Abstract
Rationale: Obstructive sleep apnea (OSA) is associated with cardiovascular morbidity and mortality, although the underlying mechanisms are not well understood.
Objectives: We aimed to determine whether more severe OSA, measured by the Respiratory Disturbance Index (RDI), is associated with subclinical myocardial injury and increased myocardial wall stress.
Methods: A total of 1,645 participants (62.5 ± 5.5 yr and 54% women) free of coronary heart disease and heart failure and participating in both the Atherosclerosis Risk in the Communities and the Sleep Heart Health Studies underwent overnight polysomnography and measurement of high-sensitivity troponin T (hs-TnT) and N-terminal pro B-type natriuretic peptide (NT-proBNP).
Measurements and Main Results: OSA severity was defined using conventional clinical categories: none (RDI ≤ 5), mild (RDI 5–15), moderate (RDI 15–30), and severe (RDI > 30). Hs-TnT, but not NT-proBNP, was associated with OSA after adjusting for 17 potential confounders (P = 0.02). Over a median of 12.4 (interquartile range, 11.6–13.1) years follow-up, hs-TnT was related to risk of death or incident heart failure in all OSA categories (P ≤ 0.05 in each category).
Conclusions: In middle-aged to older individuals, OSA severity is independently associated with higher levels of hs-TnT, suggesting that subclinical myocardial injury may play a role in the association between OSA and risk of heart failure. OSA was not associated with NT-proBNP levels after adjusting for multiple possible confounders.
Keywords: sleep disorders, troponin T, NT-proBNP, risk factors
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is associated with cardiovascular morbidity and mortality, particularly heart failure.
What This Study Adds to the Field
We found that after adjustment for potential confounders, OSA severity was significantly associated with higher levels of high-sensitivity troponin T, but not N-terminal pro B-type natriuretic peptide, suggesting that subclinical myocardial injury caused by OSA may play a role in the subsequent risk of heart failure. Our findings suggest that high-sensitivity troponin T may be an early marker of the adverse myocardial impact of OSA.
Obstructive sleep apnea (OSA) affects at least 2–6% of the US population (1) and is associated with multiple cardiovascular (CV) comorbidities (2, 3). Epidemiologic studies suggest an association between OSA and both coronary heart disease (CHD) and heart failure (HF) (4). Repetitive apneas leading to intermittent nocturnal hypoxemia and sympathetic hyperactivity are thought to result in systemic (2) and pulmonary hypertension (5), with resulting increased myocardial load, wall stress, and injury. However, a causal relationship between OSA and CV outcomes has been difficult to establish because of the strong association of OSA with other CV risk factors.
Cardiac troponins reflect myocardial injury. Previous studies have not demonstrated a significant association between OSA severity and troponin levels (6). High-sensitivity troponin T (hs-TnT) levels, measured by newer assays with a 10-fold lower detection range than traditional assays, are predictive of both CHD and HF in the general population (7). However, the relationship between OSA severity and hs-TnT levels has not been well described (8). N-terminal pro B-type natriuretic peptide (NT-proBNP) levels reflect ventricular wall stress and carry prognostic value across a spectrum of CV disease (9). Previous studies of the relationship between natriuretic peptides and OSA have shown conflicting results (10–12).
We hypothesized that more severe OSA would be significantly and independently associated with subclinical myocardial injury (elevated hs-TnT) and increased ventricular wall stress (elevated NT-proBNP). We also explored whether the relationship between OSA and these pathway biomarkers would explain the association between OSA and incident CV disease.
