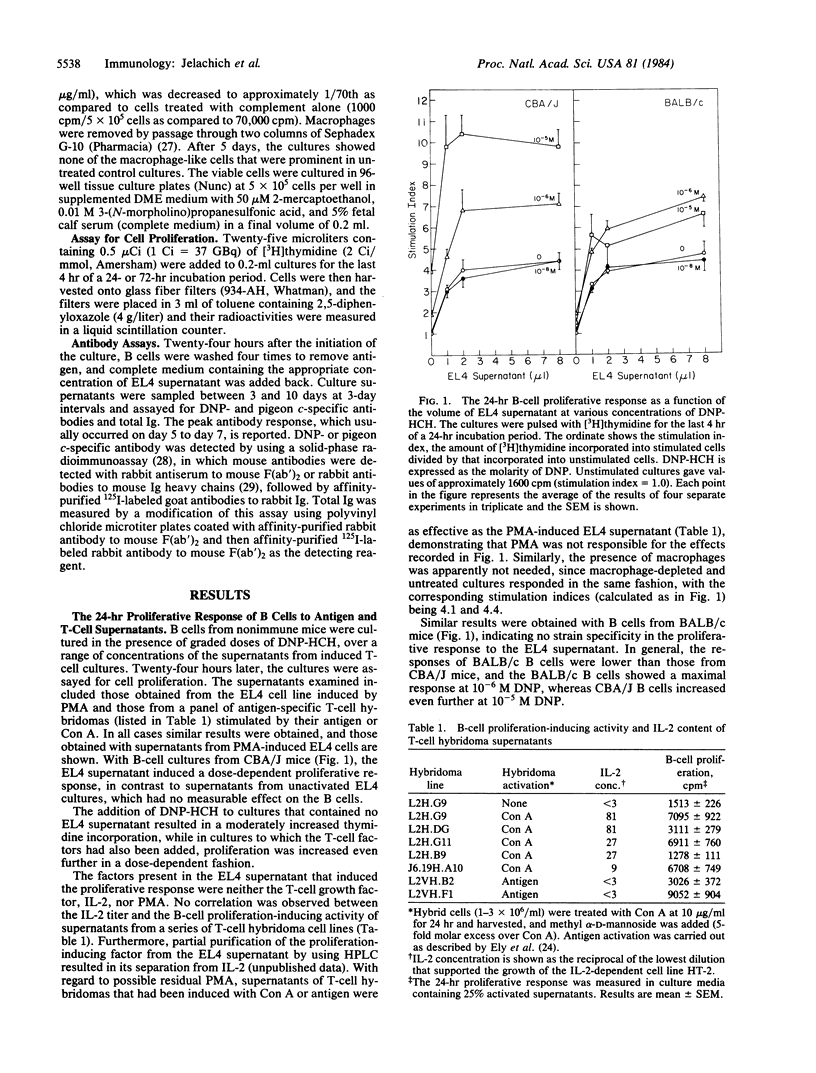
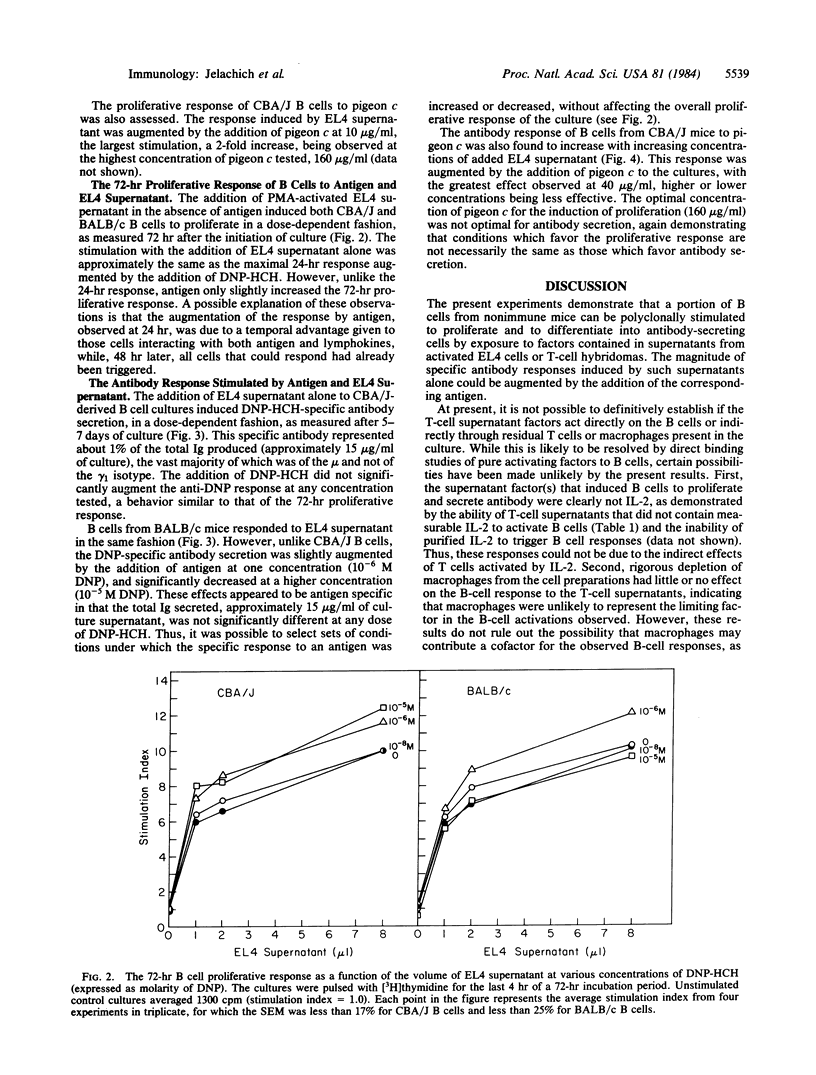
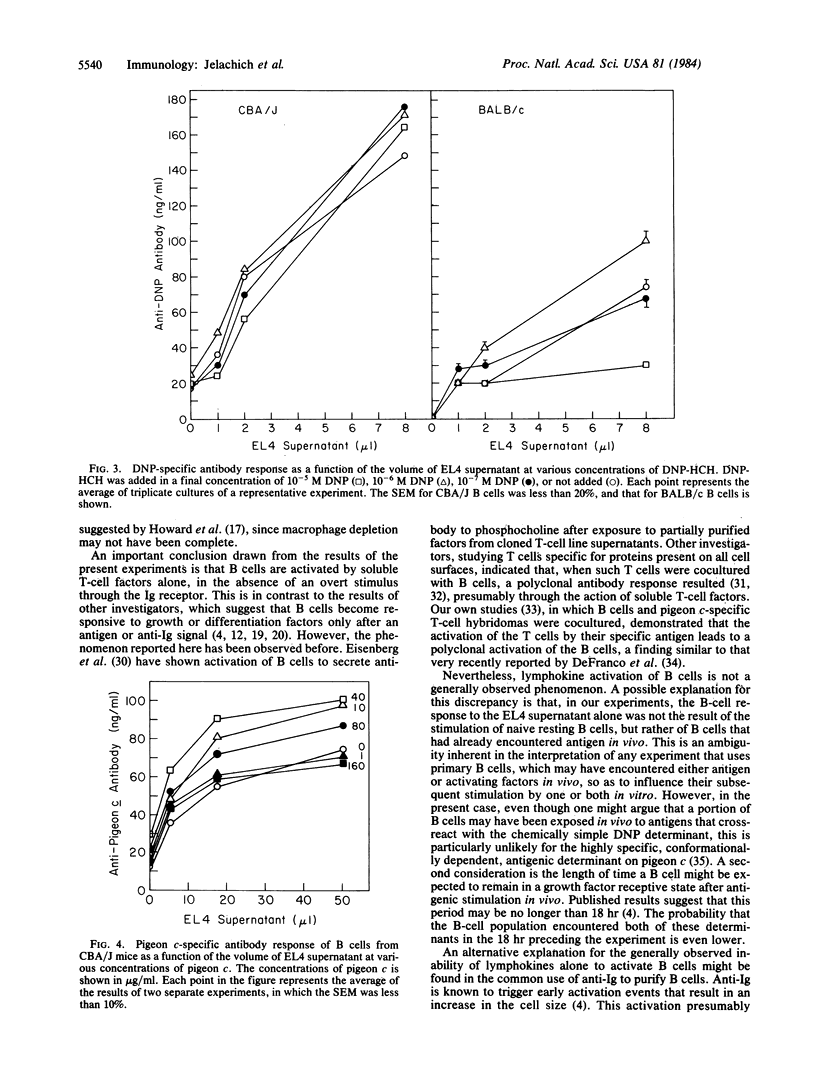
Abstract
Supernatants from phorbol 12-myristate 13-acetate-activated cultures of the mouse EL4 thymoma, or of several mouse T-cell hybridomas stimulated either by their specific antigen or by concanavalin A, induced primary splenic B cells to proliferate and differentiate to antibody-secreting cells. This effect was not due to interleukin 2 and did not require the presence of macrophages. The antibody response was polyclonal, including antibodies specific for 2,4-dinitrophenyl and pigeon cytochrome c, present in amounts of 1% or less of the total immunoglobulin produced. The addition of either of these antigens increased the amount of the corresponding specific antibody. At very high concentrations of dinitrophenyl-hemocyanin the specific response could be depressed. These observations were taken to demonstrate that soluble T-cell factors are sufficient to activate a portion of naive B cells to antibody secretion and that under these conditions in vitro the presence of antigen merely enhances the specific response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Melchers F. T cell-dependent activation of resting B cells: requirement for both nonspecific unrestricted and antigen-specific Ia-restricted soluble factors. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2497–2501. doi: 10.1073/pnas.78.4.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin A. A., Coutinho A. Specific T helper cells that activate B cells polyclonally. In vitro enrichment and cooperative function. J Exp Med. 1980 Mar 1;151(3):587–601. doi: 10.1084/jem.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. E., Gillis S., Ferm M. M., Smith K. A. The effect of T cell growth factor on the generation of cytolytic T cells. J Immunol. 1978 Dec;121(6):2168–2173. [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Brooks K., Yuan D., Uhr J. W., Krammer P. H., Vitetta E. S. Lymphokine-induced IgM secretion by clones of neoplastic B cells. Nature. 1983 Apr 28;302(5911):825–826. doi: 10.1038/302825a0. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Role of T lymphocytes in the humoral immune response. I. Proliferation of B lymphocytes in thymus-deprived mice. J Immunol. 1974 Nov;113(5):1438–1445. [PubMed] [Google Scholar]
