Abstract
Human cytomegalovirus (HCMV) gene expression during infection is characterized as a sequential process including immediate-early (IE), early (E), and late (L)-stage gene expression. The most abundantly expressed gene at the IE stage of infection is the major IE (MIE) gene that produces IE1 and IE2. IE1 has been the focus of study because it is an important protein, not only for viral gene expression but also for viral replication. It is believed that IE1 plays important roles in viral gene regulation by interacting with cellular proteins. In the current study, we performed protein array assays and identified 83 cellular proteins that interact with IE1. Among them, seven are RNA-binding proteins that are important in RNA processing; more than half are nuclear proteins that are involved in gene regulations. Tumorigenesis-related proteins are also found to interact with IE1, implying that the role of IE1 in tumorigenesis might need to be reevaluated. Unexpectedly, cytoplasmic proteins, such as Golgi autoantigen and GGA1 (both related to the Golgi trafficking protein), are also found to be associated with IE1. We also employed a coimmunoprecipitation assay to test the interactions of IE1 and some of the proteins identified in the protein array assays and confirmed that the results from the protein array assays are reliable. Many of the proteins identified by the protein array assay have not been previously reported. Therefore, the functions of the IE1-protein interactions need to be further explored in the future.
Keywords: human cytomegalovirus (HCMV), major immediate-early (MIE), IE1, protein-protein interaction, protein array
1. Introduction
Human cytomegalovirus (HCMV) has been defined as one of the major infectious agents related to AIDS. HCMV infects large populations in general and causes serious diseases in immunocompromised individuals [1,2,3]. A sequential process of viral events is characterized for CMV infection in permissive host cells [3], including viral entry, immediate-early (IE) and early gene expression, DNA replication, late (L) gene expression, viral packaging and release. Major immediate-early (MIE) gene expression is one of the earliest events during CMV infection. MIE genes are the most abundantly expressed viral genes at the IE stage and give rise to several nuclear phosphoproteins that are critical for the next stage of gene expression and viral replication [4,5,6,7,8,9,10,11,12,13,14]. Hence, the HCMV MIE gene has been the focus of study. IE1 is believed to be the first de novo viral protein produced after HCMV infection and is essential for HCMV replication when HCMV is infected at a low multiplicity of infection (MOI).
In general, IE1 has been considered as a gene activator. How IE1 activates gene expression is not fully understood. Several mechanisms that are involved in this activation (by IE1) have been described by different groups. First, IE1 interacts with nuclear corepressors such as PML, Daxx, and Sp100, thereby reducing their repressive effects on gene expression [15,16]. It was found by different groups independently that IE1 interacts with HDAC and inhibits HDAC’s activity [15,17]. Second, IE1 disperses ND10, a nuclear domain that contains many different nuclear suppressive proteins and responds to interferon stimulation [18,19,20,21]. In addition, innate cellular defense directed by interferons was disrupted by IE1 via inhibiting JAK-STAT signaling and by interacting with STAT1 [22,23,24]. The IE1 protein counteracts virus- or type I IFN-induced ISG activation via complex formation with STAT1 and STAT2 resulting in the reduced binding of ISGF3 to ISREs [22,23,24,25]. Last, IE1 is important for HCMV to arrest the cell cycle in the G1 phase that favors the infected HCMV for the cellular microenvironment. IE1 binds the Rb-related p107 protein and relieves its repression of E2F-responsive promoters [26]; IE1 also induces p53 accumulation through activating the p53 pathway by increasing the levels of p19Arf and by inducing the phosphorylation of p53 at Ser15 [27], which might also relate to the HCMV-caused “G1 arrest” of infected cells. The mechanisms used by IE1 to activate viral gene expression perhaps all depend on IE1-protein interactions. Therefore, it is important to identify the cellular proteins interacting with IE1 at a global level.
2. Results
One of the major challenges in the post-genomic era is to explore the functional elements in the human genome. It also applies to the virus-host interaction. Identifying the cellular proteins that interact with the important virus proteins will certainly contribute to the understanding of the mechanisms that the virus uses for its gene expression and replication. Protein arrays constitute a powerful tool for high throughput and multiplexed protein analysis, including protein detection, the investigation of protein interactions with various types of molecules, and the determination of protein functions [28]. Protein array technology is highly sensitive and generates large amounts of data in a single experiment with comparatively low sample consumption; therefore, it is highly economical [28]. In current studies, we used protein array assays to screen cellular proteins that interact with HCMV IE1. Here we report our experimental results.