Methods
Population
The study population was comprised of 1,645 participants in both the Atherosclerosis Risk in Communities (ARIC) Study and the Sleep Heart Health Study (SHHS) who underwent overnight home polysomnography and measurement of hs-TnT and NT-proBNP, and were free of prevalent CHD or HF at baseline assessment. ARIC is a prospective epidemiologic cohort study designed to investigate the etiology and natural history of clinical and subclinical atherosclerosis (13). A total of 15,792 middle-aged participants were enrolled between 1987 and 1989. Between 1996 and 1998, surviving participants underwent a fourth visit at which time blood samples were obtained from which soluble biomarker levels were measured (7). The SHHS is a prospective cohort study that recruited participants older than 40 years from nine cohorts, including 1,920 participants from ARIC (from sites in Minnesota and Maryland) (14). All underwent overnight home polysomnography and lung function tests between 1995 and 1998 (14). The SHHS visit and the fourth ARIC visit were performed independently of each other during the same 3-year period. Therefore, assessments of OSA severity and clinical and laboratory values were not performed at the same time (median difference of ARIC visit relative to the SHHS visit, 172 d [range −51 to 387 d]).
Demographics, clinical characteristics, and laboratory values were obtained from ARIC Visit 4 data. Pulmonary function test results were obtained from the SHHS visit. Prevalent HF or CHD was defined as either prevalent HF or CHD at ARIC Visit 1 or incident CHD or HF between ARIC Visit 1 and the later of either ARIC Visit 4 or the SHHS visit. Definitions of prevalent HF and CHD, and of incident events during follow-up, have been previously described (15–18).
Of 1,892 participants who underwent both ARIC and SHHS visits, 201 with prevalent CHD or HF at the time of the last visit performed of either SHHS or ARIC Visit 4 were excluded. Forty-six were excluded because of missing hs-TnT data, leaving 1,645 participants in the hs-TnT analysis, and one additional patient had missing NT-proBNP data leaving 1,644 participants in the NT-proBNP analysis (see Figure E1 in the online supplement).
Polysomnography
All participants underwent one overnight full polysomnography, which was centrally interpreted as previously published (14). Apnea was defined as cessation or nearly complete cessation of airflow. Hypopnea was defined as less than or equal to 70% reduction for at least 10 seconds. Only events associated with greater than or equal to 4% oxygen desaturation were included in the Respiratory Disturbance Index (RDI), a measure of OSA severity (19). The severity of OSA by RDI was defined using conventional clinical categories: none (RDI ≤ 5), mild (5 < RDI ≤ 15), moderate (15 < RDI ≤ 30), and severe (RDI > 30).
Cardiac Biomarkers
We assessed hs-TnT as a marker of subclinical myocardial injury and NT-proBNP as a marker of increased ventricular wall stress. Importantly, both biomarkers are prognostic of incident HF and mortality (7, 9). Blood samples were taken at the time of ARIC Visit 4 and plasma was stored centrally at −80°C. Hs-TnT was measured using a highly sensitive assay (Elecsys Troponin T; Roche Diagnostics, Indianapolis, IN) with a lower limit of measurement, as defined by the manufacturer, of 0.003 μg/L (7). NT-proBNP was measured using electrochemiluminescent immunoassay (Roche Diagnostics) with a lower detection limit of less than or equal to 5 pg/ml (7). Participants with undetectable NT-proBNP were considered to have a level of 2.5 pg/ml.
Clinical Outcomes
CV outcomes assessed were all-cause mortality or incident CHD, all-cause mortality or incident HF, all-cause mortality, and the composite of all-cause mortality, incident CHD, and incident HF, occurring after the later of ARIC Visit 4 or the SHHS visit.
Statistical Analysis
Summary data are presented as the mean and standard deviation for data that are normally distributed and median and interquartile range for nonnormally distributed data. Categorical variables are expressed as proportions. For regression modeling, hs-TnT and NT-proBNP were modeled separately as outcome variables. RDI was modeled continuously using the log transformed value to achieve normality and was modeled categorically using clinically defined thresholds as noted previously. NT-proBNP was modeled continuously using log transformed values. Hs-TnT was heavily skewed and was modeled as an ordinal categorical variable using five categories, as explained in the online supplement. Because the lower limit of measurement may differ from the lower limit of detection for the Roche hs-TnT assay (20) we repeated our analysis using a higher threshold, the rational and results of which are shown in the online supplement. Because many variables associated with OSA may act as either confounders or mediators of the OSA-biomarker relationship, we used several additive multivariable models adjusting for sequentially more variables. Model covariates were selected based on a priori knowledge and variables significantly associated with the predictor variable of interest in univariate analysis (see online supplement). For NT-proBNP, linear regression was used. For hs-TnT, ordinal logistic regression was used. Because an independent relationship was noted between hs-TnT and OSA measures, the association of hs-TnT levels with risk of CV events within categories of OSA severity (defined by RDI) was investigated using univariate and multivariable Cox proportional hazards models. To address potential limitations to our analysis we performed three different sensitivity analyses to support the strength of our results, as described in the online supplement. All analysis was performed using STATA 11.1 (StataCorp LP, College Station, TX).
