Abstract
Puumala virus (PUUV) causes mild to moderate cases of haemorrhagic fever with renal syndrome (HFRS), and is responsible for the majority of hantavirus infections of humans in Fennoscandia, Central and Western Europe. Although there are relatively many PUUV sequences available from different European countries, little is known about the presence of this virus in Poland. During population studies in 2009 a total of 45 bank voles were trapped at three sites in north-eastern Poland, namely islands on Dejguny and Dobskie Lakes and in a forest near Mikołajki. S and M segment-specific RT-PCR assays detected PUUV RNA in three animals from the Mikołajki site. The obtained partial S and M segment sequences demonstrated the highest similarity to the corresponding segments of a PUUV strain from Latvia. Analysis of chest cavity fluid samples by IgG ELISA using a yeast-expressed PUUV nucleocapsid protein resulted in the detection of two seropositive samples, both being also RT-PCR positive. Interestingly, at the trapping site in Mikołajki PUUV-positive bank voles belong to the Carpathian and Eastern genetic lineages within this species. In conclusion, we herein present the first molecular evidence for PUUV in the rodent reservoir from Poland.
Keywords: Puumala virus, Poland, bank vole, Clethrionomys (Myodes) glareolus
1. Introduction
Hantaviruses, family Bunyaviridae, are enveloped viruses with a trisegmented RNA genome of negative polarity [1]. These zoonotic viruses have been initially believed to be exclusively rodent-borne. However, in recent years a large number of novel hantaviruses has been identified in shrews, moles and bats [2]. Human disease seems to be caused exclusively by certain rodent-borne hantaviruses. Their transmission to humans is mediated via inhalation of aerosolized excreta from persistently infected rodent hosts. Following the distribution of the causative hantaviruses, two different clinical syndromes have been described in the Old World and New World. The Hantavirus Cardiopulmonary Syndrome (HCPS) in the Americas is characterized by a very high case fatality rate of up to 35%, whereas the case fatality rate of the Haemorrhagic Fever with Renal Syndrome (HFRS) in Europe and Asia ranges from 0.1% to 15% [3,4].
In Europe, hantavirus infections are, for a long time, found in Fennoscandia, Russia and the Balkan region. Currently, hantavirus infections have been detected with highly variable annual case numbers in human patients and were identified in different reservoir species in most of Europe [5]. In certain parts of Europe hantavirus infections are frequently reported, e.g., the central and eastern parts of Finland, the northern part of Sweden, the Ardennes region in Belgium and France, the southern, western and northwestern part of Germany, the Balkans and certain regions of European Russia. In addition, for some parts of Europe, e.g., Baltic countries, Hungary and Greece, a high seroprevalence was contrasted by a low number of reported HFRS cases. For further countries, e.g., UK and Ukraine, no comprehensive epidemiological data are available [6].
Puumala virus (PUUV) causes the vast majority of human hantavirus infections in Fennoscandia, Central and Western Europe. Although severe and even a few fatal human cases have been reported, PUUV causes usually in humans a mild to moderate form of HFRS called nephropathia epidemica with a very low case fatality rate [7,8,9]. The bank vole Clethrionomys glareolus (Myodes glareolus) [10] represents the reservoir of this hantavirus [11]. Closely related viruses have been identified in other Clethrionomys species in Asia [12,13]. Consistent with the distribution of the bank vole in the main parts of continental Europe PUUV was detected in most European countries including Finland, north Sweden, Norway, Germany, Belgium, Luxembourg, France, the Netherlands, Austria, Slovakia, Latvia, Estonia, Lithuania, Slovenia, Croatia, Bosnia and Hercegovina, Greece and the European part of Russia [7,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. An oscillation of the recorded number of human cases in previous years has been reported for Belgium, France and Germany, and was postulated to be caused by a mass reproduction of the bank vole following beech mast years [31].
Little is known about the presence of human pathogenic hantaviruses in Poland, although the reservoirs of PUUV (bank vole) and Dobrava-Belgrade virus, genotype Kurkino (striped field mouse Apodemus agrarius) are present in large areas of the country [32]. In Poland, hantavirus-caused infections are notifiable under the national epidemiological surveillance system since 2007, but the number of officially recorded cases remains low: 3–9 cases per year (which includes also cases related to hantaviruses other than PUUV) [33]. In addition, human cases were exclusively identified by serological investigations without molecular characterization [34,35,36]. A serologic survey among mammalogists revealed higher reactivity with PUUV antigen than with Hantaan virus (HTNV) antigen indicating that these persons had rather contact with PUUV or the related Tula virus (TULV) than with HTNV-related DOBV [37]. In addition, hantavirus-specific antibodies have been detected in a high-risk group such as forest workers in north-eastern Poland [38]. In contrast, few molecular studies in reservoirs confirmed the presence of hantaviruses in Poland. TULV strain Lodz was isolated in cell culture from tissue samples of the common vole Microtus arvalis trapped in a region 53 km south and 104 km west of Warsaw, where human and animal hantavirus infections were not reported [39]. In addition, a molecular survey of 60 small mammals in eastern Poland revealed the presence of hantaviruses in four species: the prevalence was in A. agrarius (n = 39) 2.6%, and in Microtus agrestis (n = 1), C. glareolus (n = 5), Sorex araneus (n = 8) 12.5%–100%. The L segment sequence analysis of 5 samples revealed nucleotide sequence identities of 77%–86% to hantavirus sequences of Fusong-Mf-731 detected in Microtus fortis from China [40]. Recently, a novel shrew-borne hantavirus (Boginia virus) was identified in the Eurasian water shrew Neomys fodiens in central Poland [41].
