Abstract
The presynaptic regions of axons accumulate synaptic vesicles, active zone proteins and periactive zone proteins. However, the rules for orderly recruitment of presynaptic components are not well understood. We systematically examined molecular mechanisms of presynaptic development in egg-laying synapses of Caenorhabditis elegans, demonstrating that two scaffolding molecules, SYD-1 and SYD-2, have key roles in presynaptic assembly. SYD-2 (liprin-α) was previously shown to regulate the size and the shape of active zones. We now show that in syd-1 and syd-2 mutants, synaptic vesicles and numerous other presynaptic proteins fail to accumulate at presynaptic sites. SYD-1 and SYD-2 function cell-autonomously at presynaptic terminals, downstream of synaptic specificity molecule SYG-1. SYD-1 is likely to act upstream of SYD-2 to positively regulate its synaptic assembly activity. These data imply a hierarchical organization of presynaptic assembly, in which transmembrane specificity molecules initiate synaptogenesis by recruiting a few key scaffolding proteins, which in turn assemble other presynaptic components.
Cellular and molecular processes during synapse formation and maturation dictate specificity and types and strength of synaptic connections between neurons, ultimately determining the functional properties of neural circuits. It is believed that synapse formation is triggered by contact between synaptic partners, which induces the transformation of a patch of unspecialized plasma membrane of the presynaptic neuron into a presynaptic apparatus. Presynaptic sites are structurally characterized by a pool of synaptic vesicles and active zones, where synaptic vesicles undergo exocytosis1. Functionally, neurotransmitter release is a multistep process, which involves coordinated actions of many presynaptic proteins. How various molecular components are organized into such complex machinery during development is an unresolved question.
A number of membrane molecules have been implicated in synapse development. Transmembrane molecules are attractive candidates for initiating presynaptic differentiation when an axon comes in contact with a potential postsynaptic target2. For example, postsynaptically expressed neuroligin is capable of clustering β-neurexin in the presynaptic neuron, which then causes accumulation of synaptic vesicles3,4. Similarly, synCAM, another homophilic trans-membrane protein, can initiate presynaptic assembly in vitro5. Other transmembrane proteins have a role in patterning synapses although they are not required for synapse formation per se. The immunoglobulin superfamily (IgSF) proteins Sdk-1 and Sdk-2 are thought to specify synaptic layer selection in the retina6. In C. elegans, a heterologous interaction between the IgSF proteins SYG-1 and SYG-2 specifies postsynaptic target recognition and the subcellular location of presynaptic sites along an axon of a particular neuron7,8.
Extracellular matrix proteins and secreted molecules have also been implicated in synaptic development. Laminin-β2, a basement membrane component, has been shown to directly bind to calcium channels and leads to assembly of functional presynaptic terminals at the vertebrate neuromuscular junction9. Fibroblast growth factors (FGFs) 22, 7 and 10 are extracellular signaling molecules that promote presynaptic differentiation and increase synapse number in vitro and in vivo10. Similarly, thrombospondins secreted by astrocytes have been shown to promote synaptogenesis11. At the fly neuromuscular junction, heparan sulfate proteoglycans syndecan and dallylike have been recently shown to regulate synapse development via their interactions with the receptor tyrosine phosphatase LAR12.
Intracellularly, a number of scaffolding molecules are thought to organize presynaptic sites. For example, bassoon, a large cytomatrix protein, is required for anchoring active zones to the plasma membrane at ribbon synapses in photoreceptors13. β-Catenin is thought to have a novel role in restricting synaptic vesicles to the presynaptic regions14. Mutant analyses in C. elegans and Drosophila melanogaster have led to identification of a number of active zone molecules important for presynaptic development and function. For example, SYD-2 (liprin-α) or LAR mutants exhibit elongated and irregular active zones in neuromuscular junctions of worms and flies15–17. Bruchpilot, another scaffolding molecule, which is the D. melanogaster homolog of ELKS-1 (ERC or CAST), was recently shown to be important for formation of T-bars and localization of calcium channels at the neuromuscular junction18,19. In C. elegans, the putative ubiquitin ligase RPM-1, the F-box protein FSN-1 and a MAP kinase cascade define a ubiquitin-mediated proteasomal pathway that attenuates presynaptic differentiation and regulates presynaptic morphology20–23. Mutations in highwire, the D. melanogaster homolog of RPM-1, cause overgrowth of neuromuscular junctions, implying a consistent negative regulatory role for highwire in synaptic growth24. Finally, at the D. melanogaster neuromuscular junction, the scaffolding protein Dap160/intersectin is thought to recruit multiple endocytotic proteins, including dynamin, endophilin, synaptojanin and AP180, to the presynaptic terminals25,26. Finally, a recent study took an elegant functional genomics approach to identify a large number of molecules that are important for presynaptic function and development of cholinergic synapses in C. elegans27.
Biochemical approaches have also identified numerous physical interactions between presynaptic components. For example, β-neurexin and synCAM interact with CASK, which is part of a tripartite complex that includes Mint and Veli5,28,29. CASK in turn interacts with SYD-2 (liprin-α)30. SYD-2 (liprin-α) binds to numerous other active zone components, including UNC-10 (RIM), ELKS-1 (ERC or CAST), GIT and LAR31–34. ELKS-1 (ERC) directly interacts with UNC-10 (RIM), piccolo and bassoon35,36.
Several important questions about presynaptic development remain unanswered. First, although an elaborate web of protein-protein interactions can be created from the biochemical data, the functional significance of these interactions in presynaptic assembly is not clear. It is possible that there are only a few molecules that are essential for presynaptic assembly, and the remaining active zone components are required for presynaptic function. Alternatively, extensive redundant weak interactions could form a web-like structure, which would predict that the absence of any particular interaction would cause only subtle effects on the overall presynaptic structure. Current data from genetic analysis favors the second model, as no single molecule has been found to be essential for the assembly of the entire presynaptic structure. A global understanding of presynaptic assembly—the molecular mechanisms by which synaptic vesicles, ion channels and other presynaptic components assemble to make a functional apparatus—is still lacking. Second, how the synaptic assembly program is linked to the synaptic target selection mechanisms is not understood.
In this work, we systematically studied a set of stereotypic en passant synapses formed by the HSNL neuron in C. elegans. We found that presynaptic differentiation at these synapses was initiated by SYG-1, a transmembrane molecule that defines the location of presynaptic sites along the HSNL axon. Localization of SYG-1 leads to local activation of a presynaptic assembly program by recruiting a key scaffolding protein, SYD-2 (liprin-α), and its positive regulator SYD-1. SYD-2 and SYD-1 are master regulators of presynaptic development that are required for the assembly of numerous presynaptic components.