Results
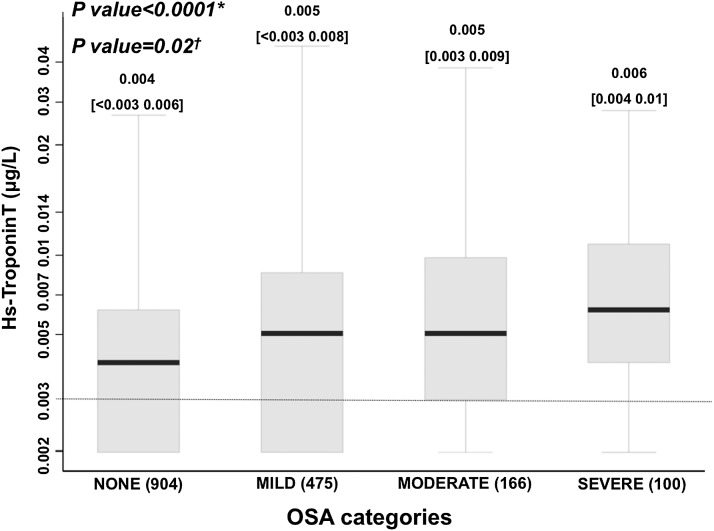
Demographic, clinical characteristics, and cardiac biomarker levels by OSA category are summarized in Table 1 (see Table E1). More severe OSA was associated with older age; male sex; higher body mass index (BMI); a higher prevalence of previous smoking, hypertension, and diabetes; and a lower estimated glomerular filtration rate. In unadjusted analysis, higher RDI was associated with higher hs-TnT levels (Spearman correlation coefficient = 0.25; P < 0.0001) (Table 2; see Table E2 and Figure E1). Similarly, based on ordinal logistic regression models, a doubling of RDI was associated with an odds ratio of 1.24 for being in a higher hs-TnT category. A change from a lower OSA category to a more severe category was associated with an odds ratio of 1.45 (1.32–1.60) for being in a higher hs-TnT category. The relationship between RDI and hs-TnT remained significant in multivariable ordinal logistic regression models adjusting for age, sex, and BMI (Model 2; P = 0.007) (Table 2; see Table E2) and in fully adjusted models (Model 5; P = 0.02) (Table 2; see Table E2). This association between RDI and hs-TnT was driven largely by significantly higher hs-TnT levels in the severe compared with no OSA group (severe vs. no OSA: age-, sex-, BMI-adjusted P = 0.03; fully adjusted P = 0.06) (Figure 1).
TABLE 1.
