be combined with a thorough cleaning of the farm and its surrounding area. With regard to Theileria annulata infection, this control option is the most beneficial. (ii) Acaricide application to animals during the summer season, targeting adults. (iii) Acaricide application during the autumn period for the control of the immature stages. (iv) Acaricide application to the walls: many field veterinarians have suggested this option but it is only partially efficient since nymphs enter deep into the cracks and crevices. It should be used if there is a very high tick burden or if there is a high risk of tick-borne diseases. (v) Manual tick removal: this method is not efficient since the ticks can feed on several other animal species in the farm. This control option can lead to a reduction of the tick population, but not a decrease in tick-borne disease incidence. (vi) Vaccination: this control option consists of injecting the protein Hd86; trials have shown a partial effect on nymphs, with no effect on adult ticks. Combination of two of these control options is recommended in regions where there are high burdens of important tick vectors. Further studies are needed to improve our knowledge on this tick species in the Maghreb region, since the number of published studies on Hyalomma scupense in this region is very limited.
Keywords: Hyalomma scupense, Tick, Biology, Control, Cattle, Vector, Maghreb
Abstract
Hyalomma scupense (syn. Hyalomma detritum) est une tique diphasique, domestique, endophile, elle est fréquente chez les bovins et secondairement chez d’autres ongulés dans la région du Maghreb. Cette espèce transmet plusieurs pathogènes, dont deux provoquent des maladies majeures : Theileria annulata et Theileria equi. Différents autres pathogènes sont également transmis par cette espèce de tique, tels que Anaplasma phagocytophilum et Ehrlichia bovis. Hyalomma scupense est commune dans les régions subhumides et semi-arides de plusieurs régions du monde, principalement dans le Maghreb. Les adultes sont observés sur les animaux en été, les larves et les nymphes se fixent sur leurs hôtes durant l’automne, mais il existe une différence régionale de la phénologie d’H. scupense. Il y a un chevauchement entre les stades immatures et les tiques adultes, et ce phénomène induit, dans certains contextes, une importante modification de l’épidémiologie des infections transmises par les tiques. Cette tique se fixe préférentiellement au niveau des quartiers postérieurs des mamelles et des cuisses. L’intensité d’infestation peut atteindre 130 tiques avec une moyenne de 60 tiques. Les veaux sont 70 fois moins infestés que les adultes. La lutte peut être réalisée par six méthodes: (i) Amélioration des locaux d’élevage par un crépissage et un lissage des façades externes et internes des murs de l’étable. Cette option de lutte doit être inévitablement accompagnée d’un nettoyage drastique de l’étable et de son pourtour. En considérant l’infection par Theileria annulata, cette option de lutte est la plus bénéfique. (ii) Application d’acaricides sur les animaux pendant la saison estivale ciblant les adultes. (iii) Application d’acaricides pendant l’automne pour la lutte contre les stades immatures. (iv) Application d’acaricides sur les murs : cette option a été proposée par plusieurs vétérinaires de terrain mais elle est seulement partiellement efficace parce que les nymphes pénètrent profondément dans les fissures et les crevasses. Elle doit être utilisée si l’infestation par les tiques est élevée ou s’il y a un risque élevé de maladies transmises par les tiques. (v) Détiquage manuel : c’est une technique qui n’est pas effective car les tiques peuvent se nourrir sur plusieurs autres espèces d’hôtes dans l’élevage. Cette option de lute peut induire une réduction de la population de tiques mais pas de l’incidence des maladies transmises par les tiques. (vi) Vaccination : cette option de lutte consiste à injecter la protéine Hd86, les essais ont montré des effets partiels sur les nymphes mais sans effets sur les tiques adultes. L’association de deux de ces options de lutte est recommandée dans les régions à forte intensité des vecteurs. Des études ultérieures sont nécessaires pour améliorer nos connaissances sur cette espèce dans la région du Maghreb puisque le nombre d’études publiées sur H. scupense dans la région du Maghreb est très réduit et la connaissance sur cette tique est fragmentaire.
Abstract
الزجاجي العين ( Hyalomma scupense ) (مرادف H. detritum ) هو نوع ثنائي الطور، أليف، مستبطن ، شائع في الماشية و بشكل ثانوي عند ذوات حوافر في مناطق أخرى بالمنطقة المغاربية. و ينقل هذه النوع العديد من الجراثيم ، منهما اثنين رئيسيين وهما : طفيلي الحمى المدارية ( Theileria annulata ) و طفيلي يرقان الخيول ( Theileria equi ). وينقل هذا النوع من القراد جراثيم أخرى نذكر منها : الانبلازما ( Anaplasma phagocytophilum ) و الإيرليخية البقرية ( Ehrlichia bovis ). الزجاجي العين ( H. scupense ) نوع شائع في المناطق شبه الرطبة وشبه القاحلة في العديد من أنحاء العالم، وخاصة في المغرب العربي. تم العثور على البالغين على جسم الحيوانات في الصيف، و تعلق اليرقات و الحوريات على مضيفاتها خلال الخريف، ولكن هناك فرقا اقليميا في دراسة الأحداث البيولوجية للزجاجي العين (H. scupense) . هناك تداخل بين المراحل الغير ناضجة و القراد البالغ، تؤدي هذه الظاهرة في بعض السياقات، الى تغيير كبير في وبائيات الأمراض المنقولة عبر القراد. يلتصق هذا النوع من القراد بشكل تفضيلي في الثديين الخلفين والفخذين. يمكن أن تصل شدة الإصابة إلى 130 قرادا بمتوسط عدد 60 من القراد. العجول هي 70 مرة أقل اصابة من بالغي الحيوانات. تتم المقاومة بستة طرق : (1) تحسين الاسطبلات من خلال التجصيص و صقل الواجهات الخارجية و الداخلية لجدران الحظيرة. يجب أن يقترن هذا الخيار حتما بتنظيف جذري للحظيرة و محيطها. اعتبارا للإصابة بطفيلي الحمى المدارية، خيار المقاومة هذا هو الأكثر فائدة. (2) استعمال مبيدات القراد على الحيوانات خلال موسم الصيف كي يستهدف البالغين من القراد. (3) استعمال مبيدات القراد في الخريف لمكافحة اليرقات و الشرانق. (4) رش مبيدات القراد على الجدران : وقد اقترح هذا الخيار من قبل العديد من الأطباء البياطرة و لكن فعاليتها نسبية لأن الشرانق تدخل بعمق في الصدوع والشقوق. ينبغي أن تستخدم هذه الطريقة إذا كانت نسبة الإصابة بالقراد مرتفعة أو إذا كان هناك خطرا كبيرا ناتجا عن وجود امراض منقولة عبر القراد. (5) قلع القراد باليد: هذه الطريقة غير ناجعة لان القراد يمكن أن يتغذى من العديد من الأنواع المضيفة الموجودة بالحضيرة. يمكن هذا الخيار من انخفاض في عدد القراد ولكن ليس في حالات الإصابة بالأمراض المنقولة عبر القراد. (6) التطعيم: يرتكز هذا الخيار علي حقن البروتين Hd86 و قد ابرزت التجارب وجود مفعول جزئي على الحوريات ولكن ليس له اي تأثير على كبار القراد. ينصح بجمع اثنين من هذه الخيارات للسيطرة في حالة وجود كثافة عالية للنواقل. هناك حاجة ماسة لدراسات إضافية حتى نحسن معرفتنا لهذا النوع في المنطقة المغاربية حيث عدد الدراسات المنشورة و المتعلقة بالزجاجي العين ( H. scupense ) في المنطقة المغاربية صغير جدا والمعرفة المتعلقة بهذا القراد مجزأة.
