Abstract
Purpose:
To evaluate the effect of bevacizumab on the mitochondrial function of human retinal pigment epithelial (ARPE-19), rat neurosensory retinal (R28) and human microvascular endothelial (HMVEC) cells in culture.
Materials and Methods:
ARPE-19 and R28 cells were treated with 0.125, 0.25, 0.50 and 1 mg/ml of bevacizumab. The HMVEC cultures were treated with 0.125, 0.25, 0.50 and 1 mg/ml of bevacizumab or 1 mg/ml of immunoglobulin G (control). Mitochondrial function assessed by mitochondrial dehydrogenase activity (MDA) was determined using the WST-1 assay.
Results:
Bevacizumab doses of 0.125 to 1 mg/ml for 5 days did not significantly affect the MDA of ARPE-19 cells. Bevacizumab treatment at 0.125 and 0.25 mg/ml (clinical dose) did not significantly affect the MDA of R28 cells; however, 0.50 and 1 mg/ml doses significantly reduced the R28 cell mitochondrial function. All doses of bevacizumab significantly reduced the MDA of proliferating and non-proliferating HMVEC.
Conclusion:
Bevacizumab exposure for 5 days was safe at clinical doses in both ARPE-19 and R28 retinal neurosensory cells in culture. By contrast, bevacizumab exposure at all doses show a significant dose-dependent decrease in mitochondrial activity in both the proliferating and non-proliferating HMVEC in vitro. This suggests a selective action of bevacizumab on endothelial cells at clinical doses.
Keywords: Bevacizumab, mitochondrial function, microvascular endothelial cells, neurosensory retinal cells, retinal pigment epithelial cells, tissue culture
Age-related macular degeneration (AMD) and diabetic retinopathy are currently two of the leading causes of visual loss in the western world.[1,2] Vascular endothelial growth factor (VEGF) has been implicated as the primary angiogenic stimulus responsible for pathologic neovascularization in many intraocular conditions associated with abnormal angiogenesis as AMD, diabetic retinopathy, and neovascular glaucoma.[3,4,5,6,7,8,9,10,11,12]
Bevacizumab (Avastin™; Genentech, Inc., San Francisco, CA), a full-length, humanized, monoclonal antibody against all isoforms of VEGF A, is currently approved for the treatment of metastatic colorectal cancer.[1] Recently, off-label intravitreal injections of bevacizumab have been shown to be safe and effective in the patients with exudative age-related macular degeneration (AMD), central retinal vein occlusion (CRVO), proliferative diabetic retinopathy (PDR), rubeosis iridis, pseudophakic cystoid macular edema (CME) and choroidal neovascular membrane (CNV) secondary to pathologic myopia.[2,3,4,5,6,7] Initial reports of in vivo rabbit and isolated bovine retina studies have described the lack of ocular toxicity associated with intravitreal bevacizumab and bevacizumab infusions, respectively.[8,9,10,11]
We have previously shown that bevacizumab did not decrease cell viability with 24 h drug exposure using the Trypan blue assay, which measures the frank cell death. The present study was designed to evaluate the effect of bevacizumab using a longer exposure times and a more sensitive assay. As such, the current study assessed changes in mitochondrial function in cultured retinal and vascular cells after 5 days exposure (the estimated drug half-life in vitreous) to bevacizumab at the clinically dose (0.25 mg/ml), half the clinical dose (0.125 mg/ml), 2× (0.50 mg/ml) and 4× (1 mg/ ml) the clinical dose. The in vitro cell lines used represent the main types of cells in the human retina (neurosensory, retinal pigment epithelium, and microvascular endothelial cells).
Materials and Methods
Cell culture
Human retinal pigment epithelial cell line (ARPE-19) was obtained from ATCCβ (Manassas, VA). Cells were grown in 1:1 mixture (vol./vol.) of Dulbecco's modified Eagle's and Ham's nutrient mixture F-12 medium (DMEM F-12, Gibcoä, Carlsbad, CA), 10 mM non-essential amino acids, 0.37% sodium bicarbonate, 0.058% L-glutamine, 10% fetal bovine serum and antibiotics (100 U/ml penicillin G, 0.1 mg/ml streptomycin sulfate, 10 μg/ml gentamicin, 2.5 μg/ml Fungizone-Amphotericin B).