In this study bank voles trapped in north-eastern Poland were investigated by serology and RT-PCR for the presence of PUUV infections.
2. Results and Discussion
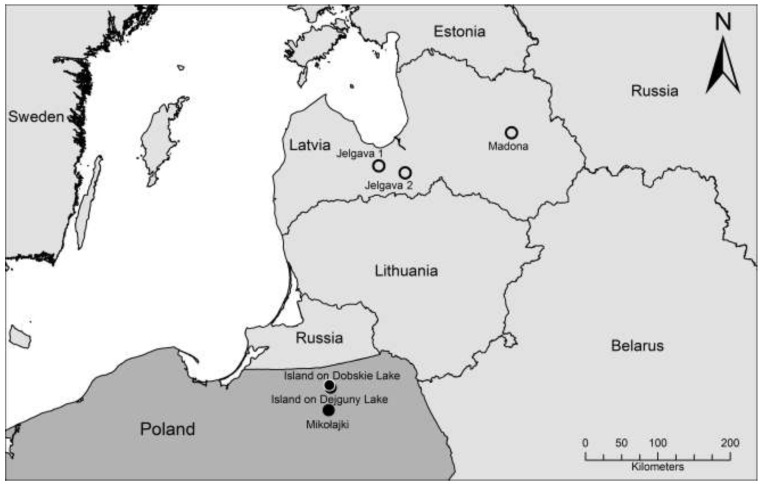
During August 2009 a total of 45 bank voles were trapped in the north-eastern part of Poland. The trapping sites were located in a forest close to Mikołajki with a distance of ca. 30 and 35 km, respectively, to the other two trapping sites on islands on Dejguny and Dobskie Lakes (Figure 1). Serological screening of chest cavity fluid from 44 of 45 bank voles by an in-house IgG enzyme-linked immunosorbent assay (ELISA) using a yeast-expressed PUUV nucleocapsid protein revealed two seropositive samples, both from animals trapped in Mikołajki forest (Table 1).
Figure 1.
Map of the north-eastern part of Poland and the surrounding countries showing the three trapping sites of bank voles in Poland described here and the three localities in Latvia where related PUUV sequences were detected [42].
Table 1.
Results of the serological and S segment RT-PCR investigations of bank voles trapped at three sites in Poland.
| Trapping site | Number of positive/total number of investigated animals | |
|---|---|---|
| Serology | S-RT-PCR | |
| Mikołajki forest | 2/15 * | 3/16 |
| Dobskie island | 0/16 | 0/16 |
| Dejguny island | 0/13 | 0/13 |
* from a single animal no chest cavity fluid for serology was available.
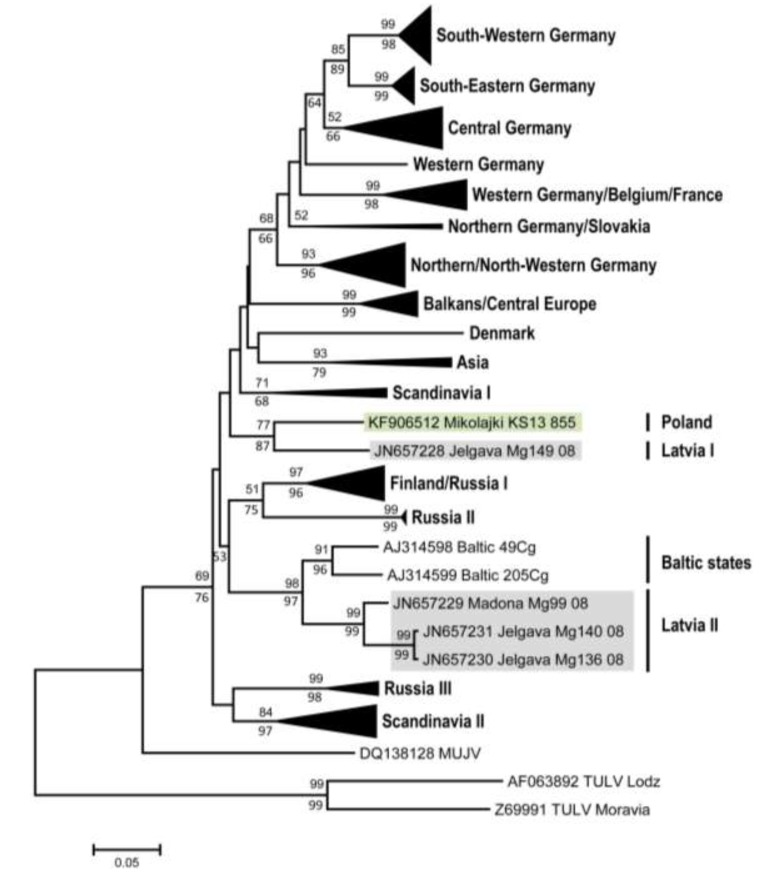
Subsequent screening of lung tissue by PUUV/TULV S segment RT-PCR test revealed three positive samples from male bank voles (with two being seropositive and one being seronegative) from the forest near Mikołajki (Table 1). Cloning of the amplification products and sequencing confirmed identical sequences of 711 nucleotides length for all three samples (accession number KF906512). A BLASTn search identified the sequence as a PUUV sequence with the highest similarity to a PUUV sequence derived from a bank vole originated from Jelgava, Latvia (Mg149/2008; JN657228; Jelgava 1, Figure 1). A phylogenetic analysis of this novel S segment sequence confirmed its closest similarity to this sequence from Jelgava 1 and showed clear a distinctness from other PUUV sequences in the Baltic region, Russia or Germany (Figure 2). The nucleotide sequence divergence of the novel sequence from Poland to the sequence from Jelgava 1 was 11.2%, whereas the sequence divergence to other two sequences from Latvia ranged from 17.5% to 19.2% (Table 2). The amino acid sequences of the novel strain and that from Jelgava 1 were identical, but the corresponding amino acid sequences from the other sites in Latvia varied by 4.6% to 5.2%. The nucleotide sequence divergence of the Mikołajki sequence to representative sequences covering the distributional range of PUUV in Europe reached 14.0% to 21.3%.