Introduction
The Maghreb region is limited by the Mediterranean Sea in the north, the Atlantic Ocean in the west and the Sahara in the south (Figure 1). The whole region is characterised by the presence of a gradient climate going from Mediterranean humid in the northern regions to Sahara in the south. High aridity concerns a large part of the Maghreb region, with a dry and hot summer and a wet and cold winter. The total agricultural surface represents 23% of the region (135,178,000 Ha), among which only 2.16% are irrigated [9].
Figure 1.
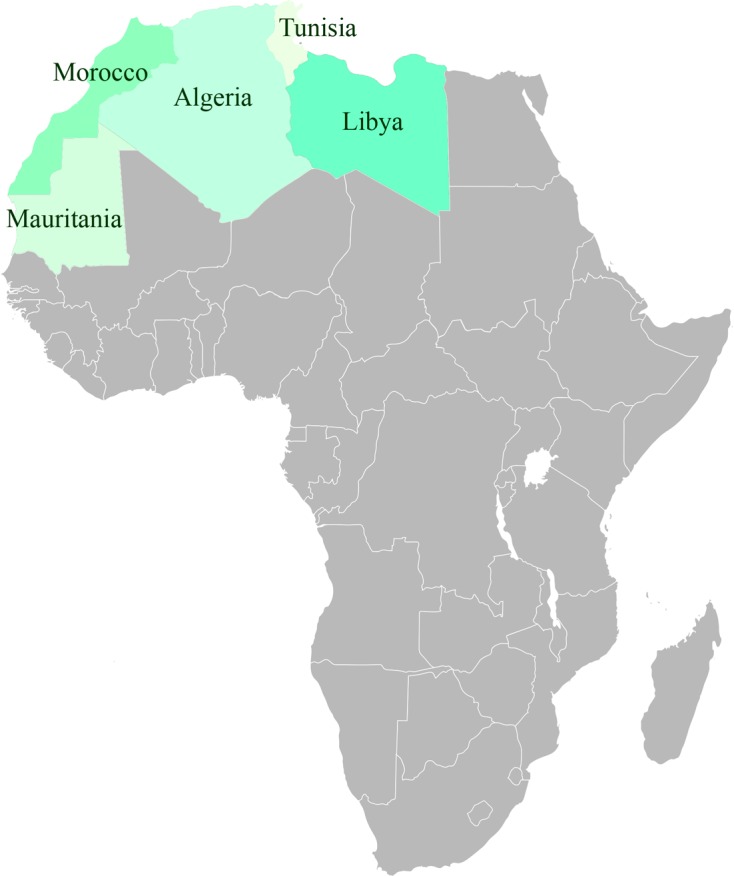
Geographic localisation of the five Maghreb countries (source: Wikipedia).
Both legal and illegal trade among all these countries is very active, including animals of different species. All these characteristics represent very important elements that deeply influence the biology and dynamics of different animals and mainly poikilotherm organisms (including ticks), strongly influenced by abiotic factors. Hyalomma scupense Schulze, 1919 is the vector of several pathogens, including Theileria annulata, the causative agent of tropical theileriosis. This protozoa is a major pathogen in the Maghreb region, causing high losses (milk yield decrease, weight loss, abortions, high treatment costs and deaths) in both endemically stable and unstable farms [21, 22]. Because of its vector role, the control of this tick species is of paramount importance in reducing both veterinary and financial impacts. The prerequisite for a successful control strategy is an excellent knowledge of the regional tick biology of each species. In this review, we present a synthesis of knowledge about the tick species H. scupense (syn. H. detritum) in the Maghreb region and different control measures that can be implemented to control it.
Systematics
There was a long-controversial issue about the possible synonymies of H. scupense Schulze, 1919 and H. detritum Schulze, 1919. It is now established that the two names correspond to the same species present in two distinct regions [13]. Filippova et al. [13] argued that due to a micro-evolutionary process, the former became monophasic whilst the latter is diphasic. Apanaskevich et al. [1] demonstrated that both belong to the same species and gave a description of the three developmental stages. According to Apanaskevich et al. [1], H. scupense Schulze, 1919 is the valid name; H. detritum Schulze, 1919 and H. mauritanicum Senevet, 1922 are synonyms and thus these names should not be used any more.
Biology
During the early 20th century, Sergent et al. [42] highlighted the importance of this tick species as a vector of Theileria annulata in the three countries colonised by France: Morocco, Algeria and Tunisia. Before the European colonisation of the Maghreb countries (Italy in Libya and France in the other four countries), there were only local cattle breeds adapted to ticks and tick-borne pathogens; the majority of cattle farms were probably in an endemically stable state with a virtual absence of clinical cases. In this context, local farmers were not aware of the vector role of ticks.
Zoogeography
Hyalomma scupense is one of the most widespread tick species of the 30 valid Hyalomma species; it is present in the Palaearctic zoogeographic region, in the humid to arid regions of 42 countries on three continents. It was reported in six African countries (Algeria, Egypt, Libya, Morocco, Sudan and Tunisia), 15 European countries (Albania, Bosnia and Herzegovina, Bulgaria, Croatia, France, Greece, Italy, Macedonia, Moldova, Montenegro, Romania, Russia [in the south of the European part and North Caucasus], Serbia, Spain and Ukraine) and twenty-one Asiatic countries (Afghanistan, Armenia, Azerbaijan, China, Georgia, India, Iran, Iraq, Israel, Jordan, Kazakhstan, Kyrgyzstan, Lebanon, Nepal, Oman, Pakistan, Syria, Tajikistan, Turkey, Turkmenistan and Uzbekistan) (Figure 2) [1, 24].
Figure 2.
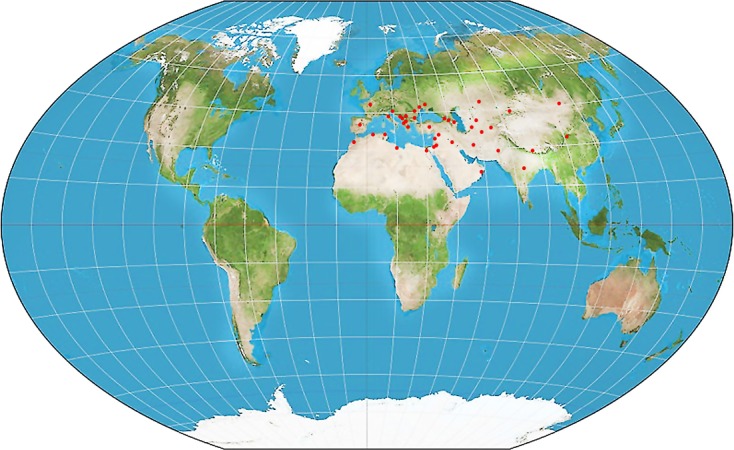
Geographic distribution of Hyalomma scupense ticks. The red dots indicate the countries where the tick is present [1, 2, 5, 13, 14, 24, 42, 45].