BASELINE DEMOGRAPHICS, CLINICAL CHARACTERISTICS, AND CARDIAC BIOMARKER LEVELS IN THE STUDY POPULATION OVERALL AND BY CATEGORY OF OSA SEVERITY
| Overall (n = 1,655) | OSA Severity |
P for Trend | ||||
|---|---|---|---|---|---|---|
| None (n = 910) | Mild (n = 476) | Moderate (n = 168) | Severe (n = 101) | |||
| Age, yr | 62.5 ± 5.5 | 61.8 ± 5.5 | 63.3 ± 5.4 | 63.4 ± 5.3 | 64.2 ± 5.3 | <0.0001 |
| Female | 897 (54%) | 595 (65%) | 208 (44%) | 59 (35%) | 35 (35%) | <0.0001 |
| White | 1,640 (99%) | 908 (99.8%) | 465 (98%) | 167 (99%) | 100 (99%) | 0.01* |
| Comorbidities | ||||||
| Hypertension | 621 (38%) | 301 (33%) | 195 (41%) | 71 (42%) | 54 (53%) | <0.0001* |
| Diabetes | 180 (11%) | 65 (7%) | 73 (15%) | 25 (15%) | 17 (17%) | <0.0001* |
| Prior stroke | 23 (1.4%) | 10 (1.1%) | 8 (1.7%) | 3 (1.8%) | 2 (2%) | 0.26* |
| Atrial fib/flutter | 13 (0.8%) | 8 (0.9%) | 3 (0.6%) | 1 (0.6%) | 1 (1%) | 0. 88* |
| Smoking | ||||||
| Current | 169 (10%) | 118 (13%) | 29 (6%) | 14 (8%) | 8 (8%) | 0.0002* |
| Former | 773 (47%) | 392 (43%) | 247 (52%) | 91 (54%) | 51 (51%) | 0.0004* |
| Asthma | 122 (7%) | 62 (7%) | 37 (8%) | 18 (11%) | 5 (5%) | 0.37* |
| COPD | 119 (7%) | 66 (7%) | 39 (8%) | 11 (7%) | 3 (3%) | 0.55* |
| BMI, kg/m2 | 28.6 ± 5.0 | 27.0 ± 4.3 | 29.6 ± 4.8 | 31.8 ± 5.3 | 33.4 ± 5.0 | <0.0001 |
| SBP, mm Hg | 126 ± 18 | 123 ± 17 | 128 ± 18 | 128 ± 18 | 130 ± 16 | <0.0001 |
| DBP, mm Hg | 71 ± 9 | 70 ± 9 | 71 ± 10 | 73 ± 10 | 73 ± 10 | <0.0001 |
| FEV1, L | 2.90 ± 0.71 | 2.84 ± 0.69 | 2.97 ± 0.71 | 3.00 ± 0.74 | 2.93 ± 0.63 | <0.0001 |
| FVC, L | 3.91 ± 0.93 | 3.85 ± 0.91 | 4.00 ± 0.93 | 4.04 ± 0.90 | 3.91 ± 0.83 | <0.0003 |
| eGFR, ml/min/1.73 m2 | 83.3 ± 13.1 | 83.9 ± 13.0 | 82.5 ± 13.4 | 83.7 ± 13.0 | 81.5 ± 12.4 | 0.03 |
| NT-proBNP, pg/ml | 67 (34–122) | 71 (39–129) | 67 (33–119) | 50 (24–95) | 52 (24–118) | <0.001† |
| Hs-TnT, μg/L | 0.004 (<0.003–0.007) | 0.004 (<0.003–0.006) | 0.005 (<0.003–0.008) | 0.005 (0.003–0.009) | 0.006 (0.004–0.010) | <0.0001‡ |
| Hs-TnT category | ||||||
| Undetectable (<0.003 μg/L) | 575 (35%) | 381 (42%) | 132 (28%) | 41 (25%) | 21 (21%) | <0.0001 |
| M: 0.003–0.005 μg/L | 370 (22%) | 198 (22%) | 117 (25%) | 39 (23%) | 16 (16%) | |
| F: 0.003–0.004 μg/L | ||||||
| M: 0.006–0.008 μg/L | 277 (17%) | 139 (15%) | 84 (18%) | 31 (19%) | 23 (23%) | |
| F: 0.005 ng/L | ||||||
| M: 0.009–0.013 μg/L | 235 (14%) | 108 (12%) | 77 (16%) | 34 (20%) | 16 (16%) | |
| F: 0.006–0.007 μg/L | ||||||
| M: ≥0.014 μg/L | 188 (11%) | 78 (9%) | 65 (14%) | 21 (13%) | 24 (24%) | |
| F: ≥0.008 μg/L | ||||||
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; hs-TnT = high-sensitivity troponin T; NT-proBNP = N-terminal pro–B-type natriuretic peptide; OSA = obstructive sleep apnea; SBP = systolic blood pressure.
Based on two sample Wilcoxon rank-sum test.
Based in logarithmic transformed values for biomarkers.
Based in nonparametric trend test.
TABLE 2.