Rat embryonal neurosensory precursor retinal (R28) cells were derived from day 6 post-natal rat retina from the laboratory of Dr. Gail M. Seigel, Buffalo, NY.[12] R28 cells express genes characteristic of neurons,[13] as well as functional neuronal properties.[14] The cell line was cultured in Dulbecco's modified Eagle's medium, high glucose (DMEM high glucose, Gibcoä, Carlsbad, CA) with 10% fetal bovine serum, 1X minimum essential medium (MEM), 10 mM non-essential amino acids, 0.37% sodium bicarbonate and 10 μg/ml gentamicin.
Human microvascular endothelial cells (HMVEC) and their tissue culture reagents were obtained from Cascade Biologics, Inc. (Portland, OR). The cells were acquired as proliferating quaternary cultures established from cryopreserved normal human microvascular endothelial cells isolated from adult human dermis. HMVEC were grown in Medium 131 supplemented with microvascular growth supplement (MVGS) to support the plating and proliferation of cells. Medium 131 is a sterile, liquid tissue culture basal medium containing the essential and non-essential amino acids, vitamins, other organic compounds, trace minerals, and inorganic salts. This medium does not contain antibiotics, antimycotics, hormones, growth factors, or proteins and is bicarbonate buffered. MVGS contains fetal bovine serum (5% v/v final concentration), hydrocortisone, recombinant human fibroblast growth factor, heparin, recombinant human epidermal growth factor, and dibutyryl cyclic AMP. Medium 131 not supplemented with MVGS was used as serum-free medium for HMVEC. Before use, the tissue culture surfaces were coated with attachment Factor (AF), a sterile 1X solution containing 0.1% gelatin. Two groups of HMVEC were evaluated: (1) Proliferating HMVEC, maintained in culture with 50 ng/ml of recombinant human vascular endothelial growth factor (VEGF, R and D Systems, Inc. Minneapolis, MN) and (2) Non-proliferating HMVEC, maintained in culture with 5 ng/ml of VEGF.
All cell types were plated onto 96-well ELISA plates (Becton Dickinson Labware, Franklin Lakes, NJ) for WST-1 assay at 2 × 104 cells/well and incubated at 37°C in 5% CO2 to reach the confluence. After incubating all the cell types for 24 h to reach the confluence, the media in the wells were replaced with the respective serum-free media containing 0.1% Bovine Serum Albumin (BSA; Sigma-Aldrich, St. Louis, MO). Cells were incubated in this serum-free media to render them quiescent (non-proliferating) condition.
Exposure to bevacizumab
ARPE-19 and R28 cells were treated with four concentrations of bevacizumab: 0.125, 0.25, 0.50 and 1 mg/ml for 5 days. Avastin is commercially available as a 100 mg or 400 mg vial containing 25 mg/ml bevacizumab. The recommended intravitreal dose of bevacizumab is 1 to 1.25 mg (0.04 to 0.05 ml) of the commercially available Avastin.[15,16] This injected drug is presumed to diffuse uniformly into the entire vitreous. The average normal vitreous volume is about 4 ml, and therefore, the dose to which the retinal cells are expected to be exposed after intravitreal injection of bevacizumab is 0.25-0.3125 mg/ml. In our study in vitro bevacizumab safety was tested at the clinical dose (0.25 mg/ml), half the clinical dose (0.125 mg/ml) and 2 and 4X the clinical dose (0.50 and 1 mg/ml, respectively). These concentrations were prepared using the serial dilutions of the drug in the respective serum-free culture medium. Untreated ARPE-19 and R28 cells incubated in serum-free medium for 5 days and ARPE-19 and R28 cells treated 5 days with 1 mg/ml of human purified immunoglobulin (IgG) (Sigma-Aldrich, St. Louis, MO) served as controls. The use of IgG allows us to compare the effect of bevacizumab (a full length humanized monoclonal antibody specific for VEGF) versus that of human IgG (a non-specific human antibody) on the cells.
