Abstract
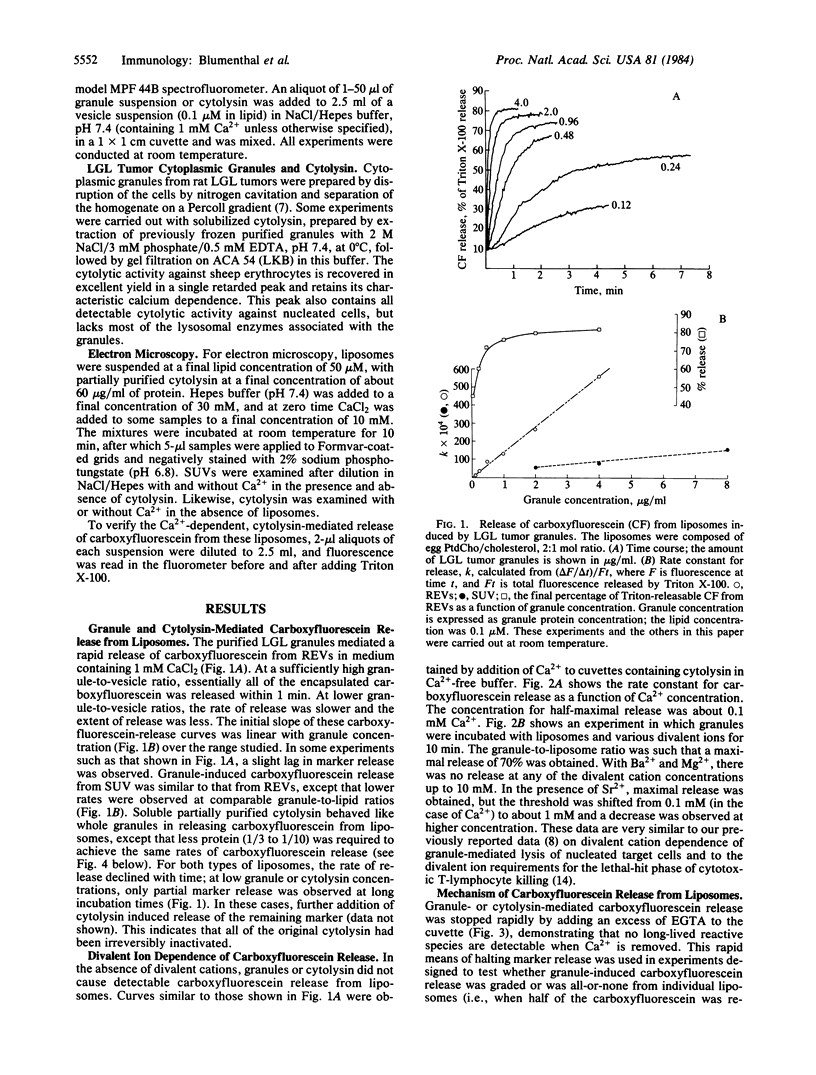
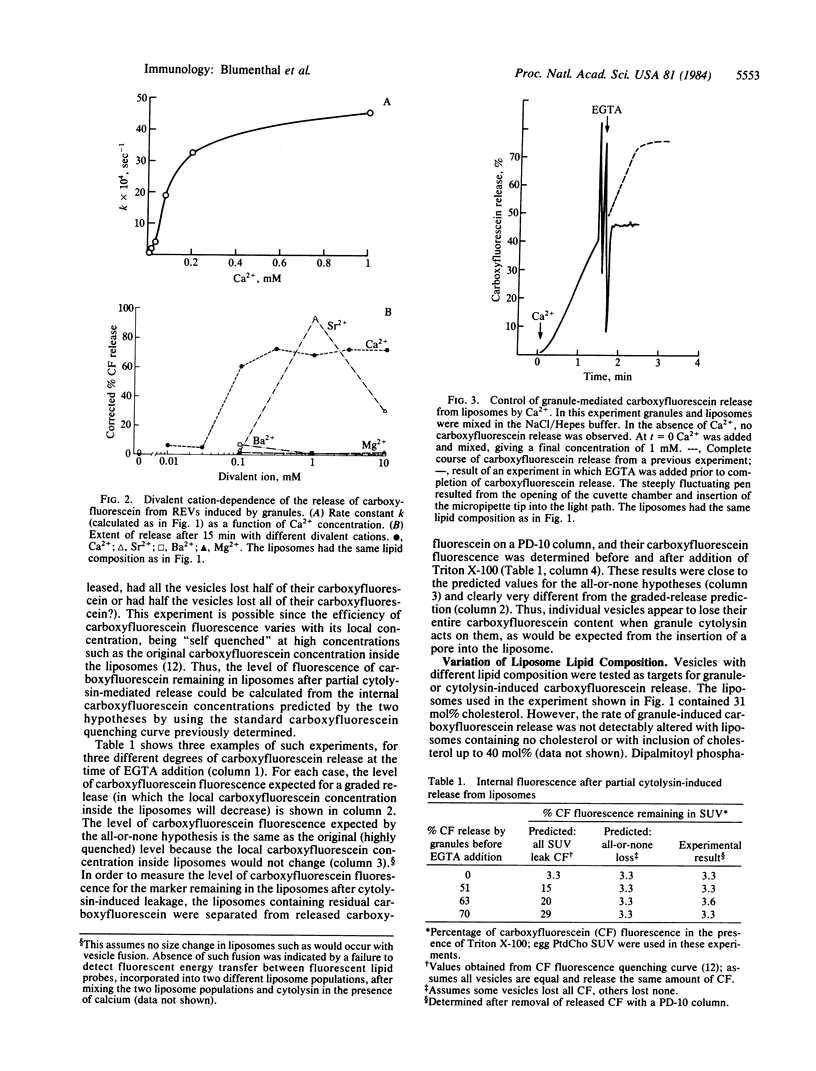
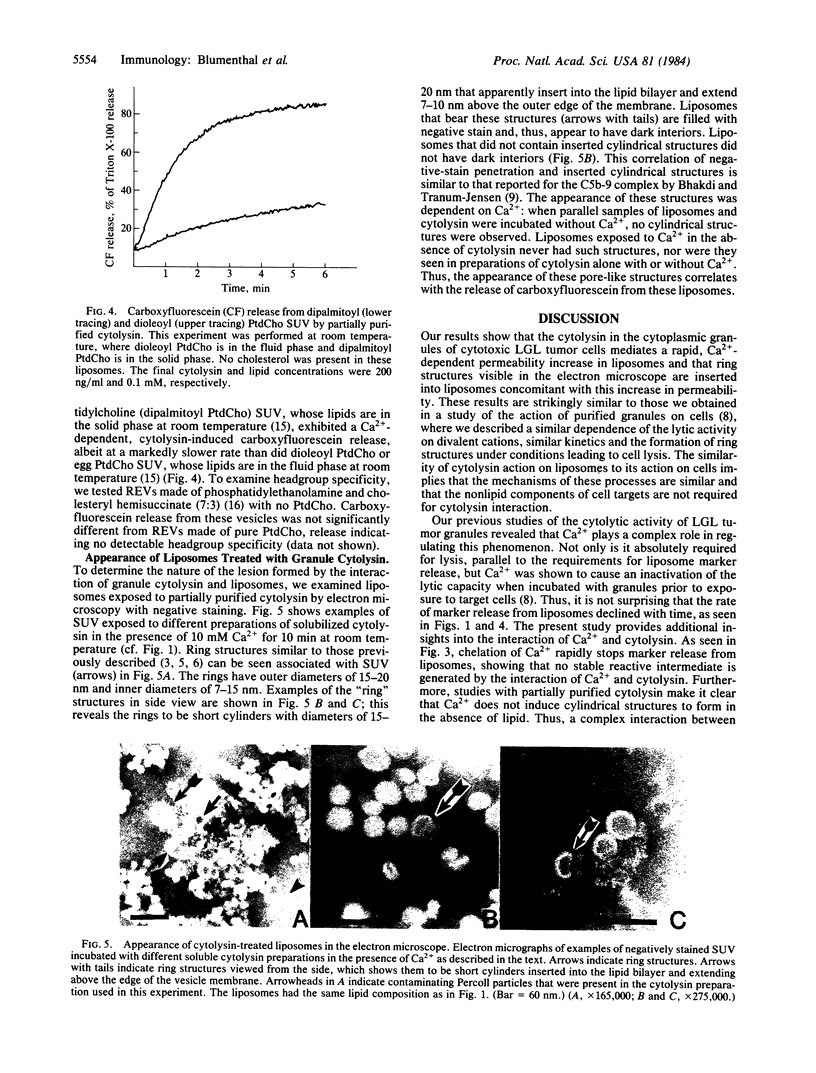
Purified cytoplasmic granules from rat large granular lymphocyte tumors having natural killer activity and/or antibody-dependent cell-mediated cytotoxicity induced a rapid, dose-dependent release of the water-soluble marker carboxyfluorescein from liposomes made of phosphatidylcholine. A solubilized, partially purified cytolytic preparation termed "cytolysin" from these granules showed identical properties. Marker release induced by granules or the cytolysin was strongly dependent on the presence of Ca2+ at a concentration of 0.1 mM or higher in the medium; Ca2+ could be replaced by higher concentration of Sr2+ but not by Ba2+ or by Mg2+. These properties strikingly parallel the lytic effects that granules and granule cytolysin exert on cells. Marker release from liposomes was stopped instantaneously when an excess of EGTA was added to the medium. The remaining carboxyfluorescein inside the liposomes was present at the original internal concentration, indicating that marker release was all-or-none from individual liposomes. Liposomes comprised of lipid in the solid phase released marker more slowly than did comparable liposomes containing fluid-phase lipids. Variation of the lipid headgroup had only minor effects on the