Figure 2.
Phylogenetic relationships of Puumala virus sequences based on 465 nt of the S segment spanning positions 436–900 (numbering based on strain Sotkamo, accession number NC_005224) with Tula virus strains Lodz AF063892 and Moravia Z69991 as outgroup. The identical novel S segment sequence from the three bank voles (KS13/855, KS13/856 and KS13/861) in Mikołajki (Poland) and relevant sequences from Latvia (see Figure 1) are highlighted. Other geographical clusters of closely related sequences were condensed to triangles with sizes proportional to sequence numbers. Support values for Neighbor-Joining or Bayesian phylogenetic analyses are reported above/below branches of main nodes if they exceeded 50 percent.
Table 2.
Nucleotide (above the diagonal) and amino acid sequence identity of the novel S segment and nucleocapsid protein sequence from Mikołajki with corresponding sequences from Latvia, Croatia, Finland, Sweden, Germany and Denmark.
| PUUV strains | Mikołajki | Jelgava 1 | Jelgava 2 | Madona | Gerovo | Konnevesi | Vindeln | Bavaria | Fyn |
|---|---|---|---|---|---|---|---|---|---|
| KS13 855 | Mg149 08 | Mg136 08 | Mg99 08 | Mg979 08 | Mg M114B 05 | L20Cg 83 | Mu CG 9 04 | 19 | |
| Mikołajki KS13 855 | 0.888 | 0.808 | 0.825 | 0.827 | 0.860 | 0.819 | 0.819 | 0.787 | |
| Jelgava 1 Mg149 08 | 1 | 0.832 | 0.823 | 0.817 | 0.843 | 0.832 | 0.819 | 0.817 | |
| Jelgava 2 Mg136 08 | 0.948 | 0.948 | 0.948 | 0.789 | 0.806 | 0.806 | 0.780 | 0.782 | |
| Madona Mg99 08 | 0.954 | 0.954 | 0.980 | 0.780 | 0.812 | 0.812 | 0.797 | 0.776 | |
| Gerovo Mg979 08 | 0.954 | 0.954 | 0.922 | 0.935 | 0.821 | 0.810 | 0.825 | 0.800 | |
| Konnevesi Mg M114B 05 | 0.967 | 0.967 | 0.941 | 0.961 | 0.948 | 0.832 | 0.815 | 0.787 | |
| Vindeln L20Cg 83 | 0.967 | 0.967 | 0.935 | 0.954 | 0.935 | 0.948 | 0.817 | 0.819 | |
| Bavaria Mu CG 9 04 | 0.974 | 0.974 | 0.935 | 0.948 | 0.961 | 0.961 | 0.948 | 0.791 | |
| Fyn 19 | 0.961 | 0.961 | 0.922 | 0.935 | 0.935 | 0.935 | 0.941 | 0.954 |
For two of the three S segment positive lung tissue samples a M segment RT-PCR amplification product of the expected size was obtained. Interestingly, both samples originated from the seropositive animals (KS13/855; KS13/856). The M segment sequences from both samples were found to be identical (accession number KF906513; length 618 nucleotides). Pairwise comparisons of a 177 nt-long segment of this sequence showed a 82.4% and 83% sequence identity to sequences from Latvia (Jelgava 1/Mg 149; JN657233) and Croatia (Gerovo Mg982; KC676635), respectively (data not shown). The corresponding amino acid sequence of 59 residues length was found to be identical to sequences from Jelgava 1 and Bavaria or showed a divergence of 5.1%–11.9% to other European PUUV strains (data not shown).