Proliferating and non-proliferating HMVEC were treated 5 days with the same four concentrations of bevacizumab. For both HMVEC groups the drug was serially diluted using Medium 131 supplemented with PSA Solution (Cascade Biologics, Portland, OR). PSA Solution (penicillin, streptomycin, and Amphotericin B solution) is a sterile, antibiotic/antimycotic solution. When added to the medium, the final mixture contained 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. Proliferating HMVEC control groups were treated with 50 ng/ml VEGF alone or with 1 mg/ml of IgG for 5 days. Non-proliferating HMVEC used as controls were treated with 5 ng/ml VEGF alone or with 1 mg/ml of IgG.
For all the three cell lines, the media in both treated and control wells were replaced with the respective media every day for 5 consecutive days.
Mitochondrial dehydrogenase activity: WST-1 assay
In order to assess mitochondrial function, mitochondrial dehydrogenase (succinate-tetrazolium-reductase) activity was determined using the WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) colorimetric assay (Roche Diagnostics, Indianapolis, IN) after 5 days of treatment with bevacizumab. Ten microliters of the formazan dye was added to each well and incubated for 2 h at 37°C. The absorbance was then measured at 490 nm on a multi-well spectrophotometer (Perkin Elmerδ, Downers Grove, IL).
Gross morphology: Phase contrast microscopy
Digital images at ×20 magnification were taken of treated and untreated ARPE-19 cultures using a Leica DM IRB inverted fluorescence microscopy and an Optronics digital system (Meyer Instruments, Houston, TX).
Statistical analysis
Data was subjected to ANOVA statistical analysis using GraphPad Prismä 3.0 version statistics program (GraphPad Software Inc., San Diego, CA). Newman-Keuls multiple comparison test was used to compare the data within each experiment. P values less than 0.05 were considered statistically significant. Error bars in the graphs represent standard error of mean (SEM) with experiments performed in triplicate.
Results
ARPE-19 cells
Bevacizumab at doses of 0.125, 0.25, 0.50 and 1 mg/ml (1/2×, 1×, ×2 and ×4 the equivalent clinical dose) did not significantly affect the mitochondrial dehydrogenase activity of ARPE-19 cells after 5 days [Fig. 1]. The mean absorbance of the untreated ARPE-19 cell control group was 1.154 ± 0.070, while the mean of the same cells with 1 mg/ml IgG was 1.248 ± 0.026 [Fig. 1]. The mean absorbance of bevacizumab- treated ARPE-19 cells after 5 day drug exposure of 0.125, 0.25, 0.50 and 1 mg/ml were 1.147 ± 0.023, 1.187 ± 0.022, 1.131 ± 0.047 and 1.062 ± 0.079, respectively. The mean absorbance of ARPE-19 cells treated with bevacizumab were not significantly different from those of controls (P > 0.05). Phase contrast microscopy also showed that ARPE-19 cells were healthy after bevacizumab treatment [Fig. 2a–c].
Figure 1.
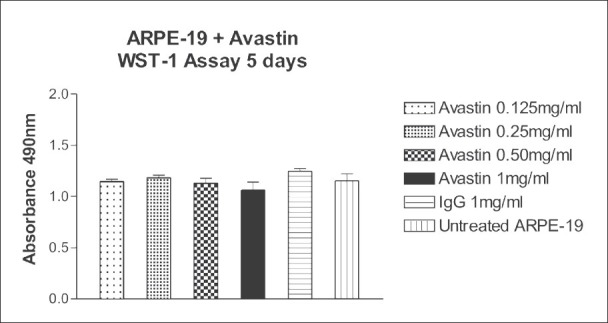
Effect of 5 days exposure of bevacizumab at 0.125, 0.25, 0.50 and 1 mg/ml on the mitochondrial dehydrogenase activity of human retinal pigment epithelial (ARPE-19) cells in culture. The mean mitochondrial dehydrogenase activity of ARPE-19 cells treated with bevacizumab was not significantly different (P > 0.05) compared to the controls
Figure 2.