cytolysin-induced marker release. Electron microscopy of liposomes exposed to cytolysin in the presence of Ca2+ showed cylindrical structures of 15-nm diameter inserted into the membrane concomitant with the penetration of negative stain into the liposome. These properties of large granular lymphocyte granule cytolysin strongly suggest that it operates through a mechanism similar to the membrane attack of complement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Dennert G., Podack E. R. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J Exp Med. 1983 May 1;157(5):1483–1495. doi: 10.1084/jem.157.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin R. R., Deteix P., Simone C. B., Henkart P. Electron microscopic demonstration of lesions in target cell membranes associated with antibody-dependent cellular cytotoxicity. Clin Exp Immunol. 1980 Dec;42(3):554–560. [PMC free article] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. III. Resolution of two distinct roles for calcium in the cytolytic process. J Immunol. 1979 Feb;122(2):482–489. [PubMed] [Google Scholar]
- Hanspal M., Ralston G. B. Purification of a trypsin-insensitive fragment of spectrin from human erythrocyte membranes. Biochim Biophys Acta. 1981 Jul 28;669(2):133–139. doi: 10.1016/0005-2795(81)90234-8. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P., Blumenthal R. Interaction of lymphocytes with lipid bilayer membranes: a model for lymphocyte-mediated lysis of target cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2789–2793. doi: 10.1073/pnas.72.7.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C., Nicolotti R. A. Immunological properties of model membranes. Annu Rev Biochem. 1977;46:49–67. doi: 10.1146/annurev.bi.46.070177.000405. [DOI] [PubMed] [Google Scholar]
- Mayer M. M. Presidential address to the American Association of Immunologists, delivered in Chicago, Illinois, April 6, 1977. Mechanism of cytolysis by lymphocytes: A comparison with complement. J Immunol. 1977 Oct;119(4):1195–1203. [PubMed] [Google Scholar]
- Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984 Jun;132(6):3197–3204. [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Simone C. B., Henkart P. Permeability changes induced in erthrocyte ghost targets by antibody-dependent cytotoxic effector cells: evidence for membrane pores. J Immunol. 1980 Feb;124(2):954–963. [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Klausner R. D., Innerarity T., Ralston E., Blumenthal R. Phase transition release, a new approach to the interaction of proteins with lipid vesicles. Application to lipoproteins. Biochim Biophys Acta. 1981 Oct 2;647(2):270–284. doi: 10.1016/0005-2736(81)90255-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]