- DeFranco A. L., Ashwell J. D., Schwartz R. H., Paul W. E. Polyclonal stimulation of resting B lymphocytes by antigen-specific T lymphocytes. J Exp Med. 1984 Mar 1;159(3):861–880. doi: 10.1084/jem.159.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Separate signals for the initiation of proliferation and differentiation in the b cell response to antigen. Transplant Rev. 1975;23:66–77. doi: 10.1111/j.1600-065x.1975.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg L., Prystowsky M. B., Dick R. F., Sosman J. A., Fitch F. W., Quintáns J. TRF requirements for in vitro PFC responses to SRBC and R36a. I. TRF is distinct from IL 2 but indistinguishable from polyclonal BCSF. J Immunol. 1984 Mar;132(3):1305–1310. [PubMed] [Google Scholar]
- Ely J. M., Prystowsky M. B., Eisenberg L., Quintans J., Goldwasser E., Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. V. Differential kinetics of IL 2, CSF, and BCSF release by a cloned T amplifier cell and its variant. J Immunol. 1981 Dec;127(6):2345–2349. [PubMed] [Google Scholar]
- Feldmann M., Basten A. Cell interactions in the immune response in vitro. 3. Specific collaboration across a cell impermeable membrane. J Exp Med. 1972 Jul 1;136(1):49–67. doi: 10.1084/jem.136.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Herber-Katz E., Hedrick S., Schwartz R. H., Paul W. E. I region-restricted antigen presentation by B cell-B lymphoma hybridomas. Nature. 1982 Jul 15;298(5871):283–284. doi: 10.1038/298283a0. [DOI] [PubMed] [Google Scholar]
- Howard M., Mizel S. B., Lachman L., Ansel J., Johnson B., Paul W. E. Role of interleukin 1 in anti-immunoglobulin-induced B cell proliferation. J Exp Med. 1983 May 1;157(5):1529–1543. doi: 10.1084/jem.157.