UNIVARIATE AND MULTIVARIABLE MODELS ASSESSING THE RELATIONSHIP BETWEEN RDI AND CARDIAC BIOMARKERS
| Hs-TnT |
NT-proBNP |
|||||
|---|---|---|---|---|---|---|
| N | Beta Coefficient ± SE | P Value | N | Beta Coefficient ± SE | P Value | |
| Unadjusted | 1,645 | 0.27 ± 0.03 | <0.0001 | 1,644 | −0.08 ± 0.02 | <0.0001 |
| Model 1 | 1,645 | 0.15 ± 0.03 | <0.0001 | 1,644 | −0.06 ± 0.02 | 0.0007 |
| Model 2 | 1,642 | 0.10 ± 0.04 | 0.007 | 1,641 | −0.04 ± 0.02 | 0.05 |
| Model 3 | 1,637 | 0.08 ± 0.04 | 0.03 | 1,636 | −0.04 ± 0.02 | 0.04 |
| Model 4 | 1,633 | 0.09 ± 0.04 | 0.02 | 1,632 | −0.03 ± 0.02 | 0.08 |
| Model 5 | 1,631 | 0.09 ± 0.04 | 0.02 | 1,630 | −0.02 ± 0.02 | 0.19 |
Definition of abbreviations: hs-TnT = high-sensitivity troponin T; NT-proBNP = N-terminal pro–B-type natriuretic peptide; RDI = Respiratory Disturbance Index.
Analysis for hs-TnT based on ordinal logistic regression. Analysis for NT-proBNP based on linear regression using logarithmic transformed values. Model 1: adjusted by age and sex. Model 2: additionally adjusted by body mass index. Model 3: additionally adjusted by smoking status, alcohol intake, hypertension, and diabetes. Model 4: additionally adjusted by pulmonary function tests (FEV1 and FVC) and chronic lung disease. Model 5: additionally adjusted by systolic blood pressure; estimated glomerular filtration rate; and blood levels of insulin, total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides. Reported regression coefficients are those associated with log (RDI).
Figure 1.
Whisker box-plot of high-sensitivity troponin T (hs-TnT) levels by obstructive sleep apnea (OSA) category. Hs-TnT is shown using a logarithmic scale. Values under the limit of detection (0.003 μg/L) are assigned to a value of 0.002 μg/L). *Unadjusted ordinal logistic regression. †Multivariable ordinal logistic regression adjusted by age, body mass index, smoking status, alcohol intake, hypertension, diabetes, chronic lung disease, pulmonary function tests, estimated glomerular filtration rate, systolic blood pressure, and blood levels of total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, and insulin (Model 5).
In unadjusted analysis, a weak, but significant negative correlation between RDI and NT-proBNP was noted (Pearson correlation coefficient, −0.11; P < 0.0001), suggesting higher RDI was associated with lower NT-proBNP. This association remained significant after adjusting for sex and age (P = 0.0007) (Table 2; see Table E2), but was greatly attenuated after additional adjustment for BMI (P = 0.05), and was no longer significant after further adjustment (P = 0.19 in Model 5) (Table 2; see Table E2).
Participants were followed up for a median of 12.4 (interquartile range, 11.6–13.1) years with a total of 222 deaths, 212 participants experiencing incident CHD events, and 122 participants experiencing incident HF. A total of 427 participants died or experienced an incident CV event during the follow-up period. Within each OSA group, higher hs-TnT level was associated with a higher hazard ratio for death or incident HF, death or incident CHD, and the composite of death, incident HF, or incident CHD, consistently from the unadjusted to the fully adjusted model (Table 3; see Table E3). For death or incident HF in particular, this relationship was most robust in the severe OSA group and least robust in the no OSA group (P for interaction = 0.04).
TABLE 3.
