Abstract
Carbohydrate oxidases are biotechnologically interesting enzymes that require a tightly or covalently bound cofactor for activity. Using the industrial workhorse Corynebacterium glutamicum as the expression host, successful secretion of a normally cytosolic FAD cofactor-containing sorbitol–xylitol oxidase from Streptomyces coelicolor was achieved by using the twin-arginine translocation (Tat) protein export machinery for protein translocation across the cytoplasmic membrane. Our results demonstrate for the first time that, also for cofactor-containing proteins, a secretory production strategy is a feasible and promising alternative to conventional intracellular expression strategies.
The secretory expression of recombinant proteins can offer significant process advantages over cytosolic production strategies, since secretion into the growth medium greatly facilitates downstream processing and therefore can significantly reduce the costs of producing a desired target protein (Quax, 1997). And, in fact, the enormous secretion capacity of certain Gram-positive bacteria (e.g. various Bacillus species) has been used since many years in industry for the production of mainly host-derived secretory proteins such as proteases and amylases, resulting in amounts of more than 20 g l−1 culture medium (Harwood and Cranenburg, 2008). In contrast, attempts to use Bacillus species for the secretory production of heterologous proteins have often failed or led to disappointing results, a fact that, among other reasons, could in many cases be attributed to the presence of multiple cell wall-associated and secreted proteases that rapidly degraded the heterologous target proteins (Li et al., 2004; Sarvas et al., 2004; Westers et al., 2011). Therefore, an increasing need exists to explore alternative host systems with respect to their ability to express and secrete problematic and/or complex heterologous proteins of biotechnological interest.
So far, the Gram-positive bacterium Corynebacterium glutamicum has been used in industry mainly for the production of amino acids and other low-molecular weight compounds (Leuchtenberger et al., 2005; Becker and Wittmann, 2011; Litsanov et al., 2012). However, various recent reports have indicated that C. glutamicum might likewise possess a great potential as an alternative host system for the secretory expression of foreign proteins. Corynebacterium glutamicum belongs to a class of diderm Gram-positive bacteria that, besides the cytoplasmic membrane, possess an additional mycolic acid-containing outer membrane-like structure that acts as an extremely efficient permeability barrier for hydrophilic compounds (Hoffmann et al., 2008; Zuber et al., 2008). Despite this fact, an efficient secretion of various heterologous proteins into the growth medium of this microorganism has been observed (e.g. Billman-Jacobe et al., 1995; Meissner et al., 2007; Kikuchi et al., 2009; Tateno et al., 2009; Tsuchidate et al., 2011).
In bacteria, two major export pathways exist for the transport of proteins across the cytoplasmic membrane that fundamentally differ with respect to the folding status of their respective substrate proteins during the actual translocation step. The general secretion (Sec) system transports its substrates in a more or less unfolded state and folding takes places on the trans side of the membrane after the actual transport event (Yuan et al., 2010; du Plessis et al., 2011). In contrast, the alternative twin-arginine translocation (Tat) system translocates its substrates in a fully folded form and therefore provides an attractive alternative for the secretory production of proteins that cannot be produced in a functional form via the Sec route (Brüser, 2007). Carbohydrate oxidases are biotechnologically interesting enzymes (van Hellemond et al., 2006) that are excluded from Sec-dependent secretion since they depend on a tightly or covalently bound cofactor for their activity and, for this reason, require that their folding and cofactor insertion has to take place in the cytosol. Because C. glutamicum has shown to be an excellent host for the Tat-dependent secretion of the cofactor-less model protein GFP (Meissner et al., 2007; Teramoto et al., 2011), we now asked whether it is likewise possible to secrete a cofactor-containing enzyme into the supernatant of C. glutamicum using the same protein export route.