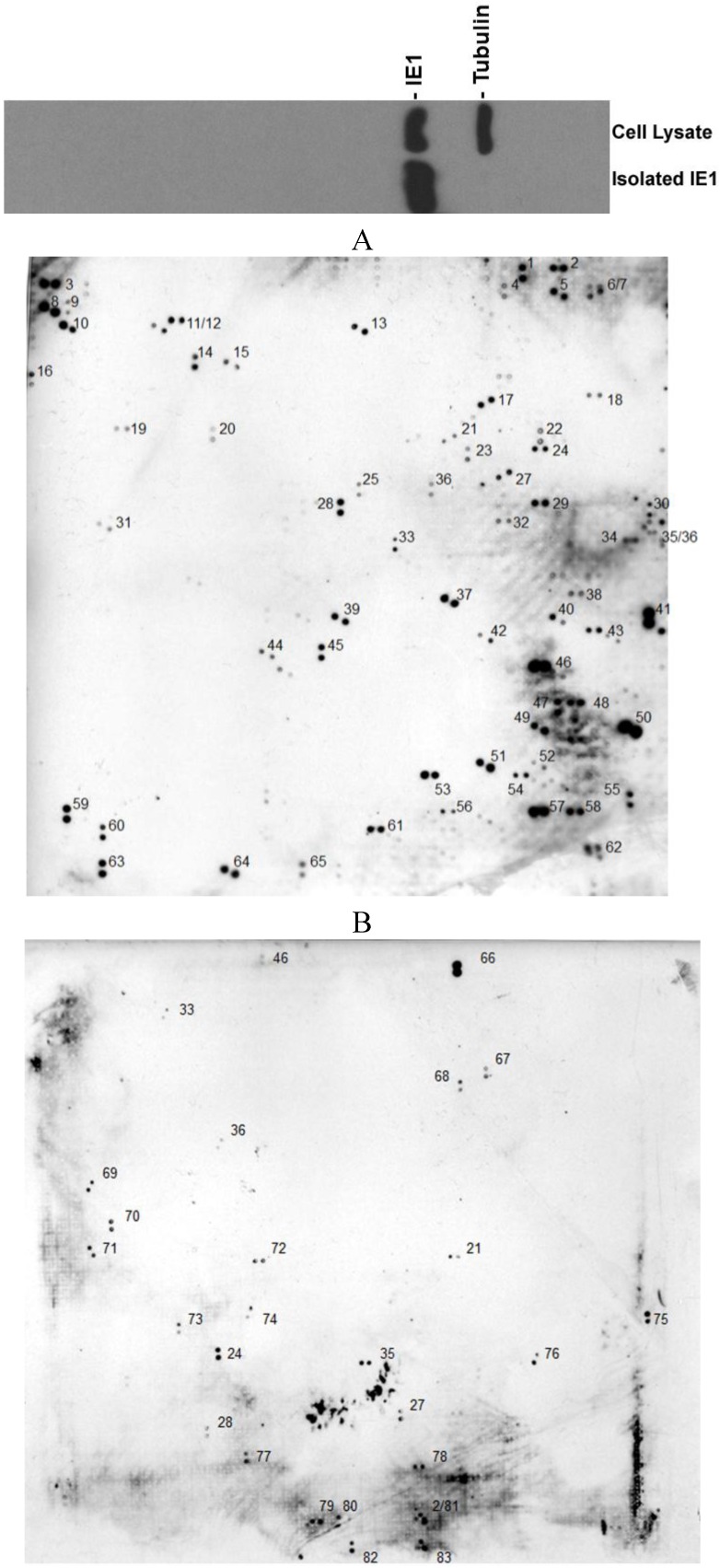
First, we isolated the IE1 from an IE1-producing cell line (previously called U373-IE1, now called U-251 MG-IE1) [29] using a specific anti-IE1 antibody that was later incubated with beads-conjugated secondary antibody after which the beads were washed in binding/wash buffer (20 mM Na2HPO4, 0.15 M NaCl, pH 7.0). The pulled-down IE1 was washed off from the secondary antibody-bound beads with elution buffer (0.1 M glycine, pH 2–3), and the eluted solution was immediately neutralized with neutralization buffer (1 M Tris, pH 8.5). The isolated IE1 was confirmed by Western blot assay as shown in the top of Figure 1. Then the isolated IE1 was incubated at room temperature for 1 hour with the 22 cm × 22 cm PVDF membranes presenting up to 7390 in situ expressed human proteins (Cat# Unipex_1, _2, library # 9027, #9028, imaGenes GmbH, Berlin, Germany). After 24 h of incubation with primary anti-IE1 antibody at 4 °C, the membrane was washed with the buffer 2 times followed by incubation with a horseradish peroxidase-coupled secondary antibody (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and detection with enhanced chemiluminescence (Pierce, Rockford, IL, USA), according to standard methods. As can be seen in Figure 1A,B, 83 cellular proteins were found to bind to IE1. In the PVDF membrane, each in situ expressed cellular protein is presented in doublets, which is why we can see 2 dots together for each protein. We set up cutoffs according to the background index. Figure 1A,B represents 2 PVDF membranes that were processed at the same time with the same samples. The 83 proteins are listed in the Table 1 with their Gene bank #, name, function, and references.
Figure 1.
Protein Array. The isolated IE1 was incubated with the two PVDF membranes, which was followed by incubation with a horseradish peroxidase-coupled secondary antibody (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and detection with enhanced chemiluminescence (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), according to standard methods. Some genes overlap between the two PVDF membranes.
Table 1.
List of the Human Cytomegalovirus (HCMV) IE1 Protein-Protein Interactions (PPIs) by Protein Array Assay. The positive spots in Figure 1A,B were listed in this table according to the manufacturer’s annotation table. The dots are all in doublets. Some genes overlap between the two PVDF membranes. Cellular Proteins Interacting with Human Cytomegalovirus IE1 (Identified by Protein Array Assays).
| GenBank entry# | Name of the protein | Function | Ref. |
|---|---|---|---|
| AF217982 | CDK5 regulatory subunit associated protein | Tumorigenesis and metastasis | [35] |
| D84294 | TPRDI (tetratricopeptide repeat) | Down syndrome | [36] |
| BC069268 | Golgi autoantigen, golgin subfamily a | Tentacular matrix | [37] |
| NM_007144 | Polycomb group ring Finger 2 (PCGF2) | Embryogenesis and tumorigenesis | [38] |
| NM_005676 | RNA binding motif protein 10 (RBM10) | RNA-related apoptosis | [39] |
| Z11584 | NuMA protein | Spindle orientation | [40] |
| BC022880 | Breast carcinoma amplified sequence 2 | Negative regulator of p53 | [41] |
| BC057387 | LSM14B, SCD6 homolog B | Regulation of translation | [42] |
| BG354577 | CDCA4 Cell division cycle associated gene 4 | Transcription regulation | [43] |
| NM_001789 | Cell division cycle 25 homolog A (CDC25A) | Cell cycle regulation | [44] |
| NM_003026 | SH3-domain GRB2-like 2 (SH3GL2) | Tumorigenesis | [45] |
| NM_203505 | GTPase activating protein binding protein 2 | Stress granule formation | [46] |
| XM_001128623 | Transcriptional regulator ATRX | Transcriptional repression | [47] |
| NM_153273 | Inositol hexaphosphate kinase 1 (IHPK1) | Type 2 diabetes | [48] |
| NM_032019 | Histone deacetylase 10 (HDAC10) | Cancer metastasis | [49] |
| NM_001040653 | ZXD family zinc finger C (ZXDC) | MHC gene transcription | [50] |
| NM_138383 | Actin-bundling protein with BAIAP2 homology (ABBA-1) | unknown | [51] |
| NM_001082486 | Adrenocortical dysplasia homolog (mouse) (ACD), transcript variant 1 | Tumorigenesis | [52] |
| AK122898 | ADP-ribosylation factor binding protein GGA1 | Trans-olgi network | [53] |
| AB208813 | RNA binding motif protein 5 | Gene splicing factor | [39] |
| NM_170677 | Meis homeobox 2 (MEIS2) | Unknown | [54] |
| NM_000038 | Adenomatosis polyposis coli (APC) | Tumor supresor | [55] |
| NM_003660 | Protein tyrosine phosphatase, receptor type f polypeptide (PTPRF), interacting protein (liprin), alpha 3 (PPFIA3) | Unknown | [56] |
| NM_012398 | Phosphatidylinositol-4-phosphate 5-kinase, type I, gamma (PIP5K1C) | Lethal contractural syndrome | [57] |
| NM_003861 | WD repeat domain 22 (WDR22) | Unknown | N/A |
| NM_017453 | Staufen, RNA binding protein | mRNA traffic | [58] |
| BC017222 | Sequestosome 1 | Signal transduction | [59] |
| NM_152586 | Ubiquitin specific peptidase 54 (USP54) | Unknown | [60] |
| NM_014868 | Ring finger protein 10 (RNF10) | Type 2 diabetes | [61] |
| BC041897 | SplA/ryanodine receptor domain and SOCS box containing 3 | Inflammation | [62] |
| AB209534 | Tumor rejection antigen (gp96) 1 | Unknown | N/A |
| NM_001080424 | Jumonji domain containing 3 (JMJD3) | Histone demethylation | [63] |