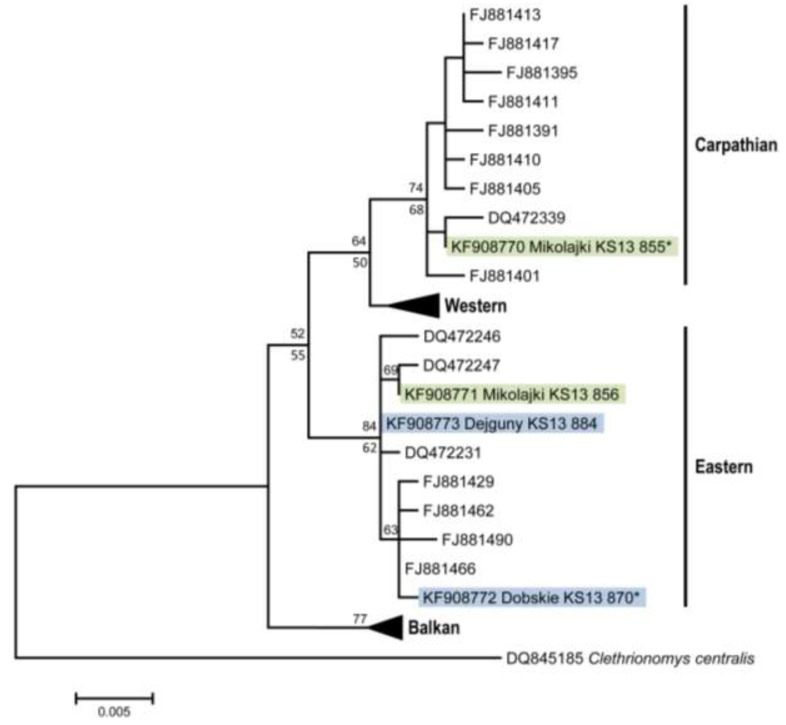
Phylogenetic analysis of cytochrome b sequences from the three PUUV-positive bank voles from Mikołajki forest and three animals from the island populations revealed the presence of representatives of the Carpathian and Eastern genetic lineages within the species (Figure 3). Interestingly, two of three PUUV-RNA-positive animals from the forest population belong to the Carpathian lineage, whereas the third one belongs to the Eastern lineage. The detection of both bank vole lineages in this region is in line with previous reports of a contact zone between these lineages in Poland [43]. The animals from the island populations belong to the Eastern lineage.
Figure 3.
Phylogenetic relationships of cytochrome b sequences (843 nt) in Clethrionomys glareolus from the study region (highlighted) relative to major phylogeographic lineages (Carpathian, Western, Eastern, Balkan; [43]) within the species. Representative sequences from all phylogeographic lineages present in the larger region (see [43]) were obtained from GenBank, and Clethrionomys (Myodes) centralis was used as outgroup. Clusters of phylogeographic lineages without novel sequences from Poland in this study were condensed to triangles with sizes proportional to sequence numbers. Support values for Neighbor-Joining or Bayesian phylogenetic algorithms exceeding 50 percent are reported above/below main branches. * Identical sequences: KS13/855 = KS13/861; KS13/870 = KS13/868.
3. Experimental Section
3.1. Rodent Trapping and Dissection
Bank voles were trapped in August 2009 in Mazurian Lake Range (north-eastern Poland) at three sites (Figure 1): an island on Dejguny Lake (54°2'N, 21°37'E, area 9.3 ha, about 250 m from mainland), an island on Dobskie Lake (54°5'N, 21°36'E) and a forest near Mikołajki (53°46'N, 21°30'E). In each of the sites, live-traps were set for 3–5 days and were checked every morning. The trapped individuals were maintained temporarily in standard plastic mouse cages in groups of up to four same-sex individuals from the same population and then transported to the Institute of Environmental Sciences (Jagiellonian University, Kraków, Poland). Immediately after arrival in the laboratory (within 1–12 days after trapping), the animals were killed by decapitation and dissected. Kidney, liver and parts of auricle were stored for toxicological and molecular analyses and the remaining carcasses were stored at −75 °C. All the procedures were carried out according to EC Directive 86/609/EEC for animal experiments and were approved by the First Local Bioethical Committee in Kraków (decision # 48/2007). For the purpose of this study, 45 frozen carcasses were transferred in dry ice to the Friedrich-Loeffler-Institut (Greifswald - Insel Riems, Germany): 7 males and 6 females from Dejguny, 7 males and 9 females from Dobskie and 9 males and 7 females from Mikołajki. The lung tissue and tail samples of these 45 carcasses were collected according to a standard protocol; chest cavity fluid was obtained by addition of 1 mL sterile phosphate-buffered saline (PBS).
3.2. Serological Investigations
The chest cavity fluid of dissected bank voles was screened by an IgG ELISA based on a yeast expressed-nucleocapsid protein of PUUV strain Bavaria [44]. The ELISA was performed according to a previously established protocol [45].
3.3. Nucleic Acid Isolation, Hantavirus RT-PCR, cytochrome b PCR and Sequence Determination
RNA was extracted from lung tissue samples of bank voles using Qiazol solution (Qiagen, Hilden, Germany). The RT-PCR amplification follows previously published protocols for amplification of partial S and M segment sequences [44,45]. For amplification 2.5 µL of RNA using 10 pmol of primers (S segment: 342 forward, 1102 reverse; M segment: C1, C2) and SuperScript III one step RT-PCR kit (Invitrogen, Darmstadt, Germany) in a final volume of 25 µL were applied under the following amplification conditions: reverse transcription at 50 °C for 45 min, inactivation of reverse transcriptase at 94 °C for 2 min, 40 cycles of denaturation at 94 °C for 30 s, annealing at 46 °C (for S segment) and at 58.5 °C (for M segment) for 30 s, and elongation at 68 °C for 1 min, and final extension at 68 °C for 10 min.