MULTIVARIABLE ADJUSTED HAZARD RATIOS FOR INCIDENT DEATH OR CARDIOVASCULAR EVENTS ASSOCIATED WITH HS-TNT LEVEL BY CATEGORY OF OSA SEVERITY
| Number of Events/Total at Risk | Hazard Ratio (95% Confidence Interval) | P Values | |
|---|---|---|---|
| CHD/death |
|
|
|
| Overall | 380/1,631 | 1.17 (1.07–1.26) | <0.001 |
| Category of OSA severity | |||
| None | 182/891 | 1.13 (1.01–1.28) | 0.04 |
| Mild | 116/472 | 1.18 (1.02–1.36) | 0.03 |
| Moderate | 49/165 | 1.32 (1.04–1.70) | 0.03 |
| Severe | 33/99 | 1.89 (1.28–2.80) | 0.001 |
| HF/death | |||
| Overall | 283/1,627 | 1.27 (1.16–1.39) | <0.0001 |
| Category of OSA severity | |||
| None | 132/891 | 1.19 (1.04–1.36) | 0.009 |
| Mild | 92/472 | 1.31 (1.11–1.53) | 0.001 |
| Moderate | 33/165 | 1.34 (1.00–1.80) | <0.05 |
| Severe | 26/99 | 2.30 (1.42–3.72) | 0.001 |
| Death | |||
| Overall | 216/1,627 | 1.19 (1.07–1.32) | 0.001 |
| Category of OSA severity | |||
| None | 101/891 | 1.12 (0.96–1.30) | 0.16 |
| Mild | 67/472 | 1.12 (0.94–1.36) | 0.23 |
| Moderate | 29/165 | 1.17 (0.85–1.63) | 0.34 |
| Severe | 19/99 | 1.86 (1.08–3.22) | 0.02 |
| CHD/HF/death | |||
| Overall | 418/1,627 | 1.22 (1.13–1.31) | <0.0001 |
| Category of OSA severity | |||
| None | 199/891 | 1.16 (1.04–1.30) | 0.009 |
| Mild | 132/472 | 1.25 (1.09–1.43) | 0.001 |
| Moderate | 51/165 | 1.39 (1.09–1.78) | 0.008 |
| Severe | 36/99 | 1.91 (1.31–2.81) | 0.001 |
Definition of abbreviations: CHD = coronary heart disease; HF = heart failure; hs-TnT = high-sensitivity troponin T; OSA = obstructive sleep apnea.
Estimates derived from multivariable Cox proportional hazards models with the following model covariates: age, sex, body mass index, smoking status, hypertension, diabetes, alcohol intake, pulmonary function variables (FEV1 and FVC), chronic obstructive pulmonary disease status, systolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, insulin level, and estimated glomerular filtration rate.
Similar results to the primary analysis were found when the analysis was repeated using 0.005 μg/L as the limit of detection, instead of 0.003 μg/L (see Tables E1–E3). Sensitivity analyses restricting the population to participants with RDI and biomarker measurement performed within 12 months of each other (n = 1,025), to participants with complete data (n = 1,630), or to participants with estimated glomerular filtration rate greater than or equal to 60 ml/min/1.73 m2 (n = 1,568) were all consistent with the results found in the overall study population. Similarly, sensitivity analysis using uniform cut-offs for hs-TnT categories for both sexes demonstrated consistent results (see online supplement).
Discussion
Among 1,655 community-dwelling participants without prevalent CHD or HF, more severe OSA was significantly associated with higher hs-TnT levels, even after adjusting for 17 potential confounders. Furthermore, hs-TnT levels were associated with incident CV disease events or death in each category of OSA severity, especially for incident HF. This association seemed to be largely driven by higher hs-TnT in participants with severe OSA compared with the no OSA group. In contrast, no association was noted between OSA and NT-proBNP levels after adjusting for potential confounders, most notably BMI.