| NM_006312 | Nuclear receptor co-repressor 2 (NCOR2) | Gene suppressor | [64] |
| AK124656 | Gamma enolase (EC 4.2.1.11) | Neural tissue development | [65] |
| AK123065 | Sperm acrosomal protein | Motility of the spermatozoon | [66] |
| NM_001098800 | Melanoma antigen family D 4 (MAGED4) | Renal cell carcinoma | [67] |
| NM_021098 | Calcium channel, voltage-dependent, T type, alpha 1H subunit (CACNA1H) | T-type Ca(2+) channel activity | [68] |
| NM_001417 | Eukaryotic translation initiation factor 4B (EIF4B) | Translation control | [69] |
| NM_002973 | Ataxin 2 (ATXN2) | Spinocerebellar ataxia type 2 | [70] |
| BC070086 | Splicing factor, arginine/serine-rich 2 | Gene splicing | [18] |
| NM_001675 | Activating transcription factor 4 (ATF4) | Gene transcription | [71] |
| AB209149 | Phenol-sulfating phenol sulfotransferase 1 | Transfer of a sulfonate moiety | [72] |
| AY509035 | Roundabout-like protein 3 (ROBO3) | Horizontal gaze palsy | [73] |
| NM_014751 | Metastasis suppressor 1 (MTSS1) | Metastasis | [74] |
| AY335491 | GON4L isoform C (GON4L) | Hematopoiesis | [75] |
| NM_014739 | BCL2-associated transcription factor 1 | Transcriptional repression | [76] |
| AB067518 | KIAA1931 protein | Unknown | N/A |
| NM_003200 | Transcription factor 3 (E2A immunoglobulin enhancer binding factors E12/E47) (TCF3) | Transcription regulation | [77] |
| AB209197 | Protein phosphatase 1 (PP1) | Multiple functions | [78] |
| NM_020226 | PR domain containing 8 (PRDM8) | Gene repressor | [79] |
| XM_166659 | OTU domain containing 1 (OTUD1) | Unknown | N/A |
| NM_001098208 | Heterogeneous nuclear ribonucleoprotein F (HNRPF) | Gene splicing | [80] |
| AB209441 | Fibroblast growth factor receptor 3 isoform 1 precursor | Development | [81] |
| NM_152643 | Kinase non-catalytic C-lobe domain (KIND) containing 1 (KNDC1) | Unknown | N/A |
| NM_001982 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (ERBB3) | Cell proliferation or differentiation | [82] |
| NM_015695 | Bromodomain and PHD finger containing, 3 (BRPF3) | Fetal liver erythropoiesis | [83] |
| NM_015906 | Tripartite motif-containing 33 (TRIM33) | Tumor suppressors | [84] |
| NM_032127 | Chromosome 11 open reading frame 56 (C11orf56) | FTS and Hook-interacting protein | [85] |
| NM_002904 | RD RNA binding protein (RDBP) | Repress RNA polymerase II | N/A |
| NM_012272 | PRP40 pre-mRNA processing factor 40 B (PRPF40B) | Gene splicing | [86] |
| NM_020967 | Nuclear receptor coactivator 5 (NCOA5) | Gene regulator | [87] |
| NM_001080495 | KIAA1856 protein (KIAA1856) | Unknown | N/A |
| NM_022748 | Tensin 3 (TNS3) | Signal transduction | [88] |
| BC063642 | Phosphodiesterase 4D interacting protein (myomegalin) | Control microtubules | [89] |
| AB209493 | Death-associated protein 6 (DAXX) | Development and Cancer | [90] |
| NM_003482 | Myeloid/lymphoid or mixed-lineage leukemia 2 (MLL2) | Lymphomagenesis | [91] |
| NM_033396 | Tankyrase 1 binding protein 1, (TNKS1BP1) | Unknown | [92] |
| AY729650 | Intersex-like protein | Unknown | [93] |
| BC110647 | Immediate-early response 2 | Unknown | N/A |
| AB014581 | KIAA0681 protein | Unknown | N/A |
| NM_004235 | Kruppel-like factor 4 (gut) (KLF4) | Transactivation and growth suppression | [94] |
| AB051455 | KIAA1668 protein | Unknown | N/A |
| AF045458 | Serine/threonine kinase ULK1 (ULK1) | Autophagy activation | [95] |
| NM_001003694 | Bromodomain and PHD finger containing, 1 (BRPF1) | Unknown | N/A |
| NM_003626 | protein tyrosine phosphatase, receptor type, f polypeptide (PTPRF), interacting protein (liprin), alpha 1 (PPFIA1) | Unknown | N/A |
| NM_006291 | tumor necrosis factor, alpha-induced protein 2 (TNFAIP2) | Unknown | N/A |
| AB209643 | smoothelin isoform b | Unknown | N/A |
| NM_002857 | peroxisomal biogenesis factor 19 (PEX19) | Peroxisomal assembly | [96] |
| AB061669 | receptor for advanced glycation end-products | Signaling and inflammation | [97] |
| NM_014678 | SAPS domain family, member 2 (SAPS2) | Unknown | N/A |
| NM_006887 | zinc finger protein 36, C3H type-like 2 (ZFP36L2) | Unknown | N/A |
| AB208876 | axin 1 isoform | Wnt signaling pathway | [98] |
| NM_004530 | matrix metallopeptidase 2 (MMP2) | Metastasis and inflammation | [99] |
Further analysis of the 83 proteins revealed some important information: First, some of them have already been reported to interact with IE1. These are ATRX (#13) [15], splicing factor, arginine/serine-rich 2 (#40) [18], and Daxx (#65) [30]. Second, some of them are reported to be important for CMV infection but they have not been shown to interact with IE1, these are CDC25A (#10) [31], ATF4 (#41) [32], BCL-2 (#46) [33], and Ataxin (#39) [34]. In addition, 7 of the proteins that interact with IE1 are RNA binding proteins: RNA binding motif protein 10 (RBM10) (#5), RNA binding motif protein 5 (#20), Staufen, RNA binding protein (#26), splicing factor, arginine/serine-rich 2 (#40), heterogeneous nuclear ribonucleoprotein F (#52), RD RNA binding protein (#59), and PRP40 pre-mRNA processing factor 40 B (#60). All the RNA-bonding proteins are important for pre-mRNA processing. To our surprise, neither HDAC1, 2, or 3 nor TRIM19 (PML) is on the list; however, HDAC10 (#15) and TRIM33 (#57) are both detected as IE1-binding proteins with high signals (Figure 1).