Amplification products were run in agarose gels and visualized by UV illumination after ethidium bromide staining. RT-PCR products of expected size were cloned into vector pCR2.1-TOPO® (TopoTA cloning kit, Invitrogen). The plasmids were purified using QIAprep spin Miniprep kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany).
For cytochrome b PCR analysis, tail tip samples of 0.5 mm from selected animals were lysed over night at 56 °C and 400 rpm in 300 µL lysis buffer (50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.45% Nonidet P40, 0.45% Tween 20) containing 3 µL proteinase K (10 mg/mL). The cytochrome b-specific PCR was performed as described previously [46].
The plasmid DNAs and cytochrome b PCR products were sequenced using the BigDye® Terminator v1.1 Cycle Sequencing Kit (Perkin-Elmer, Waltham, MA, USA) on an ABI 310 Genetic Analyser (Applied Biosystems, Foster City, CA, USA). To prove a potential quasispecies structure 13, 13 and 18 S segment plasmids of the animals KS13/855, KS13/856 and KS13/861, respectively, and 14 and 4 M segment plasmids of KS13/855 and KS13/856, respectively, were sequenced.
3.4. Sequence Comparison and Phylogenetic Analyses
An initial comparison of the novel PUUV sequences with existing data was done using the BLAST search module [47].
For phylogenetic analyses of PUUV and cytochrome b sequences, published sequences were included in the analyses in order to cover the known genetic diversity and geographical distribution of PUUV and bank voles widely, which is analogous to previous investigations [44,48,49]. For the PUUV S segment, the 465 nt alignment contained 165 sequences including four sequences from Latvia (Madona Mg99, Jelgava Mg136, Jelgava Mg140, Jelgava Mg149 [42]), and sequences of TULV strains Lodz AF063892 and Moravia Z69991 as outgroup. For the M segment, the analyses covered 177 nt with a similar geographical spread of sequences and the same outgroup strains. For the identification of the genetic lineage of the bank voles, cytochrome b sequences of the lineages Eastern, Carpathian, Balkan and Western, which have been found previously in the larger region [43], were retrieved from GenBank. Phylogenetic analyses involved the estimation of the optimal nucleotide substitution model based on the Bayesian information criterion in Mega version 5.2 [50] before tree reconstruction with Neighbor-Joining (NJ) algorithms incorporated in Mega and Bayesian algorithms incorporated in MrBayes 3.1.2. [51]. For analyses of the PUUV S segment, the GTR model with gamma shape parameter and invariable sites was used with the estimated values, and for the cytochrome b sequences (843 nt) the T3P model with gamma parameter and a fraction of invariable sites was applied. For the NJ analyses, 5,000 bootstrap replicates were performed for each data set. Bayesian analyses were run two times for each dataset with one cold and three hot chains for 5 million generations with every 10th generation sampled. The first 25% of the samples were discarded as burn-in and convergence of chains was confirmed according to standard procedures (see [52] for details).
4. Conclusions
This study shows first molecular evidence of PUUV in the north-eastern part of Poland. The finding of PUUV-positive bank voles of the Carpathian and Eastern genetic lineage demonstrates that both genetic lineages, as also the Western lineage in Germany, Belgium and France [43,44], are susceptible to PUUV infection. In conclusion, the findings have implications for the awareness of the physicians in Poland and public health measures in north-eastern Poland. Future studies will have to test for potential associations of the PUUV prevalence and bank vole population dynamics, examine reasons for the low prevalence of PUUV in bank voles from north-eastern Poland and the influence on the frequency of human infections in this part of Poland.
Acknowledgments
We thank Renata Świergosz-Kowalewska for help with planning the field work, Barbara Magłysz, Aneta Gaura and Adam Dzida for help in trappings, Sabrina Schmidt, Dörte Kaufmann, Kerstin Tauscher for their excellent technical assistance, Thorsten Schrapps and Christoph Staubach for preparation of Figure 1, and Małgorzata Sadkowska-Todys for comments on the manuscript. The field work was financed form FRISC project (Polish Ministry of Science, the EEA/Norwegian Financial Mechanism), and the Jagiellonian University (DS-758&757). H.S.A. acknowledges support by scholarship from German Academic Exchange Service (DAAD) desk number 413, Eastern and southern Africa, code number A/09/90015.