As a model protein, we chose the sorbitol–xylitol oxidase (SoXy) from Streptomyces coelicolor, a normally cytosolic enzyme that possesses a covalently bound FAD molecule as cofactor (Heuts et al., 2007; Forneris et al., 2008). FAD is incorporated into the apoprotein in a post-translational and self-catalytic process that only occurs if the polypeptide chain has adopted a correctly folded structure (Heuts et al., 2007; 2009). To direct SoXy into the Tat export pathway of C. glutamicum, we constructed a gene encoding a TorA–SoXy hybrid precursor in which SoXy is fused to the strictly Tat-specific signal peptide of the periplasmic Escherichia coli Tat substrate trimethylamine N-oxide reductase (TorA) (Fig. 1) which, in our previous study, has been proven to be a functional and strictly Tat-specific signal peptide also in C. glutamicum (Meissner et al., 2007). The corresponding torA–soxy gene was cloned into the expression vector pEKEx2 (Eikmanns et al., 1991) under the control of an IPTG-inducible Ptac promotor. After transformation of the resulting plasmid pTorA–SoXy into the C. glutamicum ATCC13032 wild-type strain, two independent colonies of the resulting recombinant C. glutamicum (pTorA–SoXy) strain and, as a control, a colony of a strain that contained the empty expression vector without insert [C. glutamicum (pEKEx2)] were grown in CGXII medium (Keilhauer et al., 1993) at 30°C for 16 h in the presence of 1 mM IPTG. Subsequently, the proteins present in the culture supernatants were analysed by SDS-PAGE followed by staining with Coomassie blue. As shown in Fig. 2, in the supernatants of the pTorA–SoXy-containing cells (lanes 3 and 4), a prominent protein band of approximately 44 kDa can be detected, the size of which is very similar to the calculated molecular mass (44.4 kDa) of SoXy. Since this band is completely lacking in the supernatant of the control strain (lane 2), this strongly suggests that this band corresponds to SoXy that has been secreted into the culture supernatant of C. glutamicum (pTorA–SoXy). And, in fact, this suggestion was subsequently confirmed in a direct way by MALDI-TOF mass spectrometry after extraction of the protein out of the gel followed by tryptic digestion (Schaffer et al., 2001) (data not shown).
Figure 1.
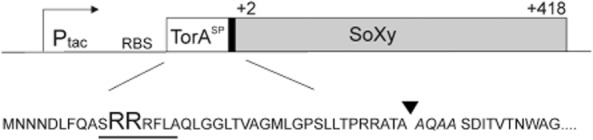
The TorA–SoXy hybrid precursor protein. Upper part: Schematic drawing of the relevant part of the pTorA–SoXy expression vector. Ptac, IPTG-inducible tac promotor. RBS, ribosome binding site. To maintain the authentic TorA signal peptidase cleavage site, the first four amino acids of the mature TorA protein (black bar) were retained in the TorA–SoXy fusion protein. White bar: TorA signal peptide (TorASP); grey bar: SoXy (amino acids 2–418). Lower part: Amino acid sequence of the signal peptide and early mature region of the TorA–SoXy hybrid precursor. The twin-arginine consensus motif of the TorA signal peptide is underlined. The four amino acids derived from mature TorA are shown in italics. The signal peptidase cleavage site is indicated by an arrowhead.
Figure 2.
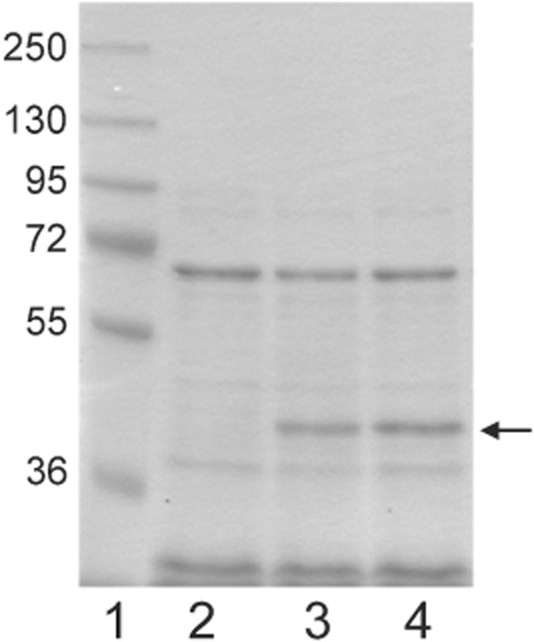
Secretion of SoXy into the growth medium of C. glutamicum. Cells of C. glutamicum ATCC13032 containing the empty vector pEKEx2 and two independently transformed colonies of C. glutamicum (pTorA–SoXy) were grown overnight in 5 ml of BHI medium (Difco) at 30°C. The cells were washed once with CGXII medium (Keilhauer et al., 1993) and inoculated to an OD600 of 0.5 in 5 ml of fresh CGXII medium containing 1 mM IPTG. After 16 h of further growth at 30°C, the supernatant fractions were prepared as described previously (Meissner et al., 2007). Samples corresponding to an equal number of cells were subjected to SDS-PAGE followed by staining with Coomassie blue. Lane 1, molecular mass marker (kDa). Lane 2, C. glutamicum (pEKEx2); lanes 3 and 4, C. glutamicum (pTorA–SoXy). The position of the secreted SoXy protein is indicated by an arrow.