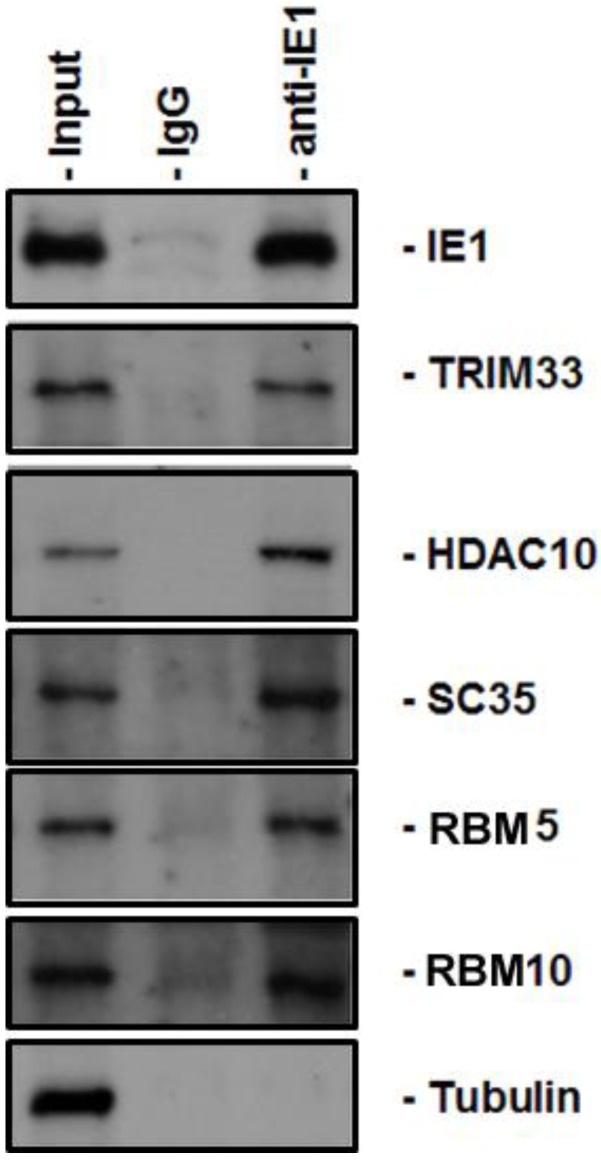
Since most of the IE1-interacting proteins in the Table 1 have not been reported, we need to validate the reliability of the results from the protein arrays. For that purpose, we performed a coimmunoprecipitation (coIP) assay using antibodies on hand to examine whether the proteins can be pulled down by the anti-IE1 antibody. The IE1-producing cell line (U-251 MG-IE1) [29] was cultured at 37 °C until it reached 95% confluence. The nuclear extract was prepared according to the protocol as described in Material and Method section. The nuclear extracts were incubated with anti-IE1 antibody or with normal IgG and then secondary antibody-conjugated beads. After several washings, the eluted complexes from the beads were examined by Western blot assay using antibodies against the proteins as indicated in Figure 2. All the proteins from the list that are examined by Western blot assay were positive. We also examined the tubulin as control and it was negative. Therefore, the results from the protein arrays are reliable.
Figure 2.
Validation of some IE1 PPIs by coIP. The nuclear extract from the IE1-producing cell line was prepared and incubated with anti-IE1 antibody followed by incubation with secondary bound beads. The pulled-down complexes were examined by Western blot using antibodies against the protein, as indicated on the right hand side.
3. Discussion
HCMV infects a large part of the population and has serious consequences for immunocompromised persons with AIDS or who have received organ transplants and for newborns after congenital infection. In fact, HCMV is the leading viral cause of congenital birth defects [1]. More than 30% of primary HCMV infections in pregnant women result in placental transmission and clinical syndromes. Congenital infection is not uncommon [100,101,102]. Of symptomatic newborns, about 12% die and half of the survivors develop mental retardation, vision loss, and/or sensorineural deafness [103]. Interference strategies commonly target the early events of the replication cycle by using approved nucleoside analogs such as ganciclovir, the nucleotide analog cidofovir, and foscarnet. However, these can lead to resistance [104]. In vitro anti-sense oligonucleotides against the HCMV immediate-early protein 2 (IE2) have proven effective [105], as has targeting the UL36 and UL37 sites [105,106]. These attempts show that targeting the HCMV IE part of the propagation cycle may be effective; however, none of these treatments are permissible or feasible for use on a potentially infected fetus. The lack of suitable treatment modalities has especially serious consequences for the congenitally infected fetus and for patients with impaired immune systems. The challenge is to find treatment modalities that do not depend on the inhibition of the DNA replication process. This has attracted our investigation to the immediate-early (IE) stage of HCMV infection. Understanding IE1’s function is our first step in the development of a practical strategy against HCMV replication via interfering with IE1 function.
In many cases, proteins play their biological role through interactions with other proteins. Especially for viral infection, viruses need to manipulate cellular function via interacting with cellular proteins. Therefore, the systematic identification and characterization of protein-protein interactions (PPIs) is considered a key strategy to understand protein function. In the case of HCMV viral immediate-early products, IE1 might be the most important protein that initiates the early interactions between the virus and cells. The number of possible contacts between protein surfaces is astronomical. In this context, protein array technology opens up new avenues for the characterization of viral proteins and the identification of molecular partners involved in virus-mediated regulatory networks in cells. The array technology used in the present study started with incubation of IE1 with the PVDF membranes that carry GST-tagged human fusion proteins. Those fusion proteins were expressed in E. coli, and after native lysis with lysozyme, crude protein extracts were prepared under non-denaturing conditions in a 384-well plate format. The crude bacterial cell extracts were used for incubation overnight with the high-density spotted PVDF membranes, which were used to detect IE1-interacting proteins.
Using the protein array, eighty-three cellular proteins in total were identified to be PPIs of HCMV IE1. Most of them were gene regulators. Only 3 IE1 PPIs from the list have been reported. However, many HCMV-mediated cell biological activities have been linked to IE1 (which link is also shown in these array assays); these activities include, among others, the interaction of IE1 with BCL-2, CDC25A, which interaction might be important for HCMV-induced “G1 arrest”. Some cellular proteins reported to interact with IE1 are not on the list, they might interact with IE1 only indirectly or the printed proteins are in different isoforms. For example, some isoforms of PML was found to interact with IE1 [15] but is not on the list because the other isoforms of PML are printed on the PVDF membranes. As shown on the Table 1, 7 RNA binding proteins are found to interact with IE1, implying that IE1 has a novel function in viral mRNA processing.
In conclusion, our current effort in characterizing IE1’s PPIs resulted in the identification of 83 cellular proteins that directly interact with HCMV IE1. The results have been partially validated by coIP assay. With the data listed in the Table 1, we will further investigate the function of the PPIs of IE1.
4. Experimental
4.1. Tissue Culture and Viruses
The IE1-producing cell lines (U-251 MG-IE1) [29] were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin.
4.2. Antibodies
The monoclonal antibody against IE1 (MAB810) and the rabbit antibodies against cellular proteins were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The rabbit antibodies included anti-SC35 (sc-28720), -TRIM33 (sc-68424), -HDAC10 (sc-130775), and -tubulin (sc-5546). Rabbit antibodies against RBM5 (ab69970) and RBM 10 (ab126112) were bought from Abcam (Cambridge, MA, USA)
4.3. Preparation of Nuclear Extracts
Nuclear extracts were obtained essentially as described previously [15]. Briefly, monolayer cells were washed with PBS and scraped into fresh Eppendorf tubes. Cell pellets were resuspended in cold buffer A (10 mM HEPES-KOH, pH 7.9, at 4 °C, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride) and incubated for 10 min at 4 °C. Then the cells were dounced with 10–20 plunges in a Kontes-B (Wheaton, DC, USA) Dounce Homogenizer (Pestle B) and poured into new bottles after centrifugation. The pellets were resuspended in cold buffer C (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride) by vortexing and incubated for 30 min at 4 °C. The pellets were dounced again with 10–20 plunges in Kontes-B (Wheaton) Dounce Homogenizer (Pestle B), and clarified extracts were transferred to fresh tubes and stored at −70 °C until use.