The geographic distribution of this tick species varies from one country to another. In Africa, the tick is abundant in Morocco, Algeria and Tunisia, mainly in northern regions (Figure 3) [3, 4, 36, 42]. Low H. scupense tick populations are present in the Gabès oases (Southern Tunisia) where several clinical cases of tropical theileriosis were reported [23]. Hyalomma scupense was reported in Libya on a single occasion by Hoogstraal in 1956 [24]. In 1992, Gabaj et al. [16] examined a total number of 1093 cattle, 1228 camels, 13242 sheep, 4513 goats, 716 horses and 28 donkeys; they found seven tick species but did not report the presence of H. scupense. Four specimens were reported on two occasions in the central provinces of Northern Sudan during the fifties of the last century [24]. On one hand, this might be an accidental introduction or, on the other hand, this tick species may have disappeared from Sudan for one reason or another.
Figure 3.

Geographic distribution of Hyalomma scupense in Africa [45] (courtesy of Walker A.R., 2003).
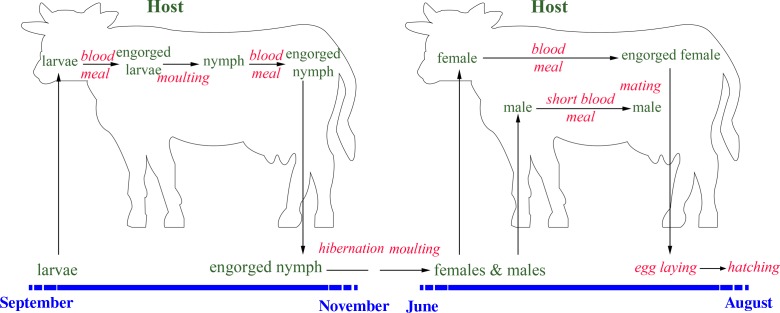
Life cycle of Hyalomma scupense
The study of the life cycle of ticks is important as a predictive tool to target the ticks where and when they are present (Figure 4). Hyalomma scupense is an endophilic, domestic tick (Figure 5); it seeks hosts and goes into diapause inside and around animal enclosures [12, 42]. In the Maghreb region, H. scupense undergoes a two-host tick activity (Figure 4); both larvae and adults seek their hosts with unimodal activity [4, 21]. Because of their small size and their moulting to nymphs on the same animal, only one field study in the Maghreb region on larvae was carried out, by Laamari et al. [28], who observed this tick on cattle during September and October in the Gharb region (extreme North-West of Morocco). Nymphs attach to cattle during the autumn: October and November [15] or September to November [28] in Morocco and October to December in Tunisia [4, 20], or September to November with a peak in mid-October in Morocco, Algeria and Tunisia [6, 15]. Larvae take a blood meal during a period of approximately 11 days, and moult on the same animal, resulting in nymphs, which feed again for approximately 11 days [42]. After the blood meal, nymphs drop off the host and go into diapause for 2–8.5 months [42] in shelters in the cattle enclosures such as cracks and crevices (Figure 6), or under a pile of rocks or dung stored by the farmer for fire-making. The overall attachment period of larvae and nymphs estimated under experimental conditions was 16.1 ± 2.4 days (range: 15–24 days) [11]. Nymphs moult during the temperature increase (late spring, early summer) into adults which seek a host (Figure 6) [20].
Figure 4.
Life cycle of Hyalomma scupense in the Maghreb region.
Figure 5.

Cattle shed where the Hyalomma scupense tick is usually present; note that the walls are not roughcast, with several cracks and crevices.
Figure 6.

Unfed adult Hyalomma scupense in a crevice.
The adults attach to animals in Tunisia and Morocco from May to August with a peak in late June in Morocco and late June/early July in Tunisia [4, 15, 28]. Gharbi et al. [20] observed a peak activity in late June with residual adults in November. In Taher, a littoral Algerian region characterised by a humid climate, Benchikh-Elfegoun et al. [3], collected adult H. scupense earlier in the year (from March to August) with a peak in June (Table 1).
Table 1.
Seasonal activity of Hyalomma scupense in the Maghreb countries (Hyalomma scupense has never been reported in Mauritania and no data about this tick species are available from Libya).
| Country | Larvae | Nymphs | Adults |
|---|---|---|---|
| Morocco | September–October [28] | October–November [15] | May–August [15] |
| September–November [6, 28] | |||
| Algeria | NA | September–November [6] | March–August [3] |
| Tunisia | NA | October–December [4] | May–August [4] |
| September–November [20] | June–November [20 1 |
The survey began in June.
NA: not available.
The presence of differences in the tick phenology has several explanations: (i) fluctuations of ambient temperature and relative humidity from one year to another [20, 21]. (ii) Variations of abiotic factors in different localities and microclimate differences from farm to farm. (iii) The management factors including host breed. The weight of each factor is difficult to estimate. The tick phenology is certainly the result of a complicated interaction among the three factors with a different weight in each case.
During summer, in farms affected by tropical theileriosis, adult H. scupense represent between 84.3 and 99.11% of the tick population in Tunisia, whilst they represent only 30.8% of the tick population in Morocco [36]. Under experimental conditions, adult ticks feed for a mean period of 9 days (range: 5–16) [29] and 12.6 days (range: 9–15) [37]. Under field conditions, the females take a blood meal during a period varying between 7 and 27 days [42]. Mating occurs on the host; females drop off and find a second shelter to lay eggs after varying periods of days (Figure 7), under experimental conditions between 7 and 26 days with a mean period of 12.3 days [29]. After a pre-oviposition period of 3–16 days, females lay 159–251 eggs/day during a period of 10 to 35 days [35]. The total number of eggs laid varies between 3625 and 7181 per female [35, 37]. Under field conditions, the eggs incubate for a period ranging between 12 and 43 days (mean: 34.8 days) [29, 37]. The authors observed that the egg-laying female shelters in crevices situated at a maximum high of 1 m; this should be considered when a control programme is implemented. The females attaching to hosts in the late summer season (after August) lay few or no eggs [12].
Figure 7.

Hyalomma scupense female laying eggs in a wall crevice (white arrow). The tick was found in a wall farm in North Tunisia at 1 m height.
Ouhelli [35] studied the impact of temperature and hygrometry on different tick stages under experimental conditions. Pre-oviposition duration is inversely correlated to hygrometry and temperature. It varies between 2.3 days (35 °C and 93% of relative hygrometry) and 7.1 days (25 °C and 25% of relative hygrometry). Oviposition duration is highly influenced by temperature; it is stopped at 16 °C, it is possible at 25 °C and has minimal duration at 35 °C and 93% hygrometry (21 days) [35].
Embryogenesis did not occur at 16 and 35 °C. It was negatively correlated with hygrometry, lasting 43, 42 and 38 days at 25, 62 and 93% of relative hygrometry, respectively. Moreover, the hatching rate increases from 70 to 72 and 76% at the same hygrometry rates. The nymphal moulting rate is blocked at 16 °C, whilst at 25% relative hygrometry and 35 °C, this rate is influenced by hygrometry; it passes from 89% (at 25% hygrometry and 25 °C) to 100% [35].