Next, the supernatant of C. glutamicum (pTorA–SoXy) was analysed for SoXy enzyme activity by measuring the production of H2O2 that is formed during the enzymatic conversion of sorbitol to fructose (Meiattini, 1983). Six hours after induction of gene expression by 1 mM IPTG, an enzymatic activity of 10.3 ± 1.6 nmol min−1 ml−1 could be determined in the supernatant of C. glutamicum (pTorA–SoXy). In contrast, no such activity was found in the supernatant of the control strain C. glutamicum (pEKEx2). From these results we conclude that we have succeeded in the secretion of enzymatically active and therefore FAD cofactor-containing SoXy into the culture supernatant of C. glutamicum.
Finally, we examined whether the secretion of SoXy had in fact occurred via the Tat pathway of C. glutamicum. Plasmid pTorA–SoXy was used to transform C. glutamcium ATCC13032 wild type and a C. glutamicum ΔTatAC mutant strain that lacks two essential components of the Tat transport machinery and therefore does not possess a functional Tat translocase (Meissner et al., 2007). The corresponding cells were grown in BHI medium (Difco) at 30°C in the presence of 1 mM IPTG for 6 h. Subsequently, the proteins present in the cellular and the supernatant fractions of the corresponding cells were analysed by SDS-PAGE followed by Western blotting using SoXy-specific antibodies. As shown in Fig. 3, polypeptides corresponding to the unprocessed TorA–SoXy precursor and some minor smaller degradation products of it can be detected in the cellular fractions of both the wild-type and the ΔTatAC deletion strains (lanes 3 and 5). In the supernatant fraction of the Tat+ wild-type strain (lane 4), but not that of the ΔTatAC strain (lane 6), a polypeptide corresponding to mature SoXy is present, clearly showing that export of SoXy in the wild-type strain had occurred in a strictly Tat-dependent manner. Another noteworthy finding is the observation that hardly any mature SoXy protein accumulated in the cellular fraction of the Tat+ wild-type strain (lane 3), indicating that SoXy is, after its Tat-dependent translocation across the cytoplasmic membrane and processing by signal peptidase, rapidly transported out of the intermembrane space across the mycolic acid-containing outer membrane into the supernatant. However, the mechanism of how proteins cross this additional permeability barrier is completely unknown so far (Bitter et al., 2009).
Figure 3.
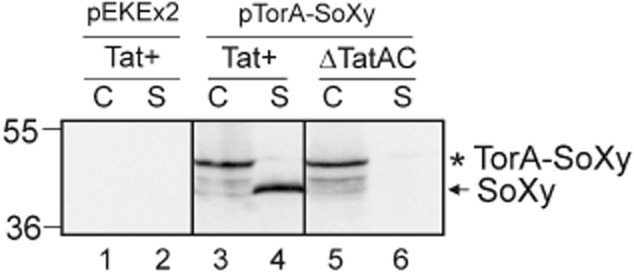
Transport of TorA–SoXy occurs in a strictly Tat-dependent manner. Plasmid pTorA–SoXy was transformed into C. glutamcium ATCC13032 (Tat+) and a C. glutamicum ΔTatAC mutant that lacks a functional Tat translocase (Meissner et al., 2007). As a control, the empty pEKEx2 expression vector was transformed into C. glutamicum ATCC13032 (Tat+). The respective strains were grown overnight in 5 ml of BHI medium (Difco) at 30°C. The cells were washed once with BHI and resuspended in 20 ml of fresh BHI medium containing 1 mM IPTG. After 6 h of further growth at 30°C, the cellular (C) and supernatant (S) fractions were prepared as described previously (Meissner et al., 2007). Samples of the C and S fractions were subjected to SDS-PAGE followed by immunoblotting using anti-SoXy antibodies as indicated at the top of the figure. Lanes 1 and 2: C. glutamicum ATCC13032 (pEKEx2); lanes 3 and 4: C. glutamicum ATCC13032 (pTorA–SoXy); lanes 5 and 6: C. glutamicum ΔTatAC (pTorA–SoXy). Asterisk: TorA–SoXy precursor; arrow: secreted SoXy protein. The positions of molecular mass markers (kDa) are indicated at the left margin of the figure.