Results
Multiple proteins localize to the presynaptic region of HSNL
The HSNL motor neuron, which regulates egg-laying in C. elegans, forms 12–20 en passant synapses onto vulval muscles and the VC4 and VC5 neurons8,37. Time-lapse experiments indicate that development of these synapses may start in late L3 larval stage, shortly after HSNL axon migrates past the vulva (G.W. and K.S., unpublished data). These synapses probably become functionally active in young adults to mediate egg laying. These HSNL synapses reside within a short stretch of the axon (∼10 μm) near the vulval opening, whereas the remainder of the HSNL axon (∼500 μm) in the ventral nerve cord is devoid of any pre- or postsynaptic specializations (Fig. 1a). This discrete location of synapses is reproducible between individuals and is in close agreement with serial electron micrograph reconstruction data8,37.
Figure 1.

GFP- or YFP-tagged known and putative presynaptic proteins localize to the synaptic region of HSNL near the vulva. (a) Schematic showing HSNL morphology (red). Boxed blowup shows HSNL details in the vulval region. Arrowhead, HSNL cell body; arrow, synapses (green) formed by HSNL onto vulval muscles and VC4 and VC5 neurons near the vulval slit (*). (b–i) Confocal images of HSNL cell body and its axon in the vulval region of adult worms expressing different fluorescently tagged proteins under the unc-86 promoter. Head is to the left and dorsal is up. (b) GFP::RAB-3. (c) GFP::SYD-1. (d) GFP::SYD-2. (e) ELKS-1::YFP. (f) GIT::YFP. (g) SAD-1::YFP. (h) UNC-57::YFP. (i) GFP::SNN-1. Note the restrictive distribution of proteins in HSNL near the vulva, with the anterior portion of the axon devoid of any fluorescence signal. Scale bar, 5 μm. (c–i) Colocalization of each presynaptic component (green) with mCHERRY::RAB-3 (red) is shown in the blowup of the synaptic region boxed with a blue dashed line in the corner of each image.
We have previously shown that the fluorescently tagged synaptic vesicle protein synaptobrevin (SNB-1::YFP) localizes to the same region of HSNL as the presynaptic sites observed by serial section electron microscopic reconstruction8,37. In order to study assembly of different presynaptic components, we fluorescently tagged a panel of known and putative presynaptic active zone proteins (SYD-2 (liprin-α) and ELKS-1 (ERC), other presynaptic proteins (SYD-1, GIT, SAD-1 kinase, UNC-57 (endophilin), Dap160 (intersectin), LIN-2 (CASK) and LIN-10 (Mint)) and synaptic vesicle–associated proteins (RAB-3 and SNN-1 (Synapsin-I)), and expressed them in HSNL under the unc-86 promoter7. We were able to visualize these fluorescent fusion proteins in live worms. Proteins that exhibited discrete localization to the presynaptic region of HSNL near the vulva included SYD-2 (liprin-α), SYD-1, ELKS-1 (ERC), GIT, SNN-1 (Synapsin-I), RAB-3 (M. Nonet, personal communication), SAD-1 kinase and UNC-57 (endophilin) (Fig. 1b–i). As the axon of the HSNL neuron is devoid of presynaptic sites except near the vulva in the ventral nerve cord, the spatially restricted localization near the vulva strongly implies that these proteins are likely to be presynaptic components. To confirm pre-synaptic localization of these molecules, we investigated their colocalization with mCHERRY::RAB-3, a monomeric red fluorescent protein fused with a synaptic vesicle–associated marker38. SYD-1, SYD-2 (liprin-α), ELKS-1 (ERC), GIT and SAD-1 showed staining patterns within the synaptic region that were nonidentical to but highly correlated with that of RAB-3 (Fig. 1b–g and Supplementary Fig. 1 online). SYD-1 showed a punctate staining pattern in the synaptic region that colocalized with RAB-3. Sparse SYD-1 puncta were also found along the axon between the HSNL cell body and synapses (Fig. 1c and Supplementary Fig. 1). UNC-57 (endophilin) and SNN-1 (Synapsin-I) showed an almost identical staining pattern to that of RAB-3, consistent with the notion that they might associate with synaptic vesicles (Fig. 1h,i and Supplementary Fig. 1). Most of these proteins have been shown to either localize or function at presynaptic specializations in C. elegans, D. melanogaster or vertebrate synapses, indicating that the molecular composition of presynaptic sites is likely to be similar across species. Candidates that failed to show discrete localization patterns in HSNL included LIN-2 (CASK), LIN-10 (Mint) and Dap160 (intersectin); these exhibited diffuse staining patterns that labeled the entire HSNL axon (data not shown). The function of these components might not be conserved in HSNL, which is one plausible explanation for their lack of localization.
SYG-1 initiates multiple aspects of presynaptic assembly
Synaptogenesis is initiated with membrane contacts between appropriate pre- and postsynaptic cells. Therefore, membrane molecules are likely to mediate synaptic target selection and might directly trigger synaptic assembly. Our previous genetic analysis of HSNL synapses showed that the transmembrane IgSF proteins SYG-1 and SYG-2 are essential for the postsynaptic target selection by HSNL and the positioning of presynaptic sites along the HSNL axon. SYG-1 localizes to the HSNL presynaptic region before synaptic vesicle accumulation through its direct interaction with SYG-2, which is expressed in the guidepost primary (1°) epithelial cells. In the absence of SYG-1 or SYG-2, SNB-1::YFP mislocalizes anteriorly to ectopic sites. Morphologically defined active zones similarly mislocalize when examined with serial electron microscopy7,8. From these data, we hypothesized that the SYG-2–SYG-1 interaction biases presynaptic assembly near the vulva. However, it was not tested whether SYG-1 is required for proper localization of active zone proteins as well as other presynaptic components besides synaptic vesicles. To address this question, we examined the localization of each presynaptic marker described in Figure 1 in syg-1 and syg-2 mutants. In wild-type N2 adults, all the presynaptic sites in HSNL are restricted to within ∼10 μm of the axon segment near the vulva (Fig. 2a). Presynaptic sites are normally absent from the portion of the HSNL axon that is more anterior to the vulva. However, in syg-1(ky652) and syg-2(ky671) mutants, all the presynaptic components showed an abnormal localization pattern (Fig. 2b–h) (data for syg-2 mutants is not shown because phenotypes in syg-1 and syg-2 mutants are indistinguishable). We quantified the relative fluorescence intensity of tagged RAB-3, ELKS-1 and GIT at the anterior location in wild-type worms and in syg-1 mutants. We found that the defects of HSNL synaptic development are twofold. First, in syg-1 mutants, fluorescence intensity of all three markers is dramatically reduced near the vulval opening where normal synapses form (Fig. 2i). Second, the localization RAB-3, ELKS-1 and GIT is no longer restricted to the normal synaptic region, but instead expands to anterior locations. Comparison of the fluorescence intensity at the anterior location between wild-type and syg-1 worms confirms that a minimal amount of synaptic signal can be found here in normal worms (Fig. 2j). These data imply that SYG-1 has an effect on the localization of presynaptic active zone components in addition to its effect on synaptic vesicles. Finally, SYD-1 and SYD-2 colocalize with RAB-3 at anterior locations in syg-1 mutants, indicating that the ectopic synapses probably represent complete presynaptic specializations (Supplementary Fig. 2 online).