The males spend more time than females on their hosts in order to take a small blood meal and mate with one or several females, and then die, but can move from one host animal to another [42]. The overall duration of the H. scupense life cycle is a single generation per year. The overlap of the two generations (immature stages and adults feeding on the same animal) plays an important role in the epidemiology of tropical theileriosis (Theileria annulata infection) since the immature stages feed and pick up the infection from cattle whilst the infective adult ticks are attached to the same host, leading to an increase in T. annulata-infected ticks.
The tick burdens can reach, as reported in Tunisia, up to 130, with a mean of 60 ticks/adult head of cattle [4, 20]. Different studies showed that calves are 70 times less infested than adult cattle [4]. This difference is possibly attributed to (i) body size, or (ii) attractive chemical substances that might be present in the rumen and eliminated with faeces in adult cattle [10]. However, Flach et al. [15] did not observe any relation between immature tick burdens and age and sex of the cattle hosts, whilst Gharbi et al. [20] reported that the highest infestation intensities were reported on cows for adult ticks (75.8) and nymphs (75.3). Some adult animals were more heavily infested than others; this is true for both immature and adult ticks [15, 20]. Indeed, during the whole season of tick activity, five per cent of cattle harbour 16.72 and 52.53% of adults and nymphs, respectively [20].
Hosts
Due to the variety of host species and their different degree of attractiveness [29], we suggest classifying hosts of H. scupense into four groups: (i) preferential hosts, represented by cattle, followed by horses. (ii) Unusual hosts, such as dromedaries; they represent only 0.02% of the overall tick population on Algerian dromedaries, and no tick was reported to infest dromedaries in a longitudinal study carried out during a one-year period in Central Tunisia [5, 18]. (iii) The exceptional hosts are infested if the size of the groups of preferred hosts is not in adequacy with the size of the host-seeking tick population. (iv) Wild hosts: the information is so meagre (no longitudinal studies have been carried out on these animals) that their biological role as hosts is unknown (Table 2) [1, 20, 24, 42].
Table 2.
| Host type | Species |
|---|---|
| Preferential hosts | Cattle |
| Horses | |
| Unusual hosts | Sheep |
| Dromedaries | |
| Buffaloes | |
| Ponies | |
| Goats | |
| Exceptional hosts | Dogs |
| Pigs | |
| Humans | |
| Wild hosts * | Argali (Ovis ammon) |
| Foxes (Vulpes spp.) | |
| Goitered gazelles (Gazella subgutturosa) | |
| Hares (Lepus spp.) | |
| Onagers (Equus hemionus) | |
| Red deer (Cervus elaphus) | |
| Roe deer (Capreolus capreolus) | |
| Striped hyenas (Hyaena hyaena) | |
| Wild boar (Sus scrofa) |
The information on the role of the wild species is very scattered and trans-sectional; they have been classified alphabetically.
Attachment sites
It is important to know the tick attachment sites for two purposes: (i) to visually monitor tick infestations in a farm, and (ii) to focus acaricide applications and manual tick removal exclusively on specific body regions, thus reducing the quantity of acaricides and labour time and increasing the effectiveness of these two control options. Ticks attach predominantly on areas with thin skin, and survive particularly in anatomical regions which are non-accessible to grooming and licking; the proportion of ticks on various areas of the body surface therefore varies widely according to the tick species and stage [33]. The posterior udder quarters of cows are the most highly infested body parts (they harbour 41.22 and 63.82% of adults and nymphs, respectively) followed by the thighs, which harbour 32.08 and 13.82% of adults and nymphs of H. scupense, respectively (Figure 8). Adults and nymphs were also observed in other regions, namely: teats (13.15 and 0.19%, respectively); inguinal region (2.73 and 15.31%, respectively); anterior udder quarters (7.54 and 4.92%, respectively). The belly (2.97%) and axilla (0.32%) were exclusively infested by adults, whilst only nymphs were present on the neck and the interscapular regions (1.95%) [20]. These results clearly indicate that application of acaricides or manual tick removal focused on the posterior udder quarters and thighs will significantly reduce tick burdens by 93.99 and 82.75% of adults and nymphs, respectively.
Figure 8.
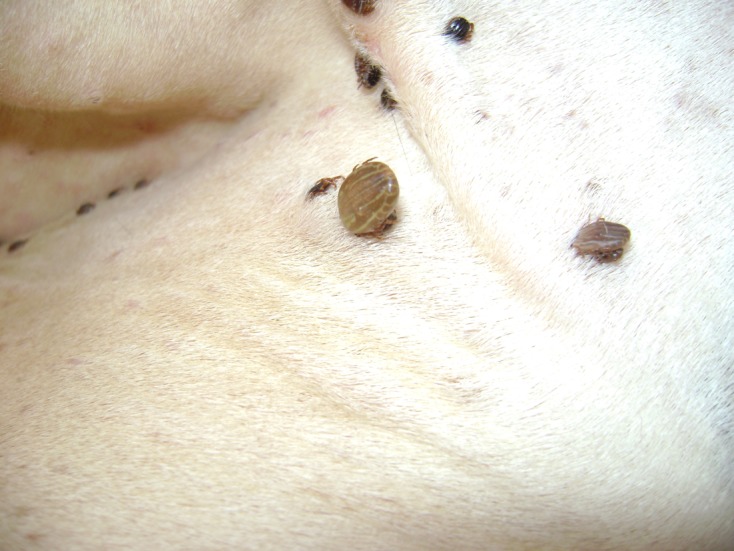
Different engorgement stages of Hyalomma scupense ticks attached to the udder of a cow.
Impact of Hyalomma scupense on their hosts
The information on both H. scupense and the pathogens it transmits is very scattered in the five Maghreb countries; intensive studies are needed to improve our knowledge on the importance of this tick species in terms of animal health and public veterinary health, animal well-being and the financial situation of the farmers by decreasing the impact of tick infestation and Hyalomma scupense-borne infections. By far the most important pathogen transmitted by H. scupense is Theileria annulata, followed by T. equi. The importance of this tick species in the Maghreb region is essentially due to its vector role of tropical theileriosis. The epidemiology of these infections is closely related to the tick vector biology [8]. Recent studies on the role of viral vectors of several Hyalomma spp. are lacking in the Maghreb [2]. However, like several other tick species (mainly Hyalomma spp.), H. scupense is a potential vector of Crimean-Congo haemorrhagic fever virus [44], but its vectorial capacity has never been proven [2]. This tick species transmits other pathogens with different levels of importance such as Coxiella burnetii, the causative agent of Q fever [2], a very prevalent infection in Tunisia. Indeed, 26% of healthy blood donors in Sousse (Central Tunisia) were seropositive in a survey carried out in 1995 [31]. The role of different tick species in the Q fever epidemiology has never been studied in the Maghreb countries (Table 3). H. scupense can also transmit other viruses such as Bhanja virus, which has never been investigated in the Maghreb region.
Table 3.