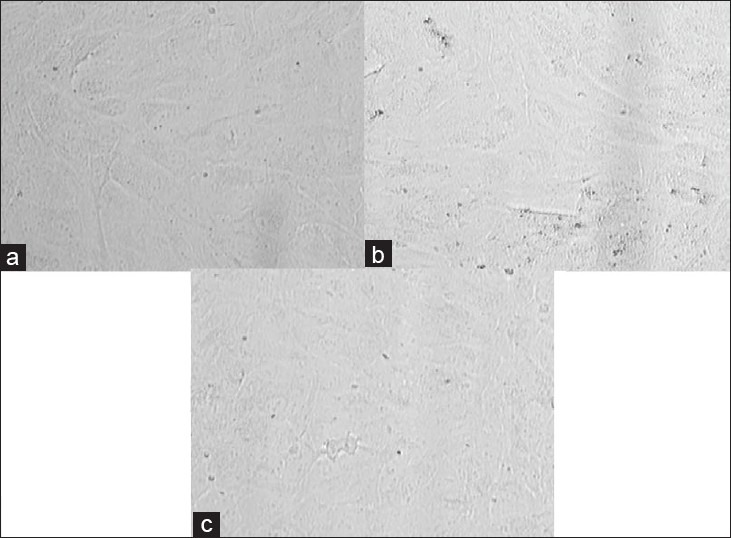
Phase contrast microscopy images of ARPE-19 cells after 5 days exposure to bevacizumab shows healthy cells. (a) Untreated control ARPE-19 cells. (b) IgG-treated (1 mg/ml) control ARPE-19 cells. (c) ARPE-19 cells treated with 1 mg/ml bevacizumab. ×40
R28 Cells
Bevacizumab at doses of 0.125 and 0.25 mg/ml (1/2× and 1× clinical dose) did not significantly affect the mitochondrial dehydrogenase activity of R28 cells after 5 days [Fig. 3]. However, 0.50 and 1 mg/ml (×2 and ×4 clinical dose) bevacizumab significantly reduced the mitochondrial dehydrogenase activity of R28 cells after 5 days exposure. The mean absorbance of the control untreated or 1 mg/ml IgG-treated R28 cells were 0.637 ± 0.055 and 0.603 ± 0.035, respectively. The mean absorbance of R28 cells, after exposure to bevacizumab concentrations of 0.125, 0.25, 0.50 and 1 mg/ml were 0.554 ± 0.014; 0.540 ± 0.021; 0.472 ± 0.015 (P < 0.05) and 0.420 ± 0.015 (P < 0.01), respectively. The mean absorbance of R28 cells treated with 0.125 and 0.25 mg/ml bevacizumab were not significantly different (P > 0.05) from those of the controls. In contrast, the mean absorbance of R28 cells treated with the higher doses of 0.50 and 1 mg/ml bevacizumab were significantly different (P < 0.05 and P < 0.01, respectively) when compared to the controls. Phase contrast microscopy also supports these results [Fig. 4a–d]. Increased numbers of rounded cells are observed at 0.5 and 1.0 mg/ml compared to untreated and IgG controls.
Figure 3.
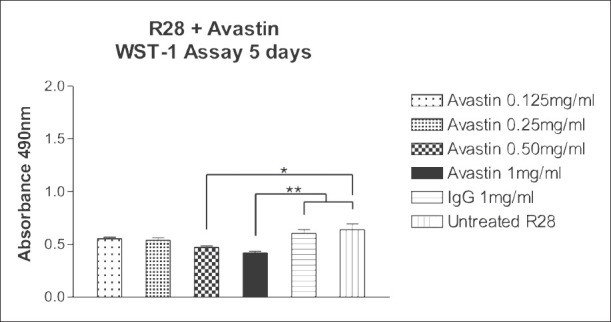
Effect of 5 days exposure of bevacizumab at 0.125, 0.25, 0.50 and 1 mg/ml on the mitochondrial dehydrogenase activity of rat neurosensory retina (R28) cells. The mean mitochondrial dehydrogenase activity of R28 cells treated with 0.125 and 0.25 mg/ml bevacizumab was not significantly different (P > 0.05) than the controls but at higher doses (0.50 and 1 mg/ml) was significantly different than the controls. *P < 0.05, **P < 0.01
Figure 4.