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Jaworski M. A., Shiozawa C., Diener E. Triggering of affinity-enriched B cells. Analysis of B cell stimulation by antigen-specific helper factor or lipopolysaccharide. I. Dissection into proliferative and differentiative signals. J Exp Med. 1982 Jan 1;155(1):248–263. doi: 10.1084/jem.155.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972 Aug 1;136(2):241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of neonatal murine B cells. J Exp Med. 1976 Jun 1;143(6):1327–1340. doi: 10.1084/jem.143.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N. A. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971 Jan;1(1):18–27. doi: 10.1002/eji.1830010104. [DOI] [PubMed] [Google Scholar]
- Mongini P. K., Paul W. E., Metcalf E. S. IgG subclass, IgE, and IgA anti-trinitrophenyl antibody production within trinitrophenyl-Ficoll-responsive B cell clones. Evidence in support of three distinct switching pathways. J Exp Med. 1983 Jan 1;157(1):69–85. doi: 10.1084/jem.157.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K., Howard M., Muraguchi A., Farrar J., Takatsu K., Hamaoka T., Paul W. E. Soluble factors involved in B cell differentiation: identification of two distinct T cell-replacing factors (TRF). J Immunol. 1983 May;130(5):2219–2224. [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C., Uhr J. W., Vitetta E. S. Activation of antigen-specific B cells: role of T cells, cytokines, and antigen in induction of growth and differentiation. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6628–6631. doi: 10.1073/pnas.80.21.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Okumura K., Metzler C. M., Tsu T. T., Herzenberg L. A., Herzenberg L. A. Two stages of B-cell memory development with different T-cell requirements. J Exp Med. 1976 Aug 1;144(2):345–357. doi: 10.1084/jem.144.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. Separable helper factors support B cell proliferation and maturation to Ig secretion. J Immunol. 1982 Aug;129(2):469–474. [PubMed] [Google Scholar]