Figure 2.

Multiple presynaptic components mislocalize in syg-1(ky652) mutants. (a) Schematic showing that the presynaptic components (green) of HSNL are normally confined to a region that contacts the guidepost epithelial cells during development. This segment of the HSNL axon is shown between the two arrowheads in HSNL (red) in wild-type N2 adults. Note that the arrow in the schematic points toward the region of the HSNL axon (anterior to the vulva) that is normally devoid of presynaptic components. Head is to the left and dorsal is up. (b) Schematic of a representative syg-1 (ky652) mutant showing reduction in the accumulation of presynaptic components (fewer green dots) near the vulval slit and anterior ectopic localization of presynaptic components (arrow). (c–h) Confocal images showing the abnormal anterior localization of multiple fluorescently tagged presynaptic components in syg-1(ky652) mutants. (c) GFP::RAB-3. (d) GFP::SYD-1. (e) GFP::SYD-2. (f) ELKS-1::YFP. (g) GIT::YFP. (h) SAD-1::YFP. (i) Quantification of the fluorescence intensity in HSNL axon near the vulva (between arrowheads) of GFP::RAB-3, ELKS-1::YFP and GIT::YFP in syg-1(ky652) mutants, compared with that in N2 worms. (j) Comparison of total fluorescence intensity within the 30 μm segment of the HSNL axon immediately anterior to the vulval region (anterior of the left arrowhead) in N2 worms relative to syg-1(ky652) mutants. There is no fluorescence above background in this anterior segment of HSNL in N2 worms. Therefore, the three N2 columns in j show zero value. Each bar represents average intensity from ten individuals and error bars are ± 2 s.e.m. Scale bar, 5 μm.
It is worth noting that the absence of SYG-1 does not lead to a complete loss of the accumulation of presynaptic proteins from the normal location near the vulva (Fig. 2). As judged from quantification of the relative fluorescent intensity near the vulva, approximately 40–60% of RAB-3, ELKS-1 and GIT is still present at the wild-type location in syg-1(ky652) mutants (Fig. 2i). These results are consistent with our previous EM data, which shows that some presynaptic sites are still present near the vulva in syg-1 mutants8. It is clear from the EM data that many of these presynaptic sites in syg-1 mutants represent synapses formed onto incorrect postsynaptic targets. Thus, there are likely to be other synaptogenic molecules like SYG-1 near the vulva that can recruit presynaptic components in the absence of SYG-1.
In order to assess more directly whether SYG-1 is sufficient to recruit presynaptic components, we performed a series of gain-of-function experiments. In wild-type N2 worms during the mid-L4 stage of development, SYG-1 localizes only to the segment of the HSNL axon that is in contact with the SYG-2–expressing 11 vulval epithelial cells (colored light blue in Fig. 3a). The region of the HSNL axon that is in contact with the secondary (2°) vulval epithelial cells is normally devoid of SYG-1 protein because the 2° cells normally do not express SYG-2. All the other presynaptic components also show minimal localization to this stretch of the axon (Fig. 1). However, when expression of SYG-2 was forced in the 2° cells under the egl-17 promoter (colored in light blue in Fig. 3b), SYG-1 localized ectopically to the region in the HSNL axon that is in contact with the 2° cells (Fig. 3d). Presynaptic components including RAB-3, GIT, SYD-2, SYD-1 and ELKS-1 also localized ectopically near the 2° epithelial cells in worms expressing egl-17::syg-2 (Fig. 3e,g,i and data not shown). Data from these gain-of-function experiments support the hypothesis that SYG-1 defines the location of presynaptic sites and is sufficient to recruit numerous presynaptic components to an axonal region that is defined by its localization. As predicted by this hypothesis, specific accumulation of synaptic components at the region in the HSNL axon that is near the 2° cells expressing egl-17::syg-2 is lost in syg-1 mutants (Fig. 3f,h,j,k). In these syg-1 mutants, presynaptic components are more anteriorly localized even in the presence of egl-17::syg-2 (Fig. 3f,h,j).
Figure 3.

SYG-1 is sufficient to recruit presynaptic components. (a) Schematic of a wild-type N2 worm at the mid-L4 stage of development. Head is to the left and dorsal is up. SYG-1 (green) normally localizes to the segment of HSNL axon (red) that is in contact with the 1° vulval epithelial cells, which express SYG-2 (blue). The region (between the two arrows) of HSNL axon that is in contact with the 2° vulval epithelial cells (gray), which normally does not express SYG-2, is devoid of SYG-1 accumulation. (b) Representative schematic, which shows that when 2° vulval epithelial cells are forced to express SYG-2 (blue) under the egl-17 promoter8 SYG-1 now localizes to the region of the HSNL axon that is near the 2° cells. (c) Confocal image showing that in N2 worms, localization of SYG-1 in HSNL is confined near the 1° vulval epithelial cells and is absent near the 2° cells (between arrows). (d,e,g,i) Representative confocal images showing various presynaptic molecules that accumulate in HSNL axon segment facing the 2° vulval epithelial cells, which express SYG-2 under the egl-17 promoter. (d) SYG-1::GFP. (e) GFP::SYD-2. (g) GFP::RAB-3. (i) GIT::YFP. (f,h,j) Presynaptic components GFP::SYD-2 (f), SNB-1::YFP (h) and GIT::YFP (j) fail to localize near the 2° epithelial cells in syg-1(ky652) mutants. In d to j, the shape of the 2° cells is visible owing to low-level expression of soluble GFP. *, vulva. Scale bar, 5 μm. (k) Quantification of number of puncta on the HSNL axon segment that contacts the 2° vulval epithelial cells. Error bars are ± 2 s.e.m.