4.4. Coimmunoprecipitation
Antibodies were coupled to protein G-Sepharose beads (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), according to the manufacturer’s instructions. After a wash with PBS-0.1% bovine serum albumin, the beads were incubated overnight at 4 °C with clarified extracts, washed again in PBS-0.1% bovine serum albumin, and resuspended in a mixture of PBS and 2× Laemmli buffer (20 µL of each). After being heated for 5 min at 95 °C, the beads were removed by centrifugation and supernatants were analyzed by SDS-PAGE and immunoblotting.
4.5. Immunoblot Analysis
Proteins were separated by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis [107] (10 to 20 µg loaded in each lane), transferred to nitrocellulose membranes (Amersham Inc., Piscataway, NJ, USA), and blocked with 5% nonfat milk for 60 min at room temperature. Membranes were incubated overnight at 4 °C with primary antibody followed by incubation with a horseradish peroxidase-coupled secondary antibody (Amersham Inc.) and detection with enhanced chemiluminescence (Pierce, Rockford, IL, USA), according to standard methods. Membranes were stripped with stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.8), washed with PBS-0.1% Tween 20, and used to detect additional proteins.
5. Conclusions
We currently used a protein array assay to identify HCMV IE1 interacting proteins. There are 83 cellular proteins that are PPIs of IE1 and most of the PPIs have not been reported previously. The interactions have been partially validated by coIP method which confirmed that the protein array assay is reliable.
Acknowledgements
This study was supported by a pilot grant from the Research Center for Minority Institutes (RCMI) program (2G12RR003050-24/8G12MD007579-27) (Q.T.), an American Cancer Society grant (RSG-090289-01-MPC) (Q.T), and NIH/NCRR U54RR022762 (Q.T.). We acknowledge the instrument support of the PSMHS Molecular Biology Core Laboratory. Finally, we are grateful to Bob Ritchie of the Ponce School of Medicine and Health Sciences/RCMI Publications Office (G12 RR003050/8G12MD007579-27) for his help with manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Sweet C. The pathogenicity of cytomegalovirus. FEMS Microbiol. Rev. 1999;23:457–482. doi: 10.1111/j.1574-6976.1999.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 2.Landolfo S., Gariglio M., Gribaudo G., Lembo D. The human cytomegalovirus. Pharmacol. Ther. 2003;98:269–297. doi: 10.1016/S0163-7258(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 3.Mocarski E.S., Jr., Shenk T., Pass R.F. Cytomegaloviruses. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2006. [Google Scholar]
- 4.Tang Q., Li L., Maul G.G. Mouse cytomegalovirus early M112/113 proteins control the repressive effect of IE3 on the major immediate-early promoter. J. Virol. 2005;79:257–263. doi: 10.1128/JVI.79.1.257-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Q., Maul G. Immediate early interactions and epigenetic defense mechanisms. In: Reddehase M.J., editor. In Cytomegaloviruses: Molecular Biology and Immunology. Hethersett, Horizon Scientific Press; Norwich, UK: 2005. [Google Scholar]
- 6.Hagemeier C., Walker S.M., Sissons P.J., Sinclair J.H. The 72K IE1 and 80K IE2 proteins of human cytomegalovirus independently trans-activate the c-Fos, c-Myc and hsp70 promoters via basal promoter elements. J. Gen. Virol. 1992;73:2385–2393. doi: 10.1099/0022-1317-73-9-2385. [DOI] [PubMed] [Google Scholar]
- 7.Liu B., Hermiston T.W., Stinski M.F. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol. 1991;65:897–903. doi: 10.1128/jvi.65.2.897-903.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scully A.L., Sommer M.H., Schwartz R., Spector D.H. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol. 1995;69:6533–6540. doi: 10.1128/jvi.69.10.6533-6540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awasthi S., Isler J.A., Alwine J.C. Analysis of splice variants of the immediate-early 1 region of human cytomegalovirus. J. Virol. 2004;78:8191–8200. doi: 10.1128/JVI.78.15.8191-8200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadanari H., Yamada R., Yamagoshi T., Ohnishi K., Matsubara K., Fukuda S., Tanaka J. The major immediate-early genes of human cytomegalovirus induce two novel proteins with molecular weights of 91 and 102 kilodaltons. Arch. Virol. 2000;145:1257–1266. doi: 10.1007/s007050070125. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J.H., Hayward G.S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier J.L., Stinski M.F. Effect of a modulator deletion on transcription of the human cytomegalovirus major immediate-early genes in infected undifferentiated and differentiated cells. J. Virol. 1997;71:1246–1255. doi: 10.1128/jvi.71.2.1246-1255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenberg R.M. The human cytomegalovirus major immediate-early gene. Intervirology. 1996;39:343–349. doi: 10.1159/000150505. [DOI] [PubMed] [Google Scholar]
- 14.Stenberg R.M., Thomsen D.R., Stinski M.F. Structural analysis of the major immediate early gene of human cytomegalovirus. J. Virol. 1984;49:190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q., Maul G.G. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J. Virol. 2003;77:1357–1367. doi: 10.1128/JVI.77.2.1357-1367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H.R., Kim D.J., Lee J.M., Choi C.Y., Ahn B.Y., Hayward G.S., Ahn J.H. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 2004;78:6527–6542. doi: 10.1128/JVI.78.12.6527-6542.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevels M., Paulus C., Shenk T. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. USA. 2004;101:17234–17239. doi: 10.1073/pnas.0407933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn J.H., Hayward G.S. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology. 2000;274:39–55. doi: 10.1006/viro.2000.0448. [DOI] [PubMed] [Google Scholar]
- 19.Ishov A.M., Stenberg R.M., Maul G.G. Human cytomegalovirus immediate early interaction with host nuclear structures: Definition of an immediate transcript environment. J. Cell Biol. 1997;138:5–16. doi: 10.1083/jcb.138.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H.R., Huh Y.H., Kim Y.E., Lee K., Kim S., Ahn J.H. N-Terminal determinants of human cytomegalovirus IE1 protein in nuclear targeting and disrupting PML-associated subnuclear structures. Biochem. Biophys. Res. Commun. 2007;356:499–504. doi: 10.1016/j.bbrc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Maul G.G., Negorev D., Bell P., Ishov A.M. Review: Properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 2000;129:278–287. doi: 10.1006/jsbi.2000.4239. [DOI] [PubMed] [Google Scholar]