To the best of our knowledge, our results represent the first documented example of the successful secretion of a normally cytosolic, cofactor-containing protein via the Tat pathway in an active form into the culture supernatant of a recombinant expression host. Our results clearly show that, also for this biotechnologically very interesting class of proteins, a secretory production strategy can be a promising alternative to conventional intracellular expression strategies. Besides for SoXy and other FAD-containing carbohydrate oxidases, for which various applications are perceived by industry such as the in situ generation of hydrogen peroxide for bleaching and disinfection performance in technical applications, their use in the food and drink industry, as well as their use in diagnostic applications and carbohydrate biosynthesis processes (Oda and Hiraga, 1998; Murooka and Yamashita, 2001; van Hellemond et al., 2006; Heuts et al., 2007), a secretory production strategy might now be an attractive option also for biotechnologically relevant enzymes that are used as biocatalysts in chemo-enzymatic syntheses and that possess cofactors other than FAD, such as pyridoxal-5′-phosphate (PLP)-dependent ω-transaminases (Mathew and Yun, 2012) or various thiamin diphosphate (TDP)-dependent enzymes (Müller et al., 2009).
Acknowledgments
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF) project ‘Genomic Design – Doubling Efficiency by Microbial Genomic Design’ 0313917C. We are very grateful to Melanie Brocker for her help with the MALDI-TOF mass spectrometry experiments and to Astrid Bida for excellent technical assistance.
Funding Information
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF) project ‘Genomic Design–Doubling Efficiency by Microbial Genomic Design’ 0313917C.
Conflict of interest
None declared.
References
- Becker J, Wittmann C. Bio-based production of chemicals, materials and fuels – Corynebacterium glutamicum as versatile cell factory. Curr Opin Microbiol. 2011;23:1–10. doi: 10.1016/j.copbio.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Billman-Jacobe H, Wang LF, Kortt A, Stewart D, Radford A. Expression and secretion of heterologous proteases by Corynebacterium glutamicum. Appl Environ Microbiol. 1995;61:1610–1613. doi: 10.1128/aem.61.4.1610-1613.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter W, Houben ENG, Luirink J, Appelmelk BJ. Type VII secretion in mycobacteria: classification in line with cell envelope structure. Trends Microbiol. 2009;17:337–338. doi: 10.1016/j.tim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Brüser T. The twin-arginine translocation system and its capability for protein secretion in biotechnological protein production. Appl Microbiol Biotechnol. 2007;76:35–45. doi: 10.1007/s00253-007-0991-z. [DOI] [PubMed] [Google Scholar]
- Eikmanns BJ, Kleinertz E, Liebl W, Sahm H. A family of Corynebacterium glutamicumEscherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene. 1991;102:93–98. doi: 10.1016/0378-1119(91)90545-m. [DOI] [PubMed] [Google Scholar]
- Forneris F, Heuts DPHM, Delvecchio M, Rovida S, Fraaije MW, Mattevi A. Structural analysis of the catalytic mechanism and stereoselectivity in Streptomyces coelicolor alditol oxidase. Biochemistry. 2008;47:978–985. doi: 10.1021/bi701886t. [DOI] [PubMed] [Google Scholar]
- Harwood CR, Cranenburgh R. Bacillus protein secretion: an unfolding story. Trends Microbiol. 2008;16:73–79. doi: 10.1016/j.tim.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hellemond van EW, Leferink NG, Heuts D, Fraaije MW, Berkel van WJ. Occurrence and biocatalytic potential of carbohydrate oxidases. Adv Appl Microbiol. 2006;60:17–54. doi: 10.1016/S0065-2164(06)60002-6. [DOI] [PubMed] [Google Scholar]
- Heuts DPH, Hellemond van EW, Janssen DB, Fraaije MW. Discovery, characterization, and kinetic analysis of an alditol oxidase from Streptomyces coelicolor. J Biol Chem. 2007;282:20283–20291. doi: 10.1074/jbc.M610849200. [DOI] [PubMed] [Google Scholar]