SYD-1 & SYD-2 (liprin-α) are necessary for presynaptic assembly
SYG-1 might directly recruit many presynaptic components in parallel. Alternatively, SYG-1 might recruit one or a few scaffold molecules, which in turn assemble multiple presynaptic components in a linear fashion. To study the molecular assembly events downstream of SYG-1, we performed a visual genetic screen in HSNL for mutants with abnormal clustering of SNB-1::YFP. We reasoned that mutations in the assembly pathway should yield a phenotype in which presynaptic components fail to accumulate at presynaptic regions. From this screen, we isolated four mutant alleles, wy5, wy11, wy18 and wy19, in all of which synaptic vesicles failed to accumulate both near the vulval region and at the anterior ectopic regions where vesicles accumulate in syg-1 and syg-2 mutants (data not shown). Using complementation tests, we found that wy18 and wy19 were allelic to syd-1, whereas wy5 and wy11 were allelic to syd-2. Additional alleles (including ky292) of syd-2 were also isolated in a similar screen of synapses in the ASI neurons39.
syd-1 encodes a presynaptic protein that is predicted to contain a Rho-GTPase activating domain and has been implicated in specifying axonal identity of DD and VD motor neurons in C. elegans40. In syd-1 (ju82) mutants, fluorescence intensity of GFP::RAB-3 in the synaptic region of the HSNL axon decreased by about 86% relative to wild-type (Fig. 4a,c,e,l; Y. Jin, personal communication). Another synaptic vesicle marker, SNB-1::YFP, also fails to accumulate in the synaptic region of syd-1 mutants in a similarly severe fashion (97% reduction in fluorescence intensity; see Fig. 5), indicating that synaptic vesicles, rather than just RAB-3, may fail to cluster at presynaptic sites. In L4-stage syd-1 mutants, when the HSNL axon is growing into the nerve ring, transient GFP::RAB-3 puncta were detected throughout the length of the growing axon without accumulation at any particular location, a pattern that was not observed in N2 worms of the same age (Fig. 4a,b,d). This observation implies that synaptic vesicles can be made and trafficked out of the HSNL cell body in syd-1 mutants, but are either not recruited to presynaptic sites, not retained at them, or both.
Figure 4.

Three presynaptic components show severe defects in localizing to the synaptic region of HSNL in syd-1 and syd-2 mutants. (a) Schematic of the HSNL (red) in mid-L4 worms. Boxed region on the right corresponds to where confocal images c,e,g and h–k were taken. Boxed region on the left denotes the region where images b,d and f were taken in different worm strains. Arrow, synaptic region in HSNL near the vulva (*); arrowhead, HSNL cell body. (b,d,f) At the mid-L4 stage of development, GFP::RAB-3 is normally absent from HSNL axon that is anterior to the vulva (b), but transiently accumulates in this region in syd-1(ju82) (d) and syd-2(ju37) (f) mutants. (c,e,g) GFP::RAB-3 accumulates near the vulva in wild-type N2 worms (c), but fails to localize in syd-1 (e) and syd-2 (g) mutants. (h–k) ELKS-1::YFP and GIT::YFP also fail to cluster near the vulva in syd-1 (h and j, respectively) and syd-2 (i and k, respectively) mutants. (l) Quantification of GFP::RAB-3, ELKS-1::YFP and GIT::YFP fluorescence intensities at the synaptic region (10 μm segment) near the vulva in syd-1 and syd-2 mutants relative to wild-type N2 worms. Each bar represents average intensity from 10 individuals and error bars are ± 2 s.e.m. Scale bar, 5 μm.
Figure 5.
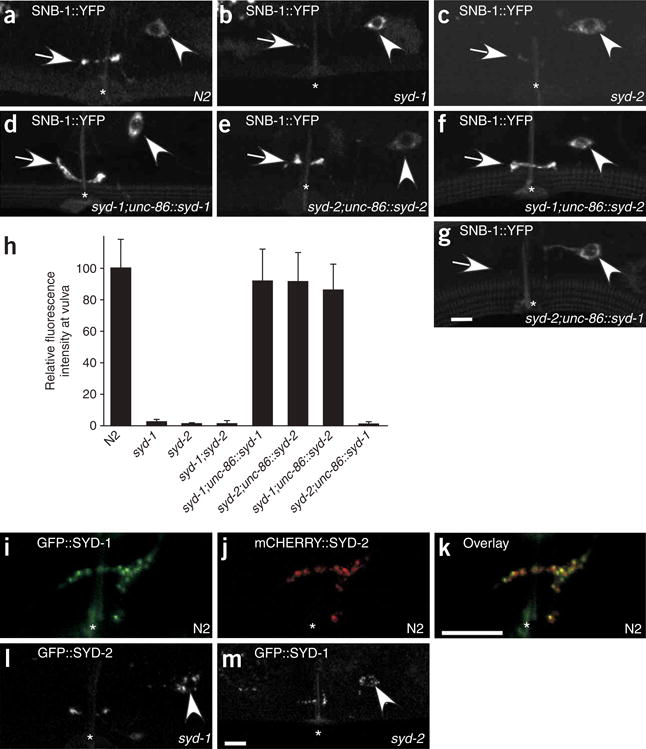
SYD-1 and SYD-2 function cell-autonomously in HSNL and SYD-2 overexpression can bypass requirement for SYD-1. (a–g,i–k) Head is to the left and dorsal is up. Arrow, synaptic region in HSNL; arrowhead, HSNL cell body; *, vulva. (a–c) In HSNL, SNB-1::YFP localizes to the synaptic region near the vulva in (a) wild-type N2 worms but fails to localize in (b) syd-1 (ju82) and (c) syd-2 (ju37) mutants. (d) Expression of SYD-1 under the unc-86 promoter, which drives expression only in the HSNs in the vulval region, fully rescues clustering of SNB-1::YFP in the synaptic region of syd-1 mutants. (e) Similarly, expressing SYD-2 in HSNL restores accumulation of SNB-1::YFP in syd-2 mutants. (f) Expressing SYD-2 in HSNL of syd-1 mutants also leads to wild-type levels of SNB-1::YFP accumulation near the vulva. (g) Expressing SYD-1 in syd-2 mutants fails to rescue SNB-1::YFP clustering. (h) Quantification of the SNB-1::YFP fluorescence intensity near the vulval region in various mutant and transgenic backgrounds relative to that in N2 worms. Each bar represents average intensity from 10 individuals, and error bars are ± 2 s.e.m. (i–k) Confocal images of a worm that expresses GFP::SYD-1 and mCHERRY::SYD-2 simultaneously. (i) GFP::SYD-1 (green) and (j) mCHERRY::SYD-2 puncta (red) colocalize in the synaptic region in HSNL (k). (l) Localization of GFP::SYD-2 in a syd-1 mutant worm. (m) Localization of GFP::SYD-1 in a syd-2 mutant worm. Scale bar, 5 μm.