- 22.Paulus C., Krauss S., Nevels M. A human cytomegalovirus antagonist of type I IFN-dependent signal transducer and activator of transcription signaling. Proc. Natl. Acad. Sci. USA. 2006;103:3840–3845. doi: 10.1073/pnas.0600007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh Y.H., Kim Y.E., Kim E.T., Park J.J., Song M.J., Zhu H., Hayward G.S., Ahn J.H. Binding STAT2 by the acidic domain of human cytomegalovirus IE1 promotes viral growth and is negatively regulated by SUMO. J. Virol. 2008;82:10444–10454. doi: 10.1128/JVI.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krauss S., Kaps J., Czech N., Paulus C., Nevels M. Physical requirements and functional consequences of complex formation between the cytomegalovirus IE1 protein and human STAT2. J. Virol. 2009;83:12854–12870. doi: 10.1128/JVI.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoblach T., Grandel B., Seiler J., Nevels M., Paulus C. Human cytomegalovirus IE1 protein elicits a type II interferon-like host cell response that depends on activated STAT1 but not interferon-gamma. PLoS Pathog. 2011;7:e1002016. doi: 10.1371/journal.ppat.1002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Huong S.M., Wang X., Huang D.Y., Huang E.S. Interactions between human cytomegalovirus IE1-72 and cellular p107: Functional domains and mechanisms of up-regulation of cyclin E/cdk2 kinase activity. J. Virol. 2003;77:12660–12670. doi: 10.1128/JVI.77.23.12660-12670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo J.P., Frame F.M., Rogoff H.A., Pickering M.T., Yurochko A.D., Kowalik T.F. Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J. Virol. 2005;79:11467–11475. doi: 10.1128/JVI.79.17.11467-11475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu S., Xie Z., Onishi A., Yu X., Jiang L., Lin J., Rho H.S., Woodard C., Wang H., Jeong J.S., et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell. 2009;139:610–622. doi: 10.1016/j.cell.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q., Maul G.G. Mouse cytomegalovirus crosses the species barrier with help from a few human cytomegalovirus proteins. J. Virol. 2006;80:7510–7521. doi: 10.1128/JVI.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves M., Woodhall D., Compton T., Sinclair J. Human cytomegalovirus IE72 protein interacts with the transcriptional repressor hDaxx to regulate LUNA gene expression during lytic infection. J. Virol. 2010;84:7185–7194. doi: 10.1128/JVI.02231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaspar M., Shenk T. Human cytomegalovirus inhibits a DNA damage response by mislocalizing checkpoint proteins. Proc. Natl. Acad. Sci. USA. 2006;103:2821–2826. doi: 10.1073/pnas.0511148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xuan B., Qian Z., Torigoi E., Yu D. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J. Virol. 2009;83:3463–3474. doi: 10.1128/JVI.02307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andoniou C.E., Andrews D.M., Manzur M., Ricciardi-Castagnoli P., Degli-Esposti M.A. A novelcheckpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J. Cell Biol. 2004;166:827–837. doi: 10.1083/jcb.200403010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tower C., Fu L., Gill R., Prichard M., Lesort M., Sztul E. Human cytomegalovirus UL97 kinase prevents the deposition of mutant protein aggregates in cellular models of Huntington’s disease and ataxia. Neurobiol. Dis. 2011;41:11–22. doi: 10.1016/j.nbd.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mak G.W., Lai W.L., Zhou Y., Li M., Ng I.O., Ching Y.P. CDK5RAP3 is a novel repressor of p14ARF in hepatocellular carcinoma cells. PLoS One. 2012;7:e42210. doi: 10.1371/journal.pone.0042210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukahara F., Hattori M., Muraki T., Sakaki Y. Identification and cloning of a novel cDNA belonging to tetratricopeptide repeat gene family from Down syndrome-critical region 21q22.2. J. Biochem. 1996;120:820–827. doi: 10.1093/oxfordjournals.jbchem.a021485. [DOI] [PubMed] [Google Scholar]
- 37.Munro S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hennig L., Derkacheva M. Diversity of Polycomb group complexes in plants: Same rules, different players? Trends Genet. 2009;25:414–423. doi: 10.1016/j.tig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland L.C., Rintala-Maki N.D., White R.D., Morin C.D. RNA binding motif (RBM) proteins: A novel family of apoptosis modulators? J. Cell Biochem. 2005;94:5–24. doi: 10.1002/jcb.20204. [DOI] [PubMed] [Google Scholar]
- 40.Seldin L., Poulson N.D., Foote H.P., Lechler T. NuMA localization, stability and function in spindle orientation involves 4.1 and Cdk1 interactions. Mol. Biol. Cell. 2013;24:3651–3662. doi: 10.1091/mbc.E13-05-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo P.C., Tsao Y.P., Chang H.W., Chen P.H., Huang C.W., Lin S.T., Weng Y.T., Tsai T.C., Shieh S.Y., Chen S.L. Breast cancer amplified sequence 2, a novel negative regulator of the p53 tumor suppressor. Cancer Res. 2009;69:8877–8885. doi: 10.1158/0008-5472.CAN-09-2023. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y.C., Schmitt M., Yang Z., Que L.G., Stewart J.C., Frampton M.W., Devlin R.B. Gene expression profile in circulating mononuclear cells after exposure to ultrafine carbon particles. Inhal. Toxicol. 2010;22:835–846. doi: 10.3109/08958378.2010.486419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tategu M., Nakagawa H., Hayashi R., Yoshida K. Transcriptional co-factor CDCA4 participates in the regulation of JUN oncogene expression. Biochimie. 2008;90:1515–1522. doi: 10.1016/j.biochi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Shen T., Huang S. The role of Cdc25A in the regulation of cell proliferation and apoptosis. Anticancer Agents Med. Chem. 2012;12:631–639. doi: 10.2174/187152012800617678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trub T., Frantz J.D., Miyazaki M., Band H., Shoelson S.E. The role of a lymphoid-restricted, Grb2-like SH3-SH2-SH3 protein in T cell receptor signaling. J. Biol. Chem. 1997;272:894–902. doi: 10.1074/jbc.272.2.894. [DOI] [PubMed] [Google Scholar]
- 46.Matsuki H., Takahashi M., Higuchi M., Makokha G.N., Oie M., Fujii M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells. 2013;18:135–146. doi: 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- 47.Salomoni P., Khelifi A.F. DAXX: Death or survival protein? Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Kamimura J., Wakui K., Kadowaki H., Watanabe Y., Miyake K., Harada N., Sakamoto M., Kinoshita A., Yoshiura K., Ohta T., et al. The IHPK1 gene is disrupted at the 3p21.31 breakpoint of t(3;9) in a family with type 2 diabetes mellitus. J. Hum. Genet. 2004;49:360–365. doi: 10.1007/s10038-004-0158-z. [DOI] [PubMed] [Google Scholar]