Author Contributions
R.G.U. designed the study, P.K., E.T.S. and M.M. collected and provided the samples, H.S.A. and S.D. run the molecular and serological analyses, G.H. performed the phylogenetic analyses, and H.S.A., S.D., P.K., M.H.G. and R.G.U. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Plyusnin A., Beaty B.J., Elliott R.M., Goldbach R., Kormelink R., Lundkvist A., Schmaljohn C.S., Tesh R.B. Bunyaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Inc.; San Diego, CA, USA: 2011. pp. 725–741. [Google Scholar]
- 2.Schlegel M., Jacob J., Krüger D.H., Rang A., Ulrich R.G. Hantavirus Emergence in Rodents, Insectivores and Bats. In: Johnson N., editor. Role of Animals in Emerging Viral Diseases. Academic Press; San Diego, CA, USA: 2014. pp. 235–291. [Google Scholar]
- 3.Krüger D.H., Schönrich G., Klempa B. Human pathogenic hantaviruses and prevention of infection. Hum. Vaccin. 2011;7:685–693. doi: 10.4161/hv.7.6.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi Z., Formenty P.B., Roth C.E. Hantavirus infection: A review and global update. J. Infect. Dev. Ctries. 2008;2:3–23. doi: 10.3855/jidc.317. [DOI] [PubMed] [Google Scholar]
- 5.Heyman P., Ceianu C.S., Christova I., Tordo N., Beersma M., Joao Alves M., Lundkvist A., Hukic M., Papa A., Tenorio A., et al. A five-year perspective on the situation of haemorrhagic fever with renal syndrome and status of the hantavirus reservoirs in Europe, 2005–2010. Euro Surveill. 2011;16:977–986. doi: 10.2807/ese.16.36.19961-en. [DOI] [PubMed] [Google Scholar]
- 6.Vaheri A., Henttonen H., Voutilainen L., Mustonen J., Sironen T., Vapalahti O. Hantavirus infections in Europe and their impact on public health. Rev. Med. Virol. 2013;23:35–49. doi: 10.1002/rmv.1722. [DOI] [PubMed] [Google Scholar]
- 7.Pilaski J., Feldmann H., Morzunov S., Rollin P.E., Ruo S.L., Lauer B., Peters C.J., Nichol S.T. Genetic identification of a new Puumala virus strain causing severe hemorrhagic fever with renal syndrome in Germany. J. Infect. Dis. 1994;170:1456–1462. doi: 10.1093/infdis/170.6.1456. [DOI] [PubMed] [Google Scholar]
- 8.Valtonen M., Kauppila M., Kotilainen P., Lahdevirta J., Svartback C.M., Kosunen O., Nurminen J., Sarkkinen H., Brummer-Korvenkontio M. Four fatal cases of nephropathia epidemica. Scand. J. Infect. Dis. 1995;27:515–517. doi: 10.3109/00365549509047057. [DOI] [PubMed] [Google Scholar]
- 9.Lahdevirta J. The minor problem of hemostatic impairment in nephropathia epidemica, the mild Scandinavian form of hemorrhagic fever with renal syndrome. Rev. Infect. Dis. 1989;11:S860–S863. doi: 10.1093/clinids/11.Supplement_4.S860. [DOI] [PubMed] [Google Scholar]
- 10.Tesakov A.S., Lebedev V.S., Bannikova A.A., Abramson N.I. Clethrionomys Tilesius, 1850 is the valid generic name for red-backed voles and Myodes Pallas, 1811 is a junior synonym of Lemmus Link, 1795. Russ. J. Theriol. 2010;9:83–86. [Google Scholar]
- 11.Brummer-Korvenkontio M., Vaheri A., Hovi T., von Bonsdorff C.H., Vuorimies J., Manni T., Penttinen K., Oker-Blom N., Lahdevirta J. Nephropathia epidemica: Detection of antigen in bank voles and serologic diagnosis of human infection. J. Infect. Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- 12.Kariwa H., Yoshizumi S., Arikawa J., Yoshimatsu K., Takahashi K., Takashima I., Hashimoto N. Evidence for the existence of Puumula-related virus among Clethrionomys rufocanus in Hokkaido, Japan. Am. J. Trop. Med. Hyg. 1995;53:222–227. doi: 10.4269/ajtmh.1995.53.222. [DOI] [PubMed] [Google Scholar]
- 13.Song K.J., Baek L.J., Moon S., Ha S.J., Kim S.H., Park K.S., Klein T.A., Sames W., Kim H.C., Lee J.S., et al. Muju virus, a novel hantavirus harboured by the arvicolid rodent Myodes regulus in Korea. J. Gen. Virol. 2007;88:3121–3129. doi: 10.1099/vir.0.83139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aberle S.W., Lehner P., Ecker M., Aberle J.H., Arneitz K., Khanakah G., Radda A., Radda I., Popow-Kraupp T., Kunz C., et al. Nephropathia epidemica and Puumala virus in Austria. Eur. J. Clin. Microbiol. Infect. Dis. 1999;18:467–472. doi: 10.1007/s100960050325. [DOI] [PubMed] [Google Scholar]
- 15.Plyusnina A., Aberle S.W., Aberle J.H., Plyusnin A. Genetic analysis of Puumala hantavirus strains from Austria. Scand. J. Infect. Dis. 2006;38:512–519. doi: 10.1080/00365540600585040. [DOI] [PubMed] [Google Scholar]
- 16.Plyusnina A., Ferenczi E., Racz G.R., Nemirov K., Lundkvist A., Vaheri A., Vapalahti O., Plyusnin A. Co-circulation of three pathogenic hantaviruses: Puumala, Dobrava, and Saaremaa in Hungary. J. Med. Virol. 2009;81:2045–2052. doi: 10.1002/jmv.21635. [DOI] [PubMed] [Google Scholar]