We next determined whether the clustering of synaptic vesicles is specifically affected in syd-1 mutants or whether the assembly of other presynaptic components is similarly compromised. All the presynaptically localized proteins that we tested (those shown in Fig. 1) failed almost completely to accumulate at presynaptic sites in syd-1 mutants, with the exception of GFP::SYD-2 (Fig. 4h,j,l; Table 1). The fluorescence intensities of ELKS-1::YFP and GIT::YFP in the synaptic region were reduced in syd-1 mutants to 3.6% and 3.4% of the wild-type intensity, respectively (Fig. 4h,j,l). Similarly, UNC-57 (endophilin) and SNN-1 (Synapsin-I) also failed to assemble at presynaptic sites in syd-1 mutants (Table 1 and data not shown). Furthermore, SAD-1 kinase, which was recently shown to be important for regulating neurotransmitter release41 was no longer concentrated in the synaptic region, but was instead diffused throughout the HSNL axon in syd-1 mutants (data not shown). These results imply that SYD-1 is absolutely required for the accumulation of synaptic vesicles as well as the localization of other presynaptic components such as ELKS-1, GIT, SAD-1, UNC-57 and SNN-1 at the developing synapses.
Table 1. Mutant analysis of presynaptic assembly in HSNL synapses.
| SYG-1::YFP | GFP::SYD-2 | GFP::SYD-1 | SAD-1::YFP | GIT::YFP | ELKS-1::YFP | GFP::RAB-3 | SNB-1::YFP | |
|---|---|---|---|---|---|---|---|---|
| syg-1 (NEPH) | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| syd-1 | – | + | NA | ++ | ++ | ++ | ++ | ++ |
| syd-2 (liprin-α) | – | NA | + | ++ | ++ | ++ | ++ | ++ |
| sad-1 (sad-1 kinase) | – | – | – | NA | + | – | + | + |
| rpm-1 (highwire) | ND | ND | ND | ND | ND | ND | – | – |
| fsn-1 | ND | ND | ND | ND | ND | ND | – | – |
| elks-1 (ERC) | – | – | – | – | – | NA | – | – |
| unc-10 (RIM) | – | – | – | – | – | – | – | – |
| git | ND | – | – | ND | NA | – | – | – |
| unc-13 (Unc-13) | – | – | – | – | – | – | – | – |
| unc-18 (Munc18) | – | – | – | – | – | – | – | – |
| unc-26 (synaptojanin) | – | – | – | – | – | – | – | ND |
| unc-2 (Ca2+ channel) | – | – | – | – | – | – | – | ND |
| unc-36 (Ca2+ channel) | – | – | – | – | – | – | – | ND |
| unc-104 (KIF1A) | – | – | – | – | – | – | ++ | ++ |
Mutants and their homologs are listed vertically on the left and presynaptic protein markers are listed across the top of the table. Phenotype (localization of the presynaptic protein marker) of a given mutant is reported using the following nomenclature: ++ severely defective; + moderately defective; – normal; ND, not determined; NA, not applicable.
Mutations in syd-2 have previously been shown to affect active zone morphology of synapses at the neuromuscular junction in both C. elegans and D. melanogaster15,16. In wy5, wy11, ky292 and the canonical allele ju37 of syd-2, GFP::RAB-3 failed to cluster in the synaptic region of HSNL. Similar to the phenotype found in syd-1 mutants, GFP::RAB-3 was scattered throughout the growing axon of L4 larvae in syd-2 mutants (Fig. 4f,g,l). These results are consistent with a recent report that there are 20–29% fewer synaptic vesicles at the fly neuromuscular junction synapses in Dliprin-α mutants42. However, the extent of synaptic vesicle loss from synapses in HSNL of syd-2 mutants is much more pronounced than that observed in D. melanogaster. The fluorescence intensity of GFP::RAB-3 in the synaptic region near the vulva in syd-2(ju37) mutants was reduced by over 95% from that in wild-type worms (Fig. 4l). We next asked whether other presynaptic proteins also failed to assemble at synapses in syd-2 mutants. Localization of all the presynaptic components to the synaptic region that we examined was affected to the same severe extent in syd-2 mutants as it was in syd-1 mutant worms, with the exception of GFP::SYD-1 localization (Fig. 4i,k,l; Table 1). These data indicate that like SYD-1, SYD-2 (liprin-α) may also be required for recruiting multiple presynaptic components to the synapse, retaining them or both.
SYD-1 & SYD-2 (liprin-α) function cell-autonomously in HSNL
SYD-1 was shown to function cell autonomously in the DD and VD motor neurons to determine the axon-dendrite polarity40. SYD-2 (liprin-α) has been shown to localize and function at both pre- and postsynaptic sites15,43. To determine whether SYD-1 and SYD-2 function presynaptically in HSNL to assemble presynaptic components, we expressed unc-86::syd-1 and unc-86::syd-2 transgenes in syd-1 and syd-2 mutants, respectively. The unc-86 promoter drives expression in a group of neurons that includes HSNL, but not in the postsynaptic HSNL targets or other surrounding tissues. The unc-86::syd-1 and unc-86::syd-2 transgenes fully restored localization of SNB-1–labeled synaptic vesicles in syd-1 and syd-2 mutants, respectively (Fig. 5a–e,h). These data are consistent with those previously reported15 that showed a cell autonomous role in presynaptic neurons for SYD-2/liprin-α.