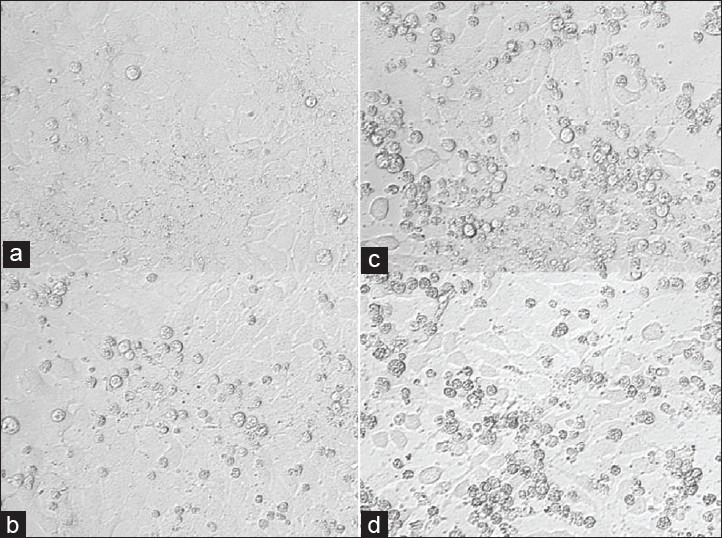
Phase contrast microscopy images of R28 cells after 5 days exposure to bevacizumab. Increased numbers of rounded cells are observed at 0.5 and 1.0 mg/ml compared to untreated and IgG controls. (a) Untreated control R28 cells. (b) IgG-treated (1 mg/ ml) control R28 cells. R28 cells treated with (c) 0.50 mg/ml and, (d) 1 mg/ ml bevacizumab. ×40
HMVEC
All four doses of bevacizumab significantly reduced the mitochondrial dehydrogenase activity of proliferating and non-proliferating HMVEC after 5 days [Figs. 5 and 6 respectively]. The mean absorbance of untreated or 1 mg/ml IgG-treated proliferating HMVEC cultures wells were 0.540 ± 0.0206 and 0.405 ± 0.016, respectively. The mean absorbance of proliferating HMVEC, after exposure to bevacizumab concentrations of 0.125, 0.25, 0.50 and 1 mg/ml were 0.350 ± 0.0096 (P < 0.01); 0.335 ± 0.0124 (P < 0.01); 0.345 ± 0.010 (P < 0.01) and 0.304 ± 0.003 (P < 0.001); respectively. The mean absorbance of proliferating HMVEC treated with bevacizumab were significantly different (P < 0.01, P < 0.001) from those of the controls.
Figure 5.
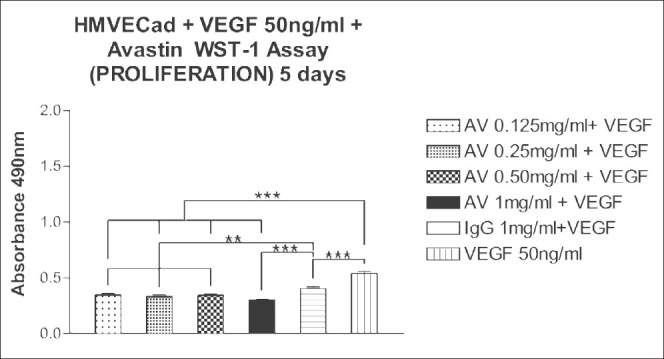
Effect of 5 days exposure of bevacizumab at 0.125, 0.25, 0.50 and 1 mg/ml on the mitochondrial dehydrogenase activity of proliferating human microvascular endothelial (HMVEC). The mean mitochondrial dehydrogenase activity of proliferating HMVEC treated with bevacizumab was significantly different than the controls for all the doses. **P < 0.01, ***P < 0.001
Figure 6.