- Heuts DPHM, Scrutton NS, McIntyre WS, Fraaije MW. What's in a covalent bond? On the role and formation of covalently bound flavin cofactors. FEBS J. 2009;276:3405–3427. doi: 10.1111/j.1742-4658.2009.07053.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhauer C, Eggeling L, Sahm H. Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol. 1993;175:5595–5603. doi: 10.1128/jb.175.17.5595-5603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Itaya H, Date M, Matsui K, Wu L-F. TatABC overexpression improves Corynebacterium glutamicum Tat-dependent protein secretion. Appl Environ Microbiol. 2009;75:603–607. doi: 10.1128/AEM.01874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchtenberger W, Huthmacher K, Drauz K. Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol. 2005;69:1–8. doi: 10.1007/s00253-005-0155-y. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou X, Lu P. Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res Microbiol. 2004;155:605–610. doi: 10.1016/j.resmic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Litsanov B, Kabus A, Brocker M, Bott M. Efficient aerobic succinate production from glucose in minimal medium with Corynebacterium glutamicum. Microb Biotechnol. 2012;5:116–128. doi: 10.1111/j.1751-7915.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Yun H. ω-Transaminases for the production of optically pure amines and unnatural amino acids. ACS Catal. 2012;2:993–1001. [Google Scholar]
- Meiattini F. Inorganic peroxides. In: Bergmeyer HU, Bergmeyer J, Graßl M, editors. Methods of Enzymatic Analysis, 3rd Edn, Vol. 7. Metabolites 2: Tri- and Dicarboxylic Acids. Purines, Pyrimidines and Derivatives, Coenzymes, Inorganic Compounds. Weinheim, Germany: Verlag Chemie; 1983. pp. 566–571. [Google Scholar]
- Meissner D, Vollstedt A, Dijl van JM, Freudl R. Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different Gram-positive bacteria. Appl Microbiol Biotechnol. 2007;76:633–642. doi: 10.1007/s00253-007-0934-8. [DOI] [PubMed] [Google Scholar]
- Müller M, Gocke D, Pohl M. Thiamin diphosphate in biological chemistry: exploitation of diverse thiamin diphosphate-dependent enzymes for asymmetric chemoenzymatic synthesis. FEBS J. 2009;276:2894–2904. doi: 10.1111/j.1742-4658.2009.07017.x. [DOI] [PubMed] [Google Scholar]
- Murooka Y, Yamashita M. Genetic and protein engineering of diagnostic enzymes, cholesterol oxidase and xylitol oxidase. J Biosci Bioeng. 2001;91:433–441. doi: 10.1263/jbb.91.433. [DOI] [PubMed] [Google Scholar]
- Oda K, Hiraga K. Sorbitol oxidase from microorganisms. Ann N Y Acad Sci. 1998;864:454–457. doi: 10.1111/j.1749-6632.1998.tb10358.x. [DOI] [PubMed] [Google Scholar]
- Plessis du DJ, Nouwen N, Driessen AJM. The Sec translocase. Biochim Biophys Acta. 2011;1808:851–865. doi: 10.1016/j.bbamem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Quax WJ. Merits of secretion of heterologous proteins from industrial microorganisms. Folia Microbiol. 1997;42:99–103. doi: 10.1007/BF02898715. [DOI] [PubMed] [Google Scholar]
- Sarvas M, Harwood CR, Bron S, Dijl van JM. Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochim Biophys Acta. 2004;1694:311–327. doi: 10.1016/j.bbamcr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Weil B, Nguyen VD, Dongmann G, Günther K, Nickolaus M, et al. A high-resolution reference map for cytoplasmic and membrane-associated proteins of Corynebacterium glutamicum. Electrophoresis. 2001;22:4404–4422. doi: 10.1002/1522-2683(200112)22:20<4404::AID-ELPS4404>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tateno T, Okada Y, Tshuchidate T, Tanaka T, Fukuda H, Kondo A. Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing alpha-amylase and lysine decarboxylase. Appl Microbiol Biotechnol. 2009;82:115–121. doi: 10.1007/s00253-008-1751-4. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Watanabe K, Suzuki N, Inui M, Yukawa H. High yield secretion of heterologous proteins in Corynebacterium glutamicum using its own Tat-type signal sequence. Appl Microbiol Biotechnol. 2011;91:677–687. doi: 10.1007/s00253-011-3281-8. [DOI] [PubMed] [Google Scholar]
- Tsuchidate T, Tateno T, Okai N, Tanaka T, Ogino C, Kondo A. Glutamate production from β-glucan using endoglucanase-secreting Corynebacterium glutamicum. Appl Microbiol Biotechnol. 90:895–901. doi: 10.1007/s00253-011-3116-7. [DOI] [PubMed] [Google Scholar]
- Westers L, Westers H, Quax WJ. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim Biophys Acta. 2011;1694:299–310. doi: 10.1016/j.bbamcr.2004.02.011. 2004. [DOI] [PubMed] [Google Scholar]
- Yuan J, Zweers JC, Dijl van JM, Dalbey RE. Protein transport across and into cell membranes in bacteria and archaea. Cell Mol Life Sci. 2010;67:179–199. doi: 10.1007/s00018-009-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffe M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol. 2008;190:5672–5680. doi: 10.1128/JB.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