To test whether SYD-1 and SYD-2/liprin-α function in HSNL to assemble the rest of the presynaptic machinery, we used egg-laying behavior as an assay for HSNL function. Both syd-1 and syd-2 mutants showed egg-laying defects that could be rescued by transgenic expression of unc-86::syd-1 and unc-86::syd-2 transgene, respectively (Supplementary Fig. 3 online). Taken together, these results strongly imply that SYD-1 and SYD-2 function cell-autonomously in the presynaptic neurons to assemble synaptic vesicles and other presynaptic components at synapses.
SYD-1 is likely to be a positive regulator of SYD-2
We were surprised to find that that overexpression of SYD-2/liprin-α under the unc-86 promoter fully restored SNB-1::YFP accumulation at synapses and rescued the egg-laying defects in syd-1 mutants (Fig. 5f,h and Supplementary Fig. 3). In contrast, overexpression of SYD-1 in syd-2 mutants was not sufficient to restore accumulation of SNB-1::YFP at synapses (Fig. 5g,h). These data indicate that SYD-1 may function upstream of SYD-2 as a positive regulator. Furthermore, GFP::SYD-1 and mCHERRY::SYD-2 showed a high degree of colocalization in the synaptic region of HSNL, implying that SYD-1 and SYD-2 function together at presynaptic terminals (Fig. 5i–k).
To understand how SYD-1 regulates SYD-2, we examined the synaptic localization of SYD-1 in syd-2 mutants and the synaptic localization of SYD-2 in syd-1 mutants. If SYD-1 functions to recruit SYD-2 to synapses, one would expect SYD-2 to not be localized in syd-1 mutants. However, we found that the fluorescence intensity of GFP::SYD-2 at synapses in syd-1(ju82) mutants decreased to 46.0 ± 8.6% of the wild-type intensity (n = 20), although the qualitative appearance of GFP::SYD-2 remained unchanged (Fig. 5l). Similarly, the total fluorescence intensity of GFP::SYD-1 puncta decreased to 50.3 ± 4.9% in syd-2(ju37) mutants compared with the wild-type value (n = 30), but the punctate appearance of the SYD-1 staining pattern was indistinguishable from the wild-type (Fig. 5m). Thus, although the localizations of SYD-1 and SYD-2 (liprin-α) are partially dependent on one another, such partial dependence cannot account for the more or less equally severe synaptic defects observed in syd-1 and syd-2 mutants. In syd-1 mutants, although there is a significant amount of SYD-2 still localized at the synapses, the severity of the presynaptic assembly defect is as dramatic as that in the syd-2 mutants. These results, taken together with the observation that overexpression of SYD-2 in syd-1 mutants can bypass the requirement for SYD-1, are consistent with a model in which SYD-1 acts as a positive regulator of SYD-2 (liprin-α). This model also suggests that SYD-1 and SYD-2 (liprin-α) are not absolutely dependent on each other to localize to presynaptic terminals.
UNC-104 (KIF1A) is required to traffic some presynaptic proteins
The localization of synaptic vesicles as well as other presynaptic molecules is affected in syd-1 and syd-2 mutants. It is possible that some or all presynaptic proteins localize to synapses through their association with synaptic vesicles (or synaptic vesicle precursors). We used unc-104 mutants to test which synaptic proteins localize to synapses by synaptic vesicle–associated mechanisms. UNC-104 (KIF1A) is required for trafficking synaptic vesicles from the cell body to the presynaptic sites in C. elegans44. Consistent with previous observations, the synaptic localization of vesicle–associated protein RAB-3 is drastically reduced in unc-104 mutants (Fig. 6a)27,38. Similarly, UNC-57 and SNN-1 are lacking almost completely from synapses in unc-104 mutants, and are instead localized to the HSNL cell body (Fig. 6b,c)27. These results indicate that UNC-57 (endophilin) and SNN-1 (Synapsin-I) may accumulate at synapses through their association with synaptic vesicles.
Figure 6.

Some, but not all, presynaptic components fail to be trafficked out of the HSNL cell body in unc-104 mutants. (a–h) Confocal images of fluorescently tagged HSNL presynaptic components in unc-104(e1265) mutants. Head is to the left and dorsal is up. Arrow, synaptic region near the vulval slit (*); arrowhead, HSNL cell body. (a–c) GFP::RAB-3 (a), UNC-57::YFP (b), and GFP::SNN-1 (c) are absent from synaptic region in HSNL axon near the vulva and instead accumulate in the cell body. (d–h) ELKS-1::YFP (d), GIT::YFP (e), SAD-1::YFP (f), GFP::SYD-1 (g) and GFP::SYD-2 (h) localize normally to the synaptic regions in HSNL near the vulva in unc-104 mutants. Scale bar, 5 μm.
The synaptic localization of GIT, SAD-1, ELKS-1, SYD-1 and SYD-2 (liprin-α) was independent of UNC-104, indicating that these proteins localize at synapses using mechanisms different from those used by synaptic vesicles (Fig. 6d–h). Therefore, SYD-1 and SYD-2 (liprin-α) are necessary for clustering synaptic vesicles and vesicle-associated proteins at presynaptic sites as well as for the assembly of active zone components. Given that the vertebrate homolog of SYD-2, liprin-α, directly interacts with ERC (ELKS-1) and GIT, it is possible that SYD-2 clusters these components to synapses in HSNL by direct binding33,34.
SYD-1 and SYD-2 act downstream of SYG-1
In order to determine whether syd-1 and syd-2 are epistatic to syg-1, we characterized the synaptic phenotype in syg-1; syd-1 and syg-1; syd-2 double mutants. SNB-1::YFP fails to accumulate at presynaptic sites, both at the wild-type location and at the ectopic anterior location, in syg-1; syd-1 and syg-1; syd-2 double mutants (Fig. 7a–f). This genetic analysis implies that syd-1 and syd-2 are epistatic to syg-1. Consistent with these results, although localization of GFP::SYD-1 and GFP::SYD-2 is affected in syg-1 mutants, SYG-1 localizes normally near the primary vulval epithelial cells during development in both syd-1 and syd-2 mutants (Fig. 7g,h).
Figure 7.