Vector role of Hyalomma scupense in the Maghreb region.
| Tick species | Transmitted pathogens | Hosts | Importance | Country | References |
|---|---|---|---|---|---|
| Hyalomma scupense | Theileria annulata | Cattle | +++ | MA, DZ, TN | [3, 8, 14, 42] |
| Theileria equi | Horses | ++ | TN | [38] | |
| Anaplasma phagocytophilum | Mammals | + | TN | [38] | |
| Anaplasma-like1, 2 | ? | Presence of DNA | MA, TN | [41] | |
| Babesia bovis 2 | Cattle | Presence of DNA | TN | [32] | |
| Ehrlichia-like1, 2 | ? | Presence of DNA | MA, TN | [41] | |
| Different species of Hyalomma spp. | Ehrlichia bovis | Cattle | + | TN | * |
| Crimean-Congo Haemorrhagic fever virus | Humans | NA | NA | [2, 44] | |
| Different Ixodid species | Coxiella burnetii | Mammals, including humans | +++ | MR, MA, DZ, TN, LY | [2, 27, 31] |
MR: Mauritania; MA: Morocco; DZ: Algeria; TN: Tunisia; LY: Libya.
NA: not available.
Sarih et al. [41] were unable to classify these isolates.
Only DNA of this pathogen was isolated without showing the vector role of H. scupense.
Unpublished data from the Laboratory of Parasitology, École Nationale de Médecine Vétérinaire de Sidi Thabet, Tunisia.
The direct effect of the tick should not be ignored, particularly when animals are heavily infested. It has been well documented with different tick species that the presence of high tick burdens can cause anaemia, damage to hides, live-weight decrease, milk yield decrease and local inflammation, leading in some cases to dermal infection and even to mastitis [26] (Figure 9).
Figure 9.
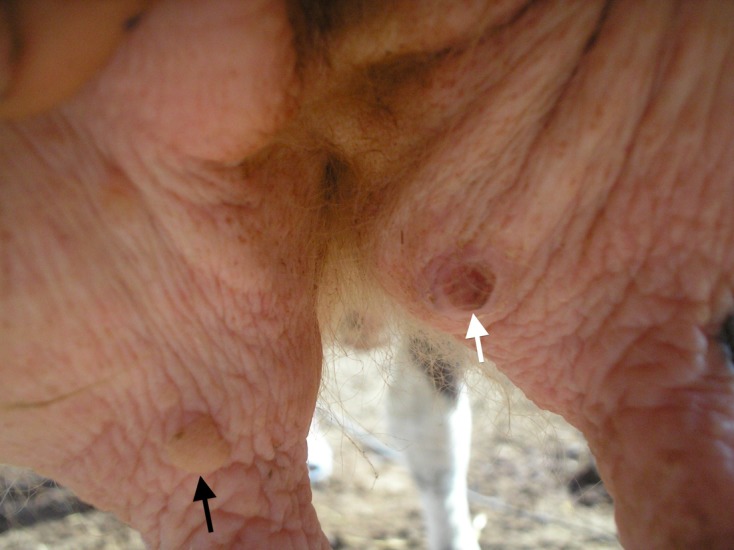
Ulcerative (white arrow) and purulent lesions (black arrow) in a cow’s udder at the tick-feeding site.
Control
We will focus in this review on control measures specific to H. scupense. For general aspects of tick control in-depth review papers should be consulted [26, 46, 47]. Control implementation and programmes should be carried out after a thorough study of both local tick phenology and epidemiology of the dominant infection(s) they transmit. This study should include an overall description of tick fauna and potential hosts (a weight should be given to each of them) and a good description of the population dynamics.
Why?
The control of H. scupense will depend on its aims; these can be divided into three objectives: (i) prevention of transmission of major tick-borne pathogens, such as T. annulata and T. equi. This is the best control option since the presence of the tick is correlated with the presence of tick-borne pathogens. (ii) Reduce the direct effect of the ticks (anaemia, skin lesions, etc.). (iii) Block the tick’s life cycle, to eliminate it from a farm or a region.
The tick-borne pathogen epidemiology should be thoroughly studied in order to improve the efficiency of any control option. In endemically unstable farms, a situation clearly associated with the presence of exotic purebred dairy cattle, the tick population is small, because of intensive tick control; eradication is recommended and feasible considering the value of the animal and the financial impact of the infection. In endemically stable state farms, a sudden decrease in the tick population can induce an emergence of clinical cases due to the loss of the equilibrium between the hosts’ immune system and the pathogen. Accordingly, in this case, the farmer should either eradicate the tick in case he intends to shift to purebred cattle breeding or keep this population and treat only during the peak of immature and adult activity without influencing the endemic stability.
How?
As H. scupense is endophilic, the control of this tick can be carried out inside the animal enclosures through targeting the immature stages and/or adult ticks. It is important to note that although this tick species is endophilic, the hosts can be infested either outdoors by peridomestic populations or with forage in animal barns [12]. In Tunisia, Darghouth and Bouattour (unpublished data) cleaned cattle manually of all ticks before grazing, but at the end of the day some of them returned from the pasture with Hyalomma scupense.
Chemical control
The presence of sheltered ticks (unfed larvae, diapausing nymphs, unfed adults and laying females) during long periods makes the control relatively easy, particularly that of the nymphs in diapause since this stage lasts for a long time. These ticks can be targeted in two ways: (i) spraying chemical acaricides on the outer and inner surfaces of the animal enclosures. This control is not very effective since the nymphs enter deep into the cracks and crevices and require a large quantity of acaricide, resulting in a high degree of pollution. It should be considered only if there is an urgent need for tick elimination, because of a high threat such as the presence of a large population of infected ticks or the presence of drug-resistant T. annulata strains, as reported by Mhadhbi et al. [34].
The on-host immature stages can be targeted by chemical acaricides; this leads to a drastic decrease in the tick population in the next season. This option is easy to implement since the ticks are attached to their hosts for a long period, particularly the immature stages, and only inexpensive acaricides are required since an acaricide with a short residual effect can be used. Adult tick control leads to an immediate decrease in tick-borne infections according to the frequency of the treatment and the residual effects of the acaricide.
Sealing cracks and crevices
This is one of the most sustainable control options, consisting of sealing all cracks and crevices by roughcasting the inner and outer wall surfaces of cattle enclosures, combined with cleaning of the surrounding areas of the enclosure in order to destroy the off-host tick stages. This action should be integrated within a strategy of barn rehabilitation; dung and litter should be removed daily from the farm and its surrounding areas. Nevertheless, if it cannot be implemented because of affordability, other control options should be implemented, but might be less efficient.
Genetic selection
Genetic resistance is one of the most used biological control methods against ticks [30]. Farmers and ranchers in Australia have been breeding for resistance to the cattle tick for many years [47]. Stachurski et al. [43] reported that when the farmer culls an Amblyomma spp.-attractive cattle in a flock, no other tick-attractive cattle appears in the same farm. Genetic control is affected by culling tick-attractive animals; the farmer should focus on tick-resistant individuals. To our knowledge, there is no study about genetic resistance to H. scupense.