- 49.Song C., Zhu S., Wu C., Kang J. Histone Deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 Expression. J. Biol. Chem. 2013;288:28021–28033. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Kandari W., Jambunathan S., Navalgund V., Koneni R., Freer M., Parimi N., Mudhasani R., Fontes J.D. ZXDC, a novel zinc finger protein that binds CIITA and activates MHC gene transcription. Mol. Immunol. 2007;44:311–321. doi: 10.1016/j.molimm.2006.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machesky L.M., Johnston S.A. MIM: A multifunctional scaffold protein. J. Mol. Med. 2007;85:569–576. doi: 10.1007/s00109-007-0207-0. [DOI] [PubMed] [Google Scholar]
- 52.Else T., Trovato A., Kim A.C., Wu Y., Ferguson D.O., Kuick R.D., Lucas P.C., Hammer G.D. Genetic p53 deficiency partially rescues the adrenocortical dysplasia phenotype at the expense of increased tumorigenesis. Cancer Cell. 2009;15:465–476. doi: 10.1016/j.ccr.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boman A.L., Zhang C., Zhu X., Kahn R.A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura T., Jenkins N.A., Copeland N.G. Identification of a new family of Pbx-related homeobox genes. Oncogene. 1996;13:2235–2242. [PubMed] [Google Scholar]
- 55.Wong M.H., Hermiston M.L., Syder A.J., Gordon J.I. Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc. Natl. Acad. Sci. USA. 1996;93:9588–9593. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katoh M. Identification and characterization of mouse Ppfia1 gene in silico. Int. J. Mol. Med. 2003;12:263–267. [PubMed] [Google Scholar]
- 57.Narkis G., Ofir R., Landau D., Manor E., Volokita M., Hershkowitz R., Elbedour K., Birk O.S. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am. J. Hum. Genet. 2007;81:530–539. doi: 10.1086/520771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miki T., Takano K., Yoneda Y. The role of mammalian Staufen on mRNA traffic: A view from its nucleocytoplasmic shuttling function. Cell Struct. Funct. 2005;30:51–56. doi: 10.1247/csf.30.51. [DOI] [PubMed] [Google Scholar]
- 59.Geetha T., Vishwaprakash N., Sycheva M., Babu J.R. Sequestosome 1/p62: Across diseases. Biomarkers. 2012;17:99–103. doi: 10.3109/1354750X.2011.653986. [DOI] [PubMed] [Google Scholar]
- 60.Rigden D.J., Liu H., Hayes S.D., Urbe S., Clague M.J. Ab initio protein modelling reveals novel human MIT domains. FEBS Lett. 2009;583:872–878. doi: 10.1016/j.febslet.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Huang K., Nair A.K., Muller Y.L., Piaggi P., Bian L., del Rosario M., Knowler W.C., Kobes S., Hanson R.L., Bogardus C., et al. Whole exome sequencing identifies variation in CYB5A and RNF10 associated with adiposity and type 2 diabetes. Obesity. 2013 doi: 10.1002/oby.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuang Z., Yao S., Xu Y., Lewis R.S., Low A., Masters S.L., Willson T.A., Kolesnik T.B., Nicholson S.E., Garrett T.J., et al. SPRY domain-containing SOCS box protein 2: Crystal structure and residues critical for protein binding. J. Mol. Biol. 2009;386:662–674. doi: 10.1016/j.jmb.2008.12.078. [DOI] [PubMed] [Google Scholar]
- 63.Svotelis A., Bianco S., Madore J., Huppe G., Nordell-Markovits A., Mes-Masson A.M., Gevry N. H3K27 demethylation by JMJD3 at a poised enhancer of anti-apoptotic gene BCL2 determines ERalpha ligand dependency. EMBO J. 2011;30:3947–3961. doi: 10.1038/emboj.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Privalsky M.L. Regulation of SMRT and N-CoR corepressor function. Curr. Top. Microbiol. Immunol. 2001;254:117–136. doi: 10.1007/978-3-662-10595-5_6. [DOI] [PubMed] [Google Scholar]
- 65.Marangos P.J., Schmechel D. The neurobiology of the brain enolases. Essays Neurochem. Neuropharmacol. 1980;4:211–247. [PubMed] [Google Scholar]
- 66.Topfer-Petersen E., Cechova D., Henschen A., Steinberger M., Friess A.E., Zucker A. Cell biology of acrosomal proteins. Andrologia. 1990;22:110–121. doi: 10.1111/j.1439-0272.1990.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 67.Kramer B.F., Schoor O., Kruger T., Reichle C., Muller M., Weinschenk T., Hennenlotter J., Stenzl A., Rammensee H.G., Stevanovic S. MAGED4-expression in renal cell carcinoma and identification of an HLA-A*25-restricted MHC class I ligand from solid tumor tissue. Cancer Biol. Ther. 2005;4:943–948. doi: 10.4161/cbt.4.9.1907. [DOI] [PubMed] [Google Scholar]
- 68.Kuwahara K., Takano M., Nakao K. Pathophysiological significance of T-type Ca2+ channels: Transcriptional regulation of T-type Ca2+ channel—Regulation of CACNA1H by neuron-restrictive silencer factor. J. Pharmacol. Sci. 2005;99:211–213. doi: 10.1254/jphs.FMJ05002X4. [DOI] [PubMed] [Google Scholar]
- 69.Shahbazian D., Parsyan A., Petroulakis E., Hershey J., Sonenberg N. eIF4B controls survival and proliferation and is regulated by proto-oncogenic signaling pathways. Cell Cycle. 2010;9:4106–4109. doi: 10.4161/cc.9.20.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonini N.M., Gitler A.D. Model organisms reveal insight into human neurodegenerative disease:Ataxin-2 intermediate-length polyglutamine expansions are a risk factor for ALS. J. Mol. Neurosci. 2011;45:676–683. doi: 10.1007/s12031-011-9548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutkowski D.T., Kaufman R.J. All roads lead to ATF4. Dev. Cell. 2003;4:442–444. doi: 10.1016/S1534-5807(03)00100-X. [DOI] [PubMed] [Google Scholar]
- 72.Bernier F., Soucy P., Luu-The V. Human phenol sulfotransferase gene contains two alternative promoters: Structure and expression of the gene. DNA Cell Biol. 1996;15:367–375. doi: 10.1089/dna.1996.15.367. [DOI] [PubMed] [Google Scholar]
- 73.Bosley T.M., Salih M.A., Jen J.C., Lin D.D., Oystreck D., Abu-Amero K.K., MacDonald D.B., al Zayed Z., al Dhalaan H., Kansu T., et al. Neurologic features of horizontal gaze palsy and progressive scoliosis with mutations in ROBO3. Neurology. 2005;64:1196–1203. doi: 10.1212/01.WNL.0000156349.01765.2B. [DOI] [PubMed] [Google Scholar]
- 74.Du P., Ye L., Li H., Yang Y., Jiang W.G. The tumour suppressive role of metastasis suppressor-1, MTSS1, in human kidney cancer, a possible connection with the SHH pathway. J. Exp. Ther. Oncol. 2012;10:91–99. [PubMed] [Google Scholar]
- 75.Lu P., Hankel I.L., Hostager B.S., Swartzendruber J.A., Friedman A.D., Brenton J.L., Rothman P.B., Colgan J.D. The developmental regulator protein Gon4l associates with protein YY1, co-repressor Sin3a, and histone deacetylase 1 and mediates transcriptional repression. J. Biol. Chem. 2011;286:18311–18319. doi: 10.1074/jbc.M110.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McPherson J.P., Sarras H., Lemmers B., Tamblyn L., Migon E., Matysiak-Zablocki E., Hakem A., Azami S.A., Cardoso R., Fish J., et al. Essential role for Bclaf1 in lung development and immune system function. Cell Death Differ. 2009;16:331–339. doi: 10.1038/cdd.2008.167. [DOI] [PubMed] [Google Scholar]
- 77.Yi F., Pereira L., Merrill B.J. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fong N.M., Jensen T.C., Shah A.S., Parekh N.N., Saltiel A.R., Brady M.J. Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J. Biol. Chem. 2000;275:35034–35039. doi: 10.1074/jbc.M005541200. [DOI] [PubMed] [Google Scholar]