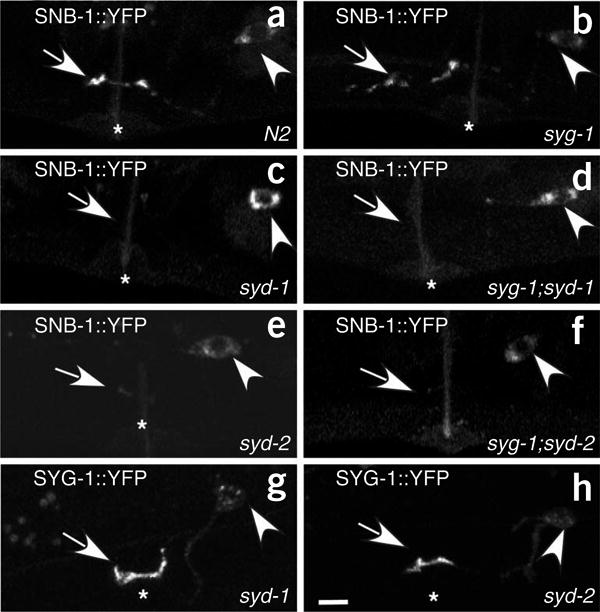
Epistasis analysis of syg-1, syd-1 and syd-2. (a–h) Confocal images of fluorescently labeled presynaptic components in HSNL near the vulva. Head is to the left and dorsal is up. Arrow, localization of presynaptic components to the synaptic region near the vulva (*). Arrowhead shows the HSNL cell body. (a) SNB-1::YFP localizes to the synaptic region of HSNL near the vulva in N2 worms. (b) SNB-1::YFP localizes to ectopic location that is anterior to the vulva in syg-1(ky652) mutants. (c,e) SNB-1::YFP fails to accumulate at the synaptic region near the vulva in (c) syd-1 (ju82) and (e) syd-2 (ju37) mutants. (d,f) SNB-1::YFP is absent from both the synaptic region near the vulva and the ectopic region of the HSNL axon anterior to the vulva in (d) syg-1;syd-1 and (f) syd-2;syg-1 double mutants. (g,h) Localization of SYG-1::GFP to synaptic region in developmental stage mid-L4 is normal in (g) syd-1 and (h) syd-2 mutants. Scale bar, 5 μm.
Ectopic localization of SYG-1 near the 2° vulval epithelial cells in worms expressing egl-17::syg-2 leads to the accumulation of multiple presynaptic components in the same region of the HSNL axon (Fig. 3). If this recruitment of presynaptic components by SYG-1 is dependent on SYD-1 and SYD-2 (liprin-α) function, then these components should fail to cluster in syd-1 and syd-2 mutants. Indeed, SYG-1-dependent recruitment of RAB-3 and GIT in worms expressing egl-17::syg-2 did not occur in syd-1 and syd-2 mutants (Supplementary Fig. 4 online). Collectively, these results indicate that SYD-1 and SYD-2 (liprin-α) may function downstream of SYG-1 to assemble multiple components of presynaptic specializations.
Many presynaptic proteins are not required for assembly
Previous studies have shown that vertebrate SYD-2 (liprin-α) directly interacts with numerous proteins, including ELKS-1 (ERC), GIT, UNC-10 (RIM) and UNC-104 (KIF1A)33,34,45. If these interactions are conserved in C. elegans, they could provide a mechanism by which SYD-2 (liprin-α) assembles ELKS-1 (ERC), GITand UNC-10 (RIM) at presynaptic sites. It is also conceivable that the localization of SYD-2 (liprin-α) depends on ELKS-1 (ERC), GIT and UNC-10 (RIM). To address whether these putative interactions are essential for the synaptic accumulation of SYD-2 (liprin-α), we examined the localization of GFP::SYD-2 in elks-1(tm1233), unc-10(e102) and git (sequence name F14F3.2, allele tm1962) mutants. GFP-SYD-2 clustered normally at presynaptic sites in all mutants, implying that ELKS-1 (ERC), UNC-10 (RIM) and GIT are not essential for the localization of SYD-2 (liprin-α) (Supplementary Fig. 5 online, Table 1). The elks-1 allele tm1233 is likely to be null, as it contains a deletion that leads to an early stop codon. The git allele tm1962 is a deletion that causes a predicted frameshift in the C-terminal half of the protein. It is possible that the N-terminal half of the protein retains a certain amount of function. We next suspected that the putative interactions between ELKS-1 (ERC) or UNC-10 (RIM) and SYD-2 (liprin-α) might be important for regulating interaction of SYD-2 (liprin-α) with other proteins such as UNC-104 (KIF1A) and GIT33,45. Biochemical assays have shown that the UNC-104 (KIF1A) and GIT interaction domains overlap with the ELKS-1 (ERC) and UNC-10 (RIM) interaction domains on vertebrate SYD-2 (liprin-α). Notably, GIT::YFP and GFP::RAB-3 showed normal synaptic localization in elks-1 and unc-10 mutants (Supplementary Fig. 5). Furthermore, the localization of GFP::SYD-1 was also not affected by elks-1 and unc-10 mutations (Supplementary Fig. 5). These data indicate that SYD-1 and SYD-2 (liprin-α) may localize to HSNL synapses by means of interactions that have not yet been identified.
To further extend our analysis of the molecular assembly, we examined the localization of synaptic vesicle markers and other presynaptic proteins in a panel of known presynaptic mutants. These mutants included calcium channel subunit mutants (unc-2 and unc-36), exocytosis mutants (unc-13, unc-10, unc-18), an endocytosis mutant (unc-26) and synaptic development mutants (sad-1, rpm-1, fsn-1) (Table 1). Consistent with the role of SAD-1 in other neurons, mutation in sad-1 partially affected accumulation of synaptic vesicle clusters39 (Supplementary Fig. 6 online). Given that clustering of synaptic vesicles in syd-2 mutants is more severely affected than it is in sad-1 mutants, and that SAD-1::YFP localization is dependent on SYD-2, SAD-1 might be one of many effectors acting downstream of SYD-2. Notably, the localization of GIT::YFP but not ELKS-1::YFP was also partially affected in sad-1 mutants. These data indicate that SAD-1 may be involved not only in the clustering of synaptic vesicles but also in some other aspects of presynaptic assembly. Consistent with the role of SYD-2 being one of the most upstream players in the assembly pathway, the localization of GFP::SYD-2 is not affected in sad-1 mutants (Supplementary Fig. 6). Although the mutants with defects in synaptic function—unc-26 (synaptojanin), unc-2 (α subunit of the calcium channel), unc-36 (α2δ subunit of the calcium channel), unc-10 (RIM), unc-13 (Munc13) and unc-18 (Munc18)—exhibited severe uncoordinated locomotion, most likely because of failed synaptic transmission, all of these mutants showed remarkably normal synaptic vesicle clustering as well as normal assembly of other presynaptic markers in HSNL (Table 1). These results indicate that the assembly of many presynaptic proteins at the synapse may be independent of each other.