To our knowledge, this community-based study is one of the first to demonstrate an independent association between sleep apnea severity and circulating levels of hs-TnT after adjusting for potential confounders. Because OSA is significantly associated with multiple established CV risk factors there has been controversy over whether OSA is causally related to incident CHD or HF (2, 3). OSA is characterized by repetitive episodes of nocturnal hypoxemia, with associated sympathetic activation, hypertension, and tachycardia. The nocturnal hypoxemia likely contributes to ischemia, as supported by several publications demonstrating electrocardiogram changes consistent with ischemia occurring in association with apneas, although this finding has not been universal (21). OSA may also cause myocardial stress and injury because of the increased load on both the right and left ventricles resulting from marked swings in intrathoracic pressure during obstructed breathing, and associated paroxysmal nocturnal and more chronic systemic and pulmonary hypertension, a mechanism supported by the association of OSA with biventricular hypertrophy (22, 23). The independent relationship found between OSA severity and higher circulating hs-TnT would therefore help inform the understanding of the association between OSA and CV outcomes by suggesting that subclinical myocardial injury may be a possible causal link.
Although troponin assays are used commonly in CV research and clinical settings, there has been little prior research that has addressed this marker in association with OSA. Previous studies have not demonstrated a significant relationship between TnT levels and OSA severity (6); however, newer hs-TnT assays detect TnT levels in a much higher proportion of disease-free subjects than traditional TnT assays (8). Similar to our study, using an hs-TnT limit of measurement of 0.003 μg/L, Randby and coworkers (8) recently evaluated the association between OSA severity and the presence of detectable hs-TnT in 505 individuals from a community-based cohort. The association of OSA with hs-TnT was not significant after adjustment of potential confounders. Importantly, unlike our study, their population was younger (30–65 yr old) and was enriched for prevalent CV disease. Possibly related to the younger age and lower prevalence of hypertension and diabetes in their population, only 43% of subjects had detectable hs-TnT, compared with 65% of subjects in our study. Additionally, they examined the association of OSA severity with the presence of measurable hs-TnT, whereas we assessed its relationship with both the presence and the magnitude of hs-TnT level, which should enhance our study’s power.
Further supporting a mechanistic link between OSA, hs-TnT, and CV events (and consistent with findings in the overall ARIC population) hs-TnT level was a significant predictor of incident CHD and HF in our study population. In addition, hs-TnT remained a significant predictor within each category of OSA severity, with greater risk associated with elevated hs-TnT levels in more severe OSA categories. The significant interaction found between OSA severity and hs-TnT levels in predicting incident HF suggests the possibility that hs-TnT levels may be a particularly important prognostic marker in patients with severe OSA.
We observed a negative association between OSA severity and NT-proBNP levels in unadjusted analysis. However, BMI is a key confounder of this relationship, because obesity is a powerful risk factor for OSA (24) but also associated with inappropriately low NT-proBNP levels (25). Indeed, in our study, the negative association between OSA severity and NT-proBNP levels was markedly attenuated after adjustment by BMI and no longer significant after further adjustment. Although previous studies have shown conflicting results (10–12), our results are concordant with those of the largest prior study by Patwardham and coworkers (11) who found no independent association between OSA severity and circulating natriuretic peptides levels in the Framingham-Offspring/SHHS study.
Several limitations of this analysis should be noted. Our analysis was cross-sectional in design and precludes conclusions regarding causality. Although multiple additive multivariable models were used, residual confounding cannot be excluded. Conversely, many potential confounders may also act as mediators between OSA and the outcomes of interest, potentially leading to greater type 2 error. Biomarkers were not measured coincident with polysomnography, with a difference between the two measurements of greater than 1 year in many participants. Although biomarker levels may change over time, individuals with intercurrent CV events between polysomnography and biomarker assessment were excluded. In addition, prior studies suggest that sleep apnea classification remains largely stable over several months to years (26, 27) suggesting that at the time of biomarkers determination OSA status was similar to that assessed at the time of the polysomnography. Additionally, a sensitivity analysis restricting the population to those with sleep variables and soluble biomarkers ascertained within 1 year of each other demonstrated concordant results with the primary analysis. Information regarding the time of collection of blood was not available. Although NT-proBNP has the longest half-life of the natriuretic peptide biomarkers, it is still short at approximately 120 minutes (28). This may diminish our ability to detect an association of OSA severity with NT-proBNP levels (11). Although our study is one of the largest to our knowledge to assess the relationship between uniformly assessed OSA severity and hs-TnT, the limited number of participants in the most severe OSA category may limit our power to detect associations.