Anti-tick vaccines
As Hyalomma scupense transmits major pathogens, has important direct effects and toxic chemical substances are used for its control, research was carried out to develop environmentally friendly control strategies. Anti-tick vaccination is one of the most sustainable control options. It is not time-consuming and easy to implement. Trials were carried out on several tick species using different antigens [46]. Galaï et al. [17], immunised cattle with Bm86 and its orthologue Hd86, two antigens of Rhipicephalus (Boophilus) microplus and H. scupense tick guts, respectively. The first Ag has been used as a commercial vaccine to protect cattle against Rhipicephalus microplus in Australia (TickGARDTM and TickGARDPLUSTM) and Latin America (GavacTM). A reduction of 59.19% in the number of H. scupense nymphs engorging on Hd86-vaccinated cattle was observed, whilst Bm86 did not show any effect. Furthermore, none of the antigens was active against adults of H. scupense, neither were cattle vaccinated with Bm86 or Hd86 antigens protected against adult tick infestations. This can be explained by a difference in the expression profile of the Hd86 gene between immature and adult ticks [40]. In view of these results, it is clear that Hd86 could be used only as a control option integrated with other measures in order to produce effective results. There is no genetic diversity in Hd86 isolated from different Tunisian regions; this is to confirm that this Ag can be used to immunise animals in several regions of Tunisia (and possibly in other countries of the Maghreb) against H. scupense [39].
Manual deticking
When the farmer keeps a small number of animals, the veterinarian can recommend daily deticking by hand and crushing. This control option is environmentally friendly and cheap to implement but requires continuous efforts from the farmer; it is tedious since the whole body of every animal in the herd should be examined. Moreover, H. scupense can feed on other alternative animal species (horses, small ruminants, etc.) in the farm (although most farms keep only one species, and this tick species is rarely found on small ruminants). Hand deticking may contribute to reducing the tick population, but is possibly the least effective control option. It is the most sustainable (no resistance, no pollution, with minimal financial costs). It might be recommended for resource-poor farmers. Further studies are needed on this control option to estimate its effectiveness under different husbandry conditions.
When?
In the specific case of H. scupense, tick control is usually intended to prevent tropical theileriosis. The veterinarians should explain to the farmers that any tick control programme should continue for several years and that any short course of action will fail even if apparent success is observed; this will improve the acceptability of any tick control option. The seasonal aspect is important for acaricide application and tick removal by hand. The treatment of different stages should consider variations in the tick life cycle in distinct bioclimatic regions. One to two weeks should be added at the beginning and end of the seasonal tick activity. Frequency of acaricide application depends on two elements:
The residual effect of the molecule; the longer this period, the more effective the molecule, but the meat and milk withdrawal period should also be considered.
The aim of the tick control. Since the tick usually transmits the pathogens on the 3rd day of attachment, 3 days are added if the aim of the control is to avoid T. annulata transmission. If the aim of this control option is to stop the detachment of the fully engorged female ticks, the minimum period of attachment is to be added to the interval between two acaricide applications.
How much?
Financial costs are very important for any control since they can be a constraint by decreasing the applicability and acceptability, even if the control option is effective. Both the willingness of each farmer to pay and his preferences should be known and discussed. The different other intangible costs such as adverse environmental impact, and toxicity to the farmer and the consumer are important but cannot easily be quantified; they have to be discussed and considered during the decision-making process.
Farm rehabilitation costs are high but beneficial in the long run. To be successfully implemented, this approach needs, on one hand, an extension programme to convince the farmers in order to adopt it and increase their willingness to pay. On the other hand, the question of government subsidies should be evaluated on a cost-benefit basis integrating the investment returns in terms of contribution to food security and rural development; the rehabilitation of enclosures requires heavy financial inputs. The financial aspects should be considered with regard to the direct effect of the tick and (mainly) to its vector role. Gharbi et al. [22] performed a cost-benefit analysis of Theileria annulata infection control in endemically unstable farms considering different control options: vaccination against T. annulata, acaricide application and enclosure rehabilitation. They showed that the most beneficial option was rehabilitation of enclosures with a benefit-cost ratio ranging between 1.62 and 3.71, whilst the ratios for vaccination and acaricides ranged from 0.20 to 1.19 and 0.32 to 0.88, respectively. In endemically stable situations, when technically feasible, enclosure rehabilitation is highly beneficial, with a benefit-cost ratio of 50.37, which is far higher than the ratio for vaccination (23.7) [19].
Who?
It is important to explain to the farmers that tick control requires a sound knowledge of both tick phenology and tick-borne infection epidemiology. The farmers should be convinced that the programme should last for many years. The tick control programme should be carried out under the supervision of veterinarians with a good extension programme and high subsidies, in collaboration with the veterinary extension workers and farmers’ unions. Indeed, the farmers represent very important partners in tick control programmes; their ignorance of tick importance can lead to control programmes’ failure. Studies carried out in Tunisia about the knowledge and practices of the animal owners showed that there is a need for great efforts to be carried out by animal health decision-makers in the Maghreb region. For instance, a survey carried out in 2004 and 2007 showed that 75.7% of Tunisian animal owners are illiterate or have primary school-level education [7, 25]. Twenty-nine out of 30 of the farmers in Northern Tunisia owning cattle suffering from tropical theileriosis are convinced that this disease is not a tick-borne disease, but is due to an increase in temperature [7]. Jmal [25] reported that 94.4% of Tunisian farmers do not take precautions when spraying their sheep with acaricides. Moreover, the meat and milk withdrawal period of acaricides is not respected by 67.5% of the stockowners.
Control decision criteria
Many criteria should be considered when deciding to implement a control option (Table 4); the weight of each criterion will depend on several factors, and the decision should be taken after convincing the farmer of the benefits of control and the importance of his participation. The willingness of the farmer to pay is essential. This willingness can be increased by the implementation of extension programmes, which will explain to the farmers the benefit of tick control. Since none of the methods is 100% efficient, integration of two control measures (or more) in a package is recommended (for example, enclosure rehabilitation and manual tick removal). Integrated pest management was applied elsewhere [46]. Its goal is to decrease the ecologically adverse effects of acaricide use whilst keeping an acceptable effectiveness and cost. To control H. scupense, we suggest the rehabilitation of enclosures integrated with acaricide application targeting immature stages. In small farms, the rehabilitation of buildings could be preceded by an acaricide application targeting both immature and adult stages. In all cases, an epidemiological study concerning tick-borne pathogens is a key feature of the success of control.
Table 4.
Advantages and limitations of different control options.
| Item | Hand tick removal | Summer treatment with chemical acaricides | Autumn treatment with chemical acaricides | Enclosure rehabilitation |
|---|---|---|---|---|
| Cost | Not quantified | ++ | + | +++ |
| Feasibility | + | + | ++ | +++ |
| Acceptability | + | +++ | +++ | + |
| Effectiveness | + | ++ | +++ | +++ |
| Benefits | + | + | ++ | +++ |
Conclusion
Hyalomma scupense is a very important tick in the Maghreb countries, as well as in several other regions in the world; it induces high losses in the cattle industry due to its direct and indirect impact. The disparity of abiotic factors (temperature and relative humidity) and management systems makes knowledge of the regional tick biology necessary to take the right decision for control of this tick species in the Maghreb countries. There are several control options; each one has its own advantages and disadvantages. A control programme should be synchronised for a whole region in a country since ticks in neighbouring farms can cause infestation. In the Maghreb region, an increase in funds allocated to research is a real priority in order to widen the knowledge on regional tick phenology and control tools to be implemented. There is a paucity of studies concerning this tick species in the Maghreb, mainly in Algeria and Libya, despite its importance. More studies are needed, with a collaborative regional programme, in the four Maghreb countries.