- 17.Ahlm C., Linderholm M., Juto P., Stegmayr B., Settergren B. Prevalence of serum IgG antibodies to Puumala virus (haemorrhagic fever with renal syndrome) in northern Sweden. Epidemiol. Infect. 1994;113:129–136. doi: 10.1017/S0950268800051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyman P., Cochez C., Ducoffre G., Mailles A., Zeller H., Abu Sin M., Koch J., van Doornum G., Koopmans M., Mossong J., et al. Haemorrhagic Fever with Renal Syndrome: An analysis of the outbreaks in Belgium, France, Germany, the Netherlands and Luxembourg in 2005. Euro Surveill. 2007;12:E15–E16. doi: 10.2807/esm.12.05.00712-en. [DOI] [PubMed] [Google Scholar]
- 19.Groen J., Gerding M.N., Jordans J.G., Clement J.P., Nieuwenhuijs J.H., Osterhaus A.D. Hantavirus infections in The Netherlands: Epidemiology and disease. Epidemiol. Infect. 1995;114:373–383. doi: 10.1017/S0950268800058003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurgaleeva R.G., Tkachenko E.A., Stepanenko A.G., Mustafin I.M., Kireev S.G., Dzagurova T.K., Dekonenko A.E., Klimchuk L.A., Minin G.D. An epidemiological analysis of hemorrhagic fever with renal syndrome morbidity in the Republic of Bashkortostan in 1997. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1999;6:45–49. [PubMed] [Google Scholar]
- 21.Sandmann S., Meisel H., Razanskiene A., Wolbert A., Pohl B., Krüger D.H., Sasnauskas K., Ulrich R. Detection of human hantavirus infections in Lithuania. Infection. 2005;33:66–72. doi: 10.1007/s15010-005-4058-8. [DOI] [PubMed] [Google Scholar]
- 22.Golovljova I., Sjolander K.B., Lindegren G., Vene S., Vasilenko V., Plyusnin A., Lundkvist A. Hantaviruses in Estonia. J. Med. Virol. 2002;68:589–598. doi: 10.1002/jmv.10231. [DOI] [PubMed] [Google Scholar]
- 23.Lundkvist A., Lindegren G., Brus Sjolander K., Mavtchoutko V., Vene S., Plyusnin A., Kalnina V. Hantavirus infections in Latvia. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:626–629. doi: 10.1007/s10096-002-0784-3. [DOI] [PubMed] [Google Scholar]
- 24.Bowen M.D., Kariwa H., Rollin P.E., Peters C.J., Nichol S.T. Genetic characterization of a human isolate of Puumala hantavirus from France. Virus Res. 1995;38:279–289. doi: 10.1016/0168-1702(95)00058-X. [DOI] [PubMed] [Google Scholar]
- 25.Cebalo L., Dusek T., Kuzman I., Markotic A. Grading the severity of disease in patients with Puumala or Dobrava virus infections from 1995 to 2000 in Croatia. Acta Med. Croat. 2003;57:355–359. [PubMed] [Google Scholar]
- 26.Leitmeyer K., Sibold C., Meisel H., Ulrich R., Labuda M., Kruger D.H. First molecular evidence for Puumala hantavirus in Slovakia. Virus Genes. 2001;23:165–169. doi: 10.1023/A:1011840104037. [DOI] [PubMed] [Google Scholar]
- 27.Korva M., Saksida A., Kejzar N., Schmaljohn C., Avsic-Zupanc T. Viral load and immune response dynamics in patients with haemorrhagic fever with renal syndrome. Clin. Microbiol. Infect. 2013;19:E358–E366. doi: 10.1111/1469-0691.12218. [DOI] [PubMed] [Google Scholar]
- 28.Papa A., Antoniadis A. Hantavirus infections in Greece—An update. Eur. J. Epidemiol. 2001;17:189–194. doi: 10.1023/A:1017987104363. [DOI] [PubMed] [Google Scholar]
- 29.Hukic M., Tulumovic D., Calkic L. The renal failure and capillary leak during the acute stage of (Dobrava) DOB and PUU (Puumala) infection. Med. Arh. 2005;59:227–230. [PubMed] [Google Scholar]
- 30.Lundkvist A., Wiger D., Horling J., Sjolander K.B., Plyusnina A., Mehl R., Vaheri A., Plyusnin A. Isolation and characterization of Puumala hantavirus from Norway: Evidence for a distinct phylogenetic sublineage. J. Gen. Virol. 1998;79:2603–2614. doi: 10.1099/0022-1317-79-11-2603. [DOI] [PubMed] [Google Scholar]
- 31.Clement J., Maes P., van Ypersele de Strihou C., van der Groen G., Barrios J.M., Verstraeten W.W., van Ranst M. Beechnuts and outbreaks of nephropathia epidemica (NE): Of mast, mice and men. Nephrol. Dial. Transplant. 2010;25:1740–1746. doi: 10.1093/ndt/gfq122. [DOI] [PubMed] [Google Scholar]
- 32.Sadkowska-Todys M., Gut W., Baumann A., Siennicka J., Litwinska B., Zielinski A. Occurrence of human hantavirus infections in Poland. Prz. Epidemiol. 2007;61:497–503. [PubMed] [Google Scholar]
- 33.Czarkowski M.P., Cielebak E., Kondej E., Staszewska E. Infectious Diseases and Poisonings in Poland in 2012. Bulletin of the National Institute of Public Health and Chief Sanitary Inspectorate; Warszawa, Poland: 2013. [Google Scholar]
