Abstract
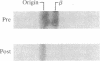
The beta-very low density lipoproteins (beta-VLDL) that accumulate in type III hyperlipoproteinemic subjects can be divided into two fractions (fraction I and fraction II), which differ in size, lipid composition, and the type of apolipoprotein B (apo-B) present in the particles. The apo-B48-containing particles (fraction I) are of intestinal origin, while apo-B100-containing particles (fraction II) are derived from the liver. Both fractions contain a defective form of apo-E referred to as apo-E2. Intravenous infusion of heparin into two subjects with type III hyperlipoproteinemia resulted in the complete removal of fraction II particles from density less than 1.006 g/ml, while fraction I particles remained at this density. In vitro studies confirmed that fraction I particles did not change density when subjected to hydrolysis with lipoprotein lipase, while fraction II particles shifted to the intermediate density lipoprotein range (approximately equal to 1.02 g/ml). When the beta-VLDL were hydrolyzed by lipoprotein lipase in the presence of density greater than 1.21 g/ml lipoprotein-deficient plasma, the addition of normal apo-E (apo-E3), but not apo-E2, resulted in a shift of fraction II particles to the low density lipoprotein (LDL) range (approximately equal to 1.05 g/ml). Fraction I particles did not undergo a shift to this higher density, supporting previous observations that apo-B48-containing particles are not converted to LDL. The demonstration that apo-B100-containing particles in type III hyperlipoproteinemic subjects could be converted to particles with the density of LDL suggests that apo-E plays a role in the normal conversion of VLDL to LDL. The mutant form of apo-E (apo-E2) found in the beta-VLDL from type III hyperlipoproteinemic subjects appears to impede this conversion, whereas the addition of normal apo-E (apo-E3) allows the processing to occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J Clin Invest. 1983 Sep;72(3):743–747. doi: 10.1172/JCI111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A., Hazzard W. R., Albers J. J., Kushwaha R. P., Brunzell J. D. Impaired very low density lipoprotein and triglyceride removal in broad beta disease: comparison with endogenous hypertriglyceridemia. Metabolism. 1978 Sep;27(9):1055–1066. doi: 10.1016/0026-0495(78)90151-8. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P. Resistance of a very low density lipoprotein subpopulation from familial dysbetalipoproteinemia to in vitro lipolytic conversion to the low density lipoprotein density fraction. J Lipid Res. 1983 Sep;24(9):1148–1159. [PubMed] [Google Scholar]
- Deckelbaum R. J., Eisenberg S., Fainaru M., Barenholz Y., Olivecrona T. In vitro production of human plasma low density lipoprotein-like particles. A model for very low density lipoprotein catabolism. J Biol Chem. 1979 Jul 10;254(13):6079–6087. [PubMed] [Google Scholar]
- Deckelbaum R. J., Eisenberg S., Oschry Y., Butbul E., Sharon I., Olivecrona T. Reversible modification of human plasma low density lipoproteins toward triglyceride-rich precursors. A mechanism for losing excess cholesterol esters. J Biol Chem. 1982 Jun 10;257(11):6509–6517. [PubMed] [Google Scholar]
- Fainaru M., Mahley R. W., Hamilton R. L., Innerarity T. L. Structural and metabolic heterogeneity of beta-very low density lipoproteins from cholesterol-fed dogs and from humans with type III hyperlipoproteinemia. J Lipid Res. 1982 Jul;23(5):702–714. [PubMed] [Google Scholar]
- Havel R. J. Familial dysbetalipoproteinemia. New aspects of pathogenesis and diagnosis. Med Clin North Am. 1982 Mar;66(2):441–454. doi: 10.1016/s0025-7125(16)31429-8. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P. Primary dysbetalipoproteinemia: predominance of a specific apoprotein species in triglyceride-rich lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2015–2019. doi: 10.1073/pnas.70.7.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Defective hepatic lipoprotein receptor binding of beta-very low density lipoproteins from type III hyperlipoproteinemic patients. Importance of apolipoprotein E. J Biol Chem. 1984 Jan 25;259(2):860–869. [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kinnunen P. K., Nikkilä E. A. An immunochemical method for the selective measurement of two triglyceride lipases in human postheparin plasma. Clin Chim Acta. 1975 Sep 16;63(3):335–347. doi: 10.1016/0009-8981(75)90055-8. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Friedlander E. J., Rall S. C., Jr, Weisgraber K. H., Mahley R. W. The receptor-binding domain of human apolipoprotein E. Binding of apolipoprotein E fragments. J Biol Chem. 1983 Oct 25;258(20):12341–12347. [PubMed] [Google Scholar]
- Kane J. P., Chen G. C., Hamilton R. L., Hardman D. A., Malloy M. J., Havel R. J. Remnants of lipoproteins of intestinal and hepatic origin in familial dysbetalipoproteinemia. Arteriosclerosis. 1983 Jan-Feb;3(1):47–56. doi: 10.1161/01.atv.3.1.47. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen P. K. Purification of bovine milk lipoprotein lipase with the aid of detergent. Med Biol. 1977 Jun;55(3):187–191. [PubMed] [Google Scholar]
- Kuusi T., Kinnunen P. K., Ehnholm C., Nikkilä E. A. A simple purification procedure for rat hepatic lipase. FEBS Lett. 1979 Feb 15;98(2):314–318. doi: 10.1016/0014-5793(79)80207-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Angelin B. Type III hyperlipoproteinemia: recent insights into the genetic defect of familial dysbetalipoproteinemia. Adv Intern Med. 1984;29:385–411. [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E and cholesterol metabolism. Klin Wochenschr. 1983 Mar 1;61(5):225–232. doi: 10.1007/BF01496128. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P. Hypolipidemia. Med Clin North Am. 1982 Mar;66(2):469–484. doi: 10.1016/s0025-7125(16)31431-6. [DOI] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Identical structural and receptor binding defects in apolipoprotein E2 in hypo-, normo-, and hypercholesterolemic dysbetalipoproteinemia. J Clin Invest. 1983 Apr;71(4):1023–1031. doi: 10.1172/JCI110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Schneider W. J., Kovanen P. T., Brown M. S., Goldstein J. L., Utermann G., Weber W., Havel R. J., Kotite L., Kane J. P., Innerarity T. L. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981 Oct;68(4):1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Langenbeck U., Beisiegel U., Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980 May;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- Utermann G., Pruin N., Steinmetz A. Polymorphism of apolipoprotein E. III. Effect of a single polymorphic gene locus on plasma lipid levels in man. Clin Genet. 1979 Jan;15(1):63–72. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Harder K. J., Mahley R. W., Milne R. W., Marcel Y. L., Sparrow J. T. The receptor-binding domain of human apolipoprotein E. Monoclonal antibody inhibition of binding. J Biol Chem. 1983 Oct 25;258(20):12348–12354. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981 Feb 17;20(4):1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]