Acknowledgments
We are grateful to Prof. Gerrit Uilenberg for his valuable comments on the manuscript, Mr. Mokhtar Dhibi, Laboratory of Parasitology, École Nationale de Médecine Vétérinaire de Sidi Thabet (Tunisia) and Ms. Joud Ltaïfi Ben Ayed for preparing the graphs. This work was supported by the “Laboratoire d’épidémiologie des infections enzootiques des herbivores en Tunisie” (Ministère de la recherche scientifique et de la promotion des compétences, Tunisia) and the Deutsche Forschungsgemeinschaft project “Molecular epidemiology network for promotion and support of delivery of live vaccines against Theileria parva and Theileria annulata infection in Eastern and Northern Africa” (AH 41/7-1). The authors declare that no competing interests exist.
Cite this article as: Gharbi M & Darghouth MA: A review of Hyalomma scupense (Acari, Ixodidae) in the Maghreb region: from biology to control. Parasite, 2014, 21, 2.
References
- 1.Apanaskevich DA, Filippova NA, Horak IG. 2010. The genus Hyalomma Koch, 1844. X. redescription of all parasitic stages of H. (Euhyalomma) scupense Schulze, 1919 (= H. detritum Schulze) (Acari: Ixodidae) and notes on its biology. Folia Parasitologica, 57, 69–78 [DOI] [PubMed] [Google Scholar]
- 2.Bakheit MA, Latif AA, Vatansever Z, Seitzer U, Ahmed J. 2012. The huge risks due to Hyalomma ticks. Arthropods as vectors of emerging diseases. Mehlhorn H, Ed.Springer: Berlin, Heidelberg, Germany [Google Scholar]
- 3.Benchikh-Elfegoun MC, Benakhla A, Bentounsi B, Bouattour A, Piarroux R. 2007. Identification et cinétique saisonnière des tiques parasites des bovins dans la région de Taher (Jijel) Algérie. Annales de Médecine Vétérinaire, 151, 209–214 [Google Scholar]
- 4.Bouattour A, Darghouth MA, Ben Miled L. 1996. Cattle infestation by Hyalomma ticks and prevalence of Theileria in H. detritum species in Tunisia. Veterinary Parasitology, 65, 233–245 [DOI] [PubMed] [Google Scholar]
- 5.Bouhous A, Aissi M, Harhoura KH. 2008. Etude des Ixodidae chez le dromadaire dans le sud algérien, région d’Adrar. Annales de Médecine Vétérinaire, 152, 52–58 [Google Scholar]
- 6.Boulkaboul A. 2003. Parasitisme des tiques (Ixodidae) des bovins à Tiaret. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux, 56, 157–162 [Google Scholar]
- 7.Chaari S. 2004. Enquête sur la theilériose tropicale dans la basse Vallée de la Mejerda, perception de la maladie et application de la lutte acaricide. Mémoire de DESS en épidémiologie animale, École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 8.Darghouth ME, Bouattour A, Ben Miled L, Kilani M, Brown CG. 1996. Epidemiology of tropical theileriosis (Theileria annulata infection of cattle) in an endemic region of Tunisia: characterisation of endemicity states. Veterinary Parasitology, 65, 199–211 [DOI] [PubMed] [Google Scholar]
- 9.Data Base of Arab Maghreb Union. http://www.maghrebarabe.org/fr/Base%20de%20donnees%20UMA%20FrontOffice.htm Accessed: 2013-06-12
- 10.Donzé G, McMahon C, Guerin PM. 2004. Rumen metabolites serve ticks to exploit large mammals. Journal of Experimental Biology, 207, 4283–4289 [DOI] [PubMed] [Google Scholar]
- 11.El Fekih O. 2011. Caractérisation du cycle biologique d’Hyalomma scupense (syn. Hyalomma detritum) : étude de la phase parasitaire des stades juvéniles sur bovins et de la mue en tiques adultes, Thèse de doctorat en médecine vétérinaire, École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 12.Euzeéby J. 1990. Protozoologie meédicale compareée : Vol III: Hemosporidioses. Fascicule 2 : Piroplasmes: Lyon, France [Google Scholar]
- 13.Filippova NA. 2003. Prototypes of Hyalomma scupense Schulze, 1918 and H. detritum Schulze, 1919 (Acari: Ixodidae) in connection with micro-evolution within the genus. Parazitologiia, 37, 455–461 [PubMed] [Google Scholar]
- 14.Flach EJ, Ouhelli H. 1992. The epidemiology of tropical theileriosis (Theileria annulata infection in cattle) in an endemic area of Morocco. Veterinary Parasitology, 44, 51–65 [DOI] [PubMed] [Google Scholar]
- 15.Flach EJ, Ouhelli H, Waddington D, Oudich M, Spooner RL. 1995. Factors influencing the transmission and incidence of tropical theileriosis (Theileria annulata infection of cattle) in Morocco. Veterinary Parasitology, 59, 177–188 [DOI] [PubMed] [Google Scholar]
- 16.Gabaj MM, Awan MA, Beesley WN. 1992. A survey of ticks on farm animals in Libya. Annals of Tropical Medicine and Parasitology, 86, 543–548 [DOI] [PubMed] [Google Scholar]
- 17.Galaï Y, Canales M, Ben Saïd M, Gharbi M, Mhadhbi M, Jedidi M, de La Fuente J, Darghouth MA. 2012. Efficacy of Hyalomma scupense (Hd86) antigen against Hyalomma excavatum and H. scupense tick infestations in cattle. Vaccine, 30, 7084–7089 [DOI] [PubMed] [Google Scholar]
- 18.Gharbi M, Moussi N, Jedidi M, Mhadhbi M, Sassi L, Darghouth MA. 2013. Population dynamics of ticks infesting the one-humped camel (Camelus dromedarius) in Central Tunisia. Ticks and Tick-Borne Diseases, 4, 488–491 [DOI] [PubMed] [Google Scholar]
- 19.Gharbi M. 2006. Vaccination contre la theilériose tropicale en Tunisie (Theileria annulata): analyse économique et essai d’immunisation par ADN. Thèse de 3 ème cycle, Institut Polytechnique de Toulouse: Toulouse, France [Google Scholar]
- 20.Gharbi M, Hayouni ME, Sassi L, Dridi W, Darghouth MA. 2013. Hyalomma scupense (Acari, Ixodidae) in Northeast Tunisia: seasonal population dynamics of nymphs and adults on field cattle. Parasite, 20, 281–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharbi M, Sassi L, Dorchies P, Darghouth MA. 2006. Infection of calves with Theileria annulata in Tunisia: economic analysis and evaluation of the potential benefit of vaccination. Veterinary Parasitology, 137, 231–241 [DOI] [PubMed] [Google Scholar]
- 22.Gharbi M, Touay A, Khayeche M, Laarif J, Jedidi M, Sassi L, Darghouth MA. 2011. Ranking control options for tropical theileriosis in at-risk dairy cattle in Tunisia, using benefit-cost analysis. Revue Scientifique et Technique de l’Organisation Internationale des Epizooties, 30, 763–778 [DOI] [PubMed] [Google Scholar]