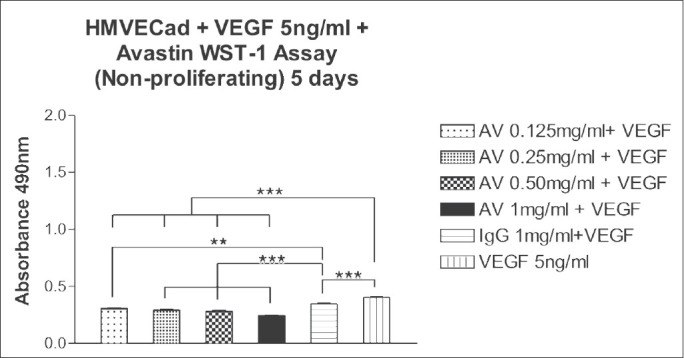
Effect of 5 days exposure of bevacizumab at 0.125, 0.25, 0.50 and 1 mg/ml on the mitochondrial dehydrogenase activity of non-proliferating HMVEC. The mean mitochondrial dehydrogenase activity of non-proliferating HMVEC treated with bevacizumab was significantly different than the controls for all the doses. **P < 0.01, ***P < 0.001
In untreated or 1 mg/ml IgG non-proliferating HMVEC cultures, the mean absorbance were 0.405 ± 0.008 and 0.347 ± 0.010, respectively. The mean absorbance of non-proliferating HMVEC, after exposure to bevacizumab concentrations of 0.125, 0.25, 0.50 and 1 mg/ml were 0.311 ± 0.0076 (P < 0.01); 0.295 ± 0.006 (P < 0.001); 0.285 ± 0.0089 (P < 0.001) and 0.248 ± 0.0025 (P < 0.001); respectively. The mean absorbance of non-proliferating HMVEC treated with bevacizumab were significantly different (P < 0.01, P < 0.001) from those of the controls.
Discussion
Since the first report in 2005 of the efficacy of intravitreal bevacizumab by Philip Rosenfeld, the clinical utilization of the drug has become widespread. However, given that the drug was not originally designed for intraocular use, the normal portfolio of pre-clinical studies evaluating this important drug remains underdeveloped. The first in vitro study evaluating the toxicity of bevacizumab on retinal cells, published by our group, suggested that bevacizumab at concentrations at or above the dose normally used in clinical practice, is safe in a short term (24 h) experiment determining the cell viability of the human retinal pigment epithelial, rat neurosensory retinal, and human microvascular endothelial cells 27. Another in vitro study confirmed our findings and found no bevacizumab cytotoxicity on rat ganglion cell line (RGC5), pig choroidal endothelial cells (CEC) and ARPE-19 cells following a one day drug exposure.[17] However, after a two day incubation with 2.5 mg/ml bevacizumab (×8-10 clinical dose), a moderate decrease in ARPE-19 cell number and viability was observed.[17] Also, bevacizumab caused a dose-dependent suppression of DNA synthesis in CEC, but the drug had no relevant antiproliferative effect on RGC5 and ARPE-19 cells at 0.8 mg/ml or less.[17]
The present study shows that all tested doses of bevacizumab (up to 4x clinical dose) did not significantly affect the mitochondrial function of human ARPE-19 cells in culture after 5 days. These results are in agreement with the report by Spitzer et al., in which there was no antiproliferative effect with concentrations of 0.8 mg/ml or less bevacizumab on ARPE-19 cells.[17] However, following 2 days of drug exposure, ARPE-19 cytotoxicity was detected at 10 times the clinical dose (2.5 mg/ml) of bevacizumab while in contrast in this study no ARPE-19 cell toxicity was observed at a longer exposure time using a lower dose of 1 mg/ml (our maximum dose and ×4 the clinical dose). In R28 cells, 0.125 mg/ml and 0.25 mg/ml (×1/2 and ×1 clinical dose) bevacizumab did not significantly affect the mitochondrial function after the same incubation period. However, 0.50 and 1 mg/ml (×2 and 4x clinical dose) bevacizumab significantly decreased the mitochondrial function of R28 cells after 5 days. This suggests that rat neurosensory retina (R28) cells are more sensitive to bevacizumab than human ARPE-19 cells. In contrast, the Spitzer group did not find cytotoxicity at 0.8 mg/ml (×3 clinical dose) or less bevacizumab when using the RGC5 cells after 2 days. This discrepancy may be due to the shorter period of drug exposure in the Spitzer experiment.