- 79.Ross S.E., McCord A.E., Jung C., Atan D., Mok S.I., Hemberg M., Kim T.K., Salogiannis J., Hu L., Cohen S., et al. Bhlhb5 and Prdm8 form a repressor complex involved in neuronal circuit assembly. Neuron. 2012;73:292–303. doi: 10.1016/j.neuron.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honore B., Rasmussen H.H., Vorum H., Dejgaard K., Liu X., Gromov P., Madsen P., Gesser B., Tommerup N., Celis J.E. Heterogeneous nuclear ribonucleoproteins H, H’, and F are members of a ubiquitously expressed subfamily of related but distinct proteins encoded by genes mapping to different chromosomes. J. Biol. Chem. 1995;270:28780–28789. doi: 10.1074/jbc.270.48.28780. [DOI] [PubMed] [Google Scholar]
- 81.Keegan K., Johnson D.E., Williams L.T., Hayman M.J. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc. Natl. Acad. Sci. USA. 1991;88:1095–1099. doi: 10.1073/pnas.88.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaiswal B.S., Kljavin N.M., Stawiski E.W., Chan E., Parikh C., Durinck S., Chaudhuri S., Pujara K., Guillory J., Edgar K.A., et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013;23:603–617. doi: 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Mishima Y., Miyagi S., Saraya A., Negishi M., Endoh M., Endo T.A., Toyoda T., Shinga J., Katsumoto T., Chiba T., et al. The Hbo1-Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood. 2011;118:2443–2453. doi: 10.1182/blood-2011-01-331892. [DOI] [PubMed] [Google Scholar]
- 84.Hatakeyama S. TRIM proteins and cancer. Nat. Rev. Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 85.Wiemann S., Weil B., Wellenreuther R., Gassenhuber J., Glassl S., Ansorge W., Bocher M., Blocker H., Bauersachs S., Blum H., et al. Toward a catalog of human genes and proteins: Sequencing and analysis of 500 novel complete protein coding human cDNAs. Genome Res. 2001;11:422–435. doi: 10.1101/gr.GR1547R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maciejewski J.P., Padgett R.A. Defects in spliceosomal machinery: A new pathway of leukaemogenesis. Br. J. Haematol. 2012;158:165–173. doi: 10.1111/j.1365-2141.2012.09158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sauve F., McBroom L.D., Gallant J., Moraitis A.N., Labrie F., Giguere V. CIA, a novel estrogen receptor coactivator with a bifunctional nuclear receptor interacting determinant. Mol. Cell Biol. 2001;21:343–353. doi: 10.1128/MCB.21.1.343-353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo S.H., Weisberg E., Chen L.B. Tensin: A potential link between the cytoskeleton and signal transduction. Bioessays. 1994;16:817–823. doi: 10.1002/bies.950161108. [DOI] [PubMed] [Google Scholar]
- 89.Roubin R., Acquaviva C., Chevrier V., Sedjai F., Zyss D., Birnbaum D., Rosnet O. Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol. Open. 2013;2:238–250. doi: 10.1242/bio.20123392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishov A.M., Vladimirova O.V., Maul G.G. Daxx-mediated accumulation of human cytomegalovirus tegument protein pp71 at ND10 facilitates initiation of viral infection at these nuclear domains. J. Virol. 2002;76:7705–7712. doi: 10.1128/JVI.76.15.7705-7712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morin R.D., Mendez-Lago M., Mungall A.J., Goya R., Mungall K.L., Corbett R.D., Johnson N.A., Severson T.M., Chiu R., Field M., et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seimiya H., Smith S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182) J. Biol. Chem. 2002;277:14116–14126. doi: 10.1074/jbc.M112266200. [DOI] [PubMed] [Google Scholar]
- 93.Kuuselo R., Savinainen K., Azorsa D.O., Basu G.D., Karhu R., Tuzmen S., Mousses S., Kallioniemi A. Intersex-like (IXL) is a cell survival regulator in pancreatic cancer with 19q13 amplification. Cancer Res. 2007;67:1943–1949. doi: 10.1158/0008-5472.CAN-06-3387. [DOI] [PubMed] [Google Scholar]
- 94.Geiman D.E., Ton-That H., Johnson J.M., Yang V.W. Transactivation and growth suppression by the gut-enriched Kruppel-like factor (Kruppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction. Nucleic Acids Res. 2000;28:1106–1113. doi: 10.1093/nar/28.5.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong P.M., Puente C., Ganley I.G., Jiang X. The ULK1 complex: Sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayerhofer P.U., Kattenfeld T., Roscher A.A., Muntau A.C. Two splice variants of human PEX19 exhibit distinct functions in peroxisomal assembly. Biochem. Biophys. Res. Commun. 2002;291:1180–1186. doi: 10.1006/bbrc.2002.6568. [DOI] [PubMed] [Google Scholar]
- 97.Kierdorf K., Fritz G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 98.Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell. Signal. 1999;11:777–788. doi: 10.1016/S0898-6568(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 99.Martignetti J.A., Aqeel A.A., Sewairi W.A., Boumah C.E., Kambouris M., Mayouf S.A., Sheth K.V., Eid W.A., Dowling O., Harris J., et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat. Genet. 2001;28:261–265. doi: 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- 100.Stagno S., Pass R.F., Cloud G., Britt W.J., Henderson R.E., Walton P.D., Veren D.A., Page F., Alford C.A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–1908. doi: 10.1001/jama.1986.03380140074025. [DOI] [PubMed] [Google Scholar]
- 101.Revello M.G., Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 2002;15:80–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Revello M.G., Zavattoni M., Furione M., Lilleri D., Gorini G., Gerna G. Diagnosis and outcomeof preconceptional and periconceptional primary human cytomegalovirus infections. J. Infect. Dis. 2002;186:553–557. doi: 10.1086/341831. [DOI] [PubMed] [Google Scholar]
- 103.Ramsay M.E., Miller E., Peckham C.S. Outcome of confirmed symptomatic congenital cytomegalovirus infection. Arch. Dis. Child. 1991;66:1068–1069. doi: 10.1136/adc.66.9.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chou S., Marousek G., Guentzel S., Follansbee S.E., Poscher M.E., Lalezari J.P., Miner R.C., Drew W.L. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J. Infect. Dis. 1997;176:786–789. doi: 10.1086/517302. [DOI] [PubMed] [Google Scholar]
- 105.Anderson K.P., Fox M.C., Brown-Driver V., Martin M.J., Azad R.F. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob. Agents Chemother. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith J.A., Pari G.S. Expression of human cytomegalovirus UL36 and UL37 genes is required for viral DNA replication. J. Virol. 1995;69:1925–1931. doi: 10.1128/jvi.69.3.1925-1931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munch K., Messerle M., Plachter B., Koszinowski U.H. An acidic region of the 89K murine cytomegalovirus immediate early protein interacts with DNA. J. Gen. Virol. 1992;73:499–506. doi: 10.1099/0022-1317-73-3-499. [DOI] [PubMed] [Google Scholar]