- 23.Gosrani A. 1999. Contribution à l’étude d’un foyer de la theilériose bovine à Theileria annulata dans l’oasis de Gabès. Thèse de doctorat en médecine vétérinaire. École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 24.Hoogstraal H. 1956. African Ixodoidea. Vol. I. Ticks of the Sudan (with special reference to Equatoria province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Research Report NM 005.050.29.27, Department of the Navy, Bureau of Medicine and Surgery, Washington, DC, 1101 p. [Google Scholar]
- 25.Jmal M. 2007. Perception du problème des helminthoses digestives et respiratoires ovines et de la gestion de la lutte contre ces parasitoses par les éleveurs : résultats d’une enquête par questionnaire dans la région d’Utique. Thèse de doctorat en médecine vétérinaire. École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 26.Jongejan F, Uilenberg G. 2004. The global importance of ticks. Parasitology, 129(Suppl.), S3–14 [DOI] [PubMed] [Google Scholar]
- 27.Kaabia N, Letaief A. 2009. La fièvre Q en Tunisie. Pathologie-Biologie, 57, 439–443 [DOI] [PubMed] [Google Scholar]
- 28.Laamari A, Mrifag R, Boukbal M, Belghyti D. 2012. Dynamique des populations de tiques parasites des bovins de la région du Gharb au Maroc. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux, 65, 57–62 [Google Scholar]
- 29.Lahmar R. 2009. Caractérisation d’une colonie d’Hyalomma scupense (Hyalomma detritum) : analyse retrospective et prospective de l’engorgement, de la ponte et du développement des oeufs. Thèse de doctorat en médecine vétérinaire. École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 30.Latif AA, Punyua DK, Nokoe S, Capstick PB. 1991. Tick infestations on Zebu cattle in western Kenya: individual host variation. Journal of Medical Entomology, 28, 114–121 [DOI] [PubMed] [Google Scholar]
- 31.Letaief AO, Yacoub S, Dupont HT, Le Cam C, Ghachem L, Jemni L, Raoult D. 1995. Seroepidemiological survey of rickettsial infections among blood donors in central Tunisia. Transactions of the Royal Society of Tropical Medicine and Hygiene, 89, 266–268 [DOI] [PubMed] [Google Scholar]
- 32.M’ghirbi Y, Hurtado A, Barandika JF, Brandika J, Khlif K, Ketata Z, Bouattour A. 2008. A molecular survey of Theileria and Babesia parasites in cattle, with a note on the distribution of ticks in Tunisia. Parasitology Research, 103, 435–442 [DOI] [PubMed] [Google Scholar]
- 33.MacLeod J, Colbo MH, Madbouly MH, Mwanaumo B. 1977. Ecological studies of ixodid ticks (Acari: Ixodidae) in Zambia. III. Seasonal activity and attachment sites on cattle, with notes on other hosts. Bulletin of Entomological Research, 67, 161–173 [Google Scholar]
- 34.Mhadhbi M, Naouach A, Boumiza A, Chaabani MF, Ben Abderazzak S, Darghouth MA. 2010. In vivo evidence for the resistance of Theileria annulata to buparvaquone. Veterinary Parasitology, 169, 241–247 [DOI] [PubMed] [Google Scholar]
- 35.Ouhelli H. 1985. Theilériose bovine à Theileria annulata (Dschunkowsky and Luhs, 1904) : recherche sur la biologie des vecteurs (Hyalomma spp.) et sur les interactions hôte-parasite. Thèse de 3ème cycle, Institut Polytechnique de Toulouse, Toulouse, France [Google Scholar]
- 36.Ouhelli H, Pandey VS. 1982. Prevalence of cattle ticks in Morocco. Tropical Animal Health and Production, 14, 151–154 [DOI] [PubMed] [Google Scholar]
- 37.Rahmouni A. 2009. Contribution à la caractérisation du développement d’une colonie d’Hyalomma scupense (syn. Hyalomma detritum) : étude de l’engorgement des femelles, de la ponte et du développement embryonnaire, Thèse de doctorat en médecine vétérinaire, École Nationale de Médecine Vétérinaire de Sidi Thabet: Sidi Thabet, Tunisia [Google Scholar]
- 38.Ros-García A, M’ghirbi Y, Hurtado A, Bouattour A. 2013. Prevalence and genetic diversity of piroplasm species in horses and ticks from Tunisia. Infection, Genetics and Evolution, 17, 33–37 [DOI] [PubMed] [Google Scholar]
- 39.Said Ben M, Galaï Y, Ahmed Ben M, Gharbi M, de la Fuente J, Jedidi M, Darghouth MA. 2013. Hd86 mRNA expression profile in Hyalomma scupense life stages, could it contribute to explain anti-tick vaccine effect discrepancy between adult and immature instars? Veterinary Parasitology, 198, 258–263 [DOI] [PubMed] [Google Scholar]
- 40.Said Ben M, Galaï Y, Canales M, Nijhof AM, Mhadhbi M, Jedidi M, de la Fuente J, Darghouth MA. 2012. Hd86, the Bm86 tick protein ortholog in Hyalomma scupense (syn. H. detritum): expression in Pichia pastoris and analysis of nucleotides and amino acids sequences variations prior to vaccination trials. Veterinary Parasitology, 183, 215–223 [DOI] [PubMed] [Google Scholar]
- 41.Sarih M, M’Ghirbi Y, Bouattour A, Gern L, Baranton G, Postic D. 2005. Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. Journal of Clinical Microbiology, 43, 1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sergent E, Donatien A, Parrot L, Lestoquard F. 1945. Etudes sur les piroplasmoses bovines. Institut Pasteur d’Algérie: Algies, Algeria [Google Scholar]
- 43.Stachurski F. 1993. Variability of cattle infestation by Amblyomma variegatum and its possible utilisation for tick control. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux, 46, 341–348 [PubMed] [Google Scholar]
- 44.Tekin S, Bursali A, Mutluay N, Keskin A, Dundar E. 2012. Crimean-Congo hemorrhagic fever virus in various ixodid tick species from a highly endemic area. Veterinary Parasitology, 186, 546–552 [DOI] [PubMed] [Google Scholar]
- 45.Walker AR, Bouattour A, Camicas J-L, Peña AE, Latif AA, Pegram R, Preston P. 2003. Ticks of domestic animals in Africa: a guide to identification of species, Ed.Bioscience Reports: Edinburgh, UK [Google Scholar]
- 46.Willadsen P. 2006. Tick control: thoughts on a research agenda. Veterinary Parasitology, 138, 161–168 [DOI] [PubMed] [Google Scholar]
- 47.Young AS, Groocock CM, Kariuki DP. 1988. Integrated control of ticks and tick-borne diseases of cattle in Africa. Parasitology, 96(Pt 2), 403–432 [DOI] [PubMed] [Google Scholar]