- 34.Knap J.P., Brzostek T., Raczka A., Burzynski W., Litarska U. A case of haemorrhagic fever with renal syndrome (HFRS) Pol. Merkur Lek. 2006;21:474–476. [PubMed] [Google Scholar]
- 35.Nowakowska A., Heyman P., Knap J.P., Burzynski W., Witas M. The first established focus of hantavirus infection in Poland, 2007. Ann. Agric. Environ. Med. 2009;16:79–85. [PubMed] [Google Scholar]
- 36.Panasiak W., Wleklik M., Oraczewska A., Luczak M. Serological studies of haemorrhagic fever with renal syndrome (HFRS) in Poland. Preliminary report. Acta Microbiol. Pol. 1989;38:63–67. [PubMed] [Google Scholar]
- 37.Gut W., Siennicka J., Sadkowska-Todys M., Gozdowska J., Litwinska B. The cross and unspecific reactions in serological examination for antibodies against hantavirus Puumala. Prz. Epidemiol. 2007;61:489–495. [PubMed] [Google Scholar]
- 38.Grygorczuk S., Pancewicz S., Zajkowska J., Kondrusik M., Swierzbinska R., Moniuszko A., Pawlak-Zalewska W. Detection of anti-hantavirus antibodies in forest workers in the north-east of Poland. Prz. Epidemiol. 2008;62:531–537. [PubMed] [Google Scholar]
- 39.Song J.W., Baek L.J., Song K.J., Skrok A., Markowski J., Bratosiewicz-Wasik J., Kordek R., Liberski P.P., Yanagihara R. Characterization of Tula virus from common voles (Microtus arvalis) in Poland: Evidence for geographic-specific phylogenetic clustering. Virus Genes. 2004;29:239–247. doi: 10.1023/B:VIRU.0000036384.50102.cf. [DOI] [PubMed] [Google Scholar]
- 40.Wojcik-Fatla A., Zajac V., Knap J.P., Sroka J., Cisak E., Sawczyn A., Dutkiewicz J. A small-scale survey of hantavirus in mammals from eastern Poland. Ann. Agric. Environ. Med. 2013;20:283–286. [PubMed] [Google Scholar]
- 41.Gu S.H., Markowski J., Kang H.J., Hejduk J., Sikorska B., Liberski P.P., Yanagihara R. Boginia virus, a newfound hantavirus harbored by the Eurasian water shrew (Neomys fodiens) in Poland. Virol. J. 2013;10:160. doi: 10.1186/1743-422X-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razzauti M., Plyusnina A., Niemimaa J., Henttonen H., Plyusnin A. Co-circulation of two Puumala hantavirus lineages in Latvia: A Russian lineage described previously and a novel Latvian lineage. J. Med. Virol. 2012;84:314–318. doi: 10.1002/jmv.22263. [DOI] [PubMed] [Google Scholar]
- 43.Wojcik J.M., Kawalko A., Markova S., Searle J.B., Kotlik P. Phylogeographic signatures of northward post-glacial colonization from high-latitude refugia: A case study of bank voles using museum specimens. J. Zool. 2010;281:249–262. [Google Scholar]
- 44.Mertens M., Kindler E., Emmerich P., Esser J., Wagner-Wiening C., Wolfel R., Petraityte-Burneikiene R., Schmidt-Chanasit J., Zvirbliene A., Groschup M.H., et al. Phylogenetic analysis of Puumala virus subtype Bavaria, characterization and diagnostic use of its recombinant nucleocapsid protein. Virus Genes. 2011;43:177–191. doi: 10.1007/s11262-011-0620-x. [DOI] [PubMed] [Google Scholar]
- 45.Essbauer S., Schmidt J., Conraths F.J., Friedrich R., Koch J., Hautmann W., Pfeffer M., Wolfel R., Finke J., Dobler G., et al. A new Puumala hantavirus subtype in rodents associated with an outbreak of Nephropathia epidemica in South-East Germany in 2004. Epidemiol. Infect. 2006;134:1333–1344. doi: 10.1017/S0950268806006170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlegel M., Ali H.S., Stieger N., Groschup M.H., Wolf R., Ulrich R.G. Molecular identification of small mammal species using novel cytochrome B gene-derived degenerated primers. Biochem. Genet. 2012;50:440–447. doi: 10.1007/s10528-011-9487-8. [DOI] [PubMed] [Google Scholar]
- 47.Basic Local Alignment Search Tool (BLAST) [(accessed on 14 October 2013)]. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi/
- 48.Braaker S., Heckel G. Transalpine colonisation and partial phylogeographic erosion by dispersal in the common vole (Microtus arvalis) Mol. Ecol. 2009;18:2518–2531. doi: 10.1111/j.1365-294X.2009.04189.x. [DOI] [PubMed] [Google Scholar]
- 49.Ettinger J., Hofmann J., Enders M., Tewald F., Oehme R.M., Rosenfeld U.M., Ali H.S., Schlegel M., Essbauer S., Osterberg A., et al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg. Infect. Dis. 2012;18:1461–1464. doi: 10.3201/eid1809.111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 52.Fink S., Fischer M.C., Excoffier L., Heckel G. Genomic scans support repetitive continental colonization events during the rapid radiation of voles (Rodentia: Microtus): The utility of AFLPs versus mitochondrial and nuclear sequence markers. Syst. Biol. 2010;59:548–572. doi: 10.1093/sysbio/syq042. [DOI] [PubMed] [Google Scholar]