We also found in this study that all doses of bevacizumab significantly affected the mitochondrial function of proliferating and non-proliferating HMVEC after 5 days. A dose-dependent decrease in mitochondrial dehydrogenase activity of HMVEC with bevacizumab was found in this study and is similar to the findings of Spitzer et al.,[17] who reported the dose dependent inhibition of DNA synthesis in CEC using bevacizumab. In vitro studies on human umbilical vein endothelial cells (HUVEC) have demonstrated that bevacizumab completely blocks both proliferating and non-proliferating VEGF-induced endothelial cells after a period of several days.[18] The mechanism of action of bevacizumab is believed to be through neutralization of secreted VEGF as there was no cell or complement-mediated cytotoxicity in either VEGF-producing or -targeting cells. Low concentrations (5 ng/ml) of VEGF have been shown to protect cultured human dermal microvascular endothelial cells from undergoing senescence without inducing the malignant transformation.[19] In our study, this low concentration of VEGF was used for maintaining the non-proliferating HMVEC.
In experimental studies in the monkeys, the half-life in vitreous of 125I-labeled full-length humanized rhuMAb HER2 antibody (148 kDa) was 5.6 days, compared to 3.2 days for the 125I-labeled humanized rhuMAb VEGF Fab antibody (48.3 kDa). Thus, in our study, bevacizumab (149 kDa) induced cell toxicity was evaluated after 5 days, the approximate half-life of bevacizumab, a physiologically and pharmacokinetically relevant time-point. However, to prevent the adverse effect of serum deprivation on the cells during the 5 day experiments, media was replaced daily in all wells, treated and controls, with the respective media and the drug when appropriate. Consequently, there may be a slightly higher drug exposure in this in vitro study than would be expected after an in vivo intravitreal injection.
In conclusion, we found that bevacizumab, at standard clinical doses, is safe to the retinal cells in vitro. We noted an increased sensitivity to bevacizumab in the retinal neurosensory cells at supranormal doses but not in the RPE cells. Finally, we noted significant effects of bevacizumab at all doses on vascular endothelial cells consistent with the selective action of this drug.
Footnotes
Source of Support: Supported by the Discovery Eye Foundation, Iris and B. Gerald Cantor Foundation, Henry L Guenther Foundation, Gilbert Foundation, Ko Family Foundation, Research To Prevent Blindness, Pan-American Association of Ophthalmology Foundation (David and Julianna Pyott Pan-American - Retinal Research Fellowship).
Conflict of Interest: None declared.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–4. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Mason JO, Albert MA, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26:356–7. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2007;91:157–60. doi: 10.1136/bjo.2006.096776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–61. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–9. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006;142:162–4. doi: 10.1016/j.ajo.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Lüke M, Warga M, Ziemssen F, Gelisken F, Grisanti S, Schneider T, et al. Effects of bevacizumab on retinal function in isolated vertebrate retina. Br J Ophthalmol. 2006;90:1178–82. doi: 10.1136/bjo.2006.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In vitro Cell Dev Biol Anim. 1996;32:66–8. doi: 10.1007/BF02723034. [DOI] [PubMed] [Google Scholar]
- 13.Seigel GM, Sun W, Wang J, Hershberger DH, Campbell LM, Salvi RJ. Neuronal gene expression and function in the growth-stimulated R28 retinal precursor cell line. Curr Eye Res. 2004;28:257–69. doi: 10.1076/ceyr.28.4.257.27831. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Seigel GM, Salvi RJ. Retinal precursor cells express functional ionotropic glutamate and GABA receptors. Neuroreport. 2002;13:2421–4. doi: 10.1097/00001756-200212200-00009. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336–9. [PubMed] [Google Scholar]
- 17.Spitzer MS, Wallenfels-Thilo B, Sierra A, Yoeruek E, Peters S, Henke-Fahle S, et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006;90:1316–21. doi: 10.1136/bjo.2006.095190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab: A humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–45. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene. 1997;14:2025–32. doi: 10.1038/sj.onc.1201033. [DOI] [PubMed] [Google Scholar]