- Pierce S. K., Cancro M. P., Klinman N. R. Individual antigen-specific T lymphocytes: helper function in enabling the expression of multiple antibody isotypes. J Exp Med. 1978 Sep 1;148(3):759–765. doi: 10.1084/jem.148.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce S. K., Klinman N. R. Allogeneic carrier-specific enhancement of hapten-specific secondary B-cell responses. J Exp Med. 1976 Nov 2;144(5):1254–1262. doi: 10.1084/jem.144.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Vaux D. L., Clark-Lewis I., Schrader J. W., Nossal G. J. Proliferation and differentiation of single hapten-specific B lymphocytes is promoted by T-cell factor(s) distinct from T-cell growth factor. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6350–6354. doi: 10.1073/pnas.79.20.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E., Isakson P. C., Kappler J. W., Marrack P., Krammer P. H., Vitetta E. S. T cell-derived B cell growth and differentiation factors. Dichotomy between the responsiveness of B cells from adult and neonatal mice. J Exp Med. 1983 Feb 1;157(2):600–612. doi: 10.1084/jem.157.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K. The carrier effect and cellular cooperation in the induction of antibodies. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):385–392. doi: 10.1098/rspb.1971.0002. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Marrack P., Kappler J. W. Helper signals in the plaque-forming cell response to protein-bound haptens. J Exp Med. 1983 Aug 1;158(2):317–333. doi: 10.1084/jem.158.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Paige C. J., Schreier M. H. B cell maturation factor (BMF): a lymphokine or family of lymphokines promoting the maturation of B lymphocytes. J Immunol. 1984 Jan;132(1):209–222. [PubMed] [Google Scholar]
- Speck N. A., Pierce S. K. The induction of B cells refractory to antibody-specific immunoregulation. Eur J Immunol. 1982 May;12(5):449–452. doi: 10.1002/eji.1830120518. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Zubler R. H., Kanagawa O. Requirement for three signals in B cell responses. II. Analysis of antigen- and Ia-restricted T helper cell-B cell interaction. J Exp Med. 1982 Aug 1;156(2):415–429. doi: 10.1084/jem.156.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