Worms mutant for rpm-1 and fsn-1, two genes that affect presynaptic development of the DD and VD motor neurons, do not have defects in synaptic vesicle accumulation in HSNL. In contrast, the loss of synaptic vesicle clusters at synapses in the DD and VD neurons in syd-1 and syd-2 mutants is much less marked than in HSNL15,40. This difference indicates that different synapses may use diverse molecules during their development. At least four other types of neurons—PLM, ALM, AVE and DA9—have the same strong dependence on syd-2 for clustering synaptic vesicles as HSNL (data not shown).
Discussion
Our genetic analysis of molecular components in HSNL implies a hierarchical organization of presynaptic assembly with three layers (Supplementary Fig. 7 online). In the first layer, the transmembrane adhesion molecules SYG-1 and SYG-2 define the synaptic targets and the subcellular location of presynaptic sites in HSNL axon. In the second layer, SYD-1 and SYD-2 (liprin-α) function together downstream of SYG-1 to assemble synaptic vesicles and other presynaptic components. SYG-1 is only expressed and required in a subset of neurons, whereas SYD-1 and SYD-2 (liprin-α) are more ubiquitously expressed in neurons, so SYD-1 and SYD-2 (liprin-α) are also likely to function downstream of other synaptogenic molecules. Furthermore, SYG-1 and SYG-2 are transiently present at the synaptic junctions at early stages of synaptogenesis, whereas SYD-1 and SYD-2 (liprin-α) persist at synapses even in adults. In the third layer, synaptic vesicles and other presynaptic proteins may be organized by specific regulators, but their accumulation at presynaptic regions is mostly independent of each other.
SYG-1 initiates multiple aspects of presynaptic assembly
We have previously shown that SYG-1 and SYG-2 are essential in patterning the synaptic connections of HSNL. SYG-1 and SYG-2 are both adhesion molecules that are present on the cell surface of HSNL and the primary vulval epithelial cells, respectively. In syg mutants, HSNL fails to form synapses onto appropriate target cells, instead forming synapses on abnormal cells. We showed that these ectopic synapses not only contain synaptic vesicles but also many other presynaptic molecules, implying that the ectopic synapses are bona fide presynaptic specializations.
These data imply that presynaptic specializations can form in the absence of SYG-1 and that the function of SYG-1 is to ensure that synapses are formed between certain cells and at particular locations. Consistent with this model, the presence of SYG proteins at HSNL synapses is only detectable during the early phase of synapse formation—presumably when synaptic target selection takes place. This transient localization of SYG proteins is also in marked contrast with the persistent synaptic localization of structural and functional pre-synaptic proteins, such as SYD-1, SYD-2 (liprin-α), SAD-1, ELKS-1 and GIT. Furthermore, this model is supported by genetic epistasis analysis, which showed that SYD-1 and SYD-2 (liprin-α) function downstream of SYG-1. It also implies that SYD-1 and SYD-2 (liprin-α) can be recruited and assembled by other potential synaptogenic molecules to form ectopic synapses in HSNL.
SYD-1 and SYD-2/liprin-α are key assembly molecules in HSNL
During the construction of functional presynaptic specializations, multiple components must accumulate at designated regions of the axon. Within the presynaptic regions, these components are further organized into subsynaptic zones to achieve the complex electrophysiological and biochemical functions of presynaptic terminals. The multiple synaptic components can reach synapses through vesicular transport or passive diffusion. For example, synaptic vesicles are trafficked by the kinesin motor UNC-104 (KIF1A) (ref. 44). It has also been proposed that certain dense-core vesicles may carry some of the presynaptic active zone proteins during synapse formation46,47. Other proteins may localize to synapses through direct interactions with synaptic scaffolding molecules. For example, Dap160 (intersectin) recruits the presynaptic endocytosis machinery dynamin, endophilin and synaptojanin in the D. melanogaster neuromuscular junction25,26. It appears that SYD-1 and SYD-2 (liprin-α) are required for recruiting vesicularly transported components such as synaptic vesicle precursors, as well as synaptic vesicle–independent proteins such as ELKS-1, GIT and SAD-1 kinase. These data indicate that SYD-1 and SYD-2 (liprin-α) may be key assembly molecules in the development of presynaptic sites in HSNL.
How do SYD-1 or SYD-2 assemble synaptic vesicles? The synaptic vesicle phenotypes in syd-1 or syd-2 mutants are different from the phenotype found in unc-104 mutants. Although synaptic vesicle precursors are missing from the HSNL presynaptic region in all three mutants, the localization of vesicles is dramatically different. Synaptic vesicles are exclusively found in the HSNL cell body in unc-104 mutants, consistent with the notion that UNC-104 (KIF1A) transports vesicle precursor from the cell body to synaptic locations. In syd-1 and syd-2 mutants, RAB-3–labeled puncta were found throughout the developing HSNL axon, indicating that synaptic vesicles are transported out of the cell body. Notably, unlike the case in wild-type worms, HSNL axons failed to cluster vesicles near synapses in the syd mutants. Instead, vesicles were transported along the axon to beyond their normal locations. This phenotype can be explained by an ‘unloading’ defect in syd mutants, in which synaptic vesicle precursors fail to dissociate from the microtubules and accumulate at HSNL synapses. The SYD-2 ortholog liprin-α has been shown to bind to UNC-104 (KIF1A) directly. Therefore, at least two potential models can be proposed. First, SYD-2 (liprin-α) functions on synaptic vesicles through its interaction with UNC-104 (KIF1A) to regulate the activity of the motor, as suggested by a previous study42. Second, SYD-2 localizes to synapses first and functions at the presynaptic sites to ‘unload’ synaptic vesicles through the regulation of motor functions. We favor the latter model based on data from HSNL because SYD-2 localizes to presynaptic sites independent of UNC-104. Finally, SYD-1 could regulate the interaction of SYD-2 and UNC-104.
It is worth noting that the phenotypes of syd-2 are different in different neurons. In inhibitory GABAergic neuromuscular synapses in the DD and VD neurons, the same syd-2 mutation causes expansion of presynaptic active zones with normal numbers of synaptic vesicles15. It is not yet known if other molecular markers localize to these syd-2 synapses. Furthermore, RPM-1 and FSN-1, two genes essential for the presynaptic development of DD and VD synapses, appear to be dispensable for HSNL synapses. Therefore, the exact roles of each molecule might