Conclusions
After adjustment for potential confounders, OSA severity is associated with higher levels of hs-TnT in middle-aged to older individuals, suggesting that subclinical myocardial injury caused by OSA may play a role in the subsequent risk of HF. Further research is needed to identify the role of subclinical ischemia in OSA, and the potential usefulness of monitoring hs-TnT levels as a prognostic marker in individuals with OSA but without prevalent CV disease.
Acknowledgments
Acknowledgment
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Supported by NHLBI cooperative agreements NHLBI-HC-11-08 (Brigham and Women’s Hospital), U01HL53940 (University of Washington), and U01HL53934 (University of Minnesota). The work for this manuscript was also supported by NHLBI grant 1K08HL116792-01A1 (A.M.S.)
Author Contributions: All of the authors have substantially contributed to the conception, analysis, interpretation of the data, and/or critical revisions for intellectual content. G.Q.R., A.M.S., S.R., B.C., and S.D.S. were responsible for the conception, design, analysis, and interpretation of data. G.Q.R. and A.M.S. were responsible for drafting the manuscript. S.R., S.D.S., N.P., and C.M.B. were responsible for revising the manuscript critically for important intellectual content.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201309-1572OC on October 24, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Loke YK, Brown JWL, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alchanatis M, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB. Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration. 2001;68:566–572. doi: 10.1159/000050574. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Svatikova A, Wolk R, Olson EJ, Duenwald CJ, Jaffe AS, Somers VK. Cardiac troponin T in obstructive sleep apnea. Chest. 2004;125:2097–2100. doi: 10.1378/chest.125.6.2097. [DOI] [PubMed] [Google Scholar]
- 7.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randby A, Namtvedt SK, Einvik G, Hrubos-Strøm H, Hagve T-A, Somers VK, Omland T. Obstructive sleep apnea is associated with increased high-sensitivity cardiac troponin T levels. Chest. 2012;142:639–646. doi: 10.1378/chest.11-1779. [DOI] [PubMed] [Google Scholar]
- 9.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, White HD, Fox KAA, Califf RM, Braunwald E Investigators. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 10.Kita H, Ohi M, Chin K, Noguchi T, Otsuka N, Tsuboi T, Itoh H, Nakao K, Kuno K. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7:199–207. doi: 10.1046/j.1365-2869.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Patwardham AA, Larson MG, Levy D, Benjamin EJ, Leip EP, Keyes MJ, Wang TJ, Gottlieb DJ, Vasan RS. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29:1301–1306. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]
- 12.Hübner RH, El Mokhtari NE, Freitag S, Rausche T, Göder R, Tiroke A, Lins M, Simon R, Bewig B. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med. 2008;102:134–142. doi: 10.1016/j.rmed.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 13.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 15.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 16.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 17.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 18.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 19.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 20.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412:748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Peled N, Abinader EG, Pillar G, Sharif D, Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol. 1999;34:1744–1749. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 22.Chami HA, Devereux RB, Gottdiener JS, Mehra R, Roman MJ, Benjamin EJ, Gottlieb DJ. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117:2599–2607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidry UC, Mendes LA, Evans JC, Levy D, O’Connor GT, Larson MG, Gottlieb DJ, Benjamin EJ. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med. 2001;164:933–938. doi: 10.1164/ajrccm.164.6.2001092. [DOI] [PubMed] [Google Scholar]
- 24.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 25.Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2012;17:81–96. doi: 10.1007/s10741-011-9249-z. [DOI] [PubMed] [Google Scholar]
- 26.Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, Redline S, Sanders M, Young T Sleep Heart Health Study (SHHS) Research Group. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography—the Sleep Heart Health Study. [corrected] Sleep. 2002;25:843–849. [PubMed] [Google Scholar]
- 27.Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 28.Kemperman H, van den Berg M, Kirkels H, de Jonge N. B-type natriuretic peptide (BNP) and N-terminal proBNP in patients with end-stage heart failure supported by a left ventricular assist device. Clin Chem. 2004;50:1670–1672. doi: 10.1373/clinchem.2003.030510. [DOI] [PubMed] [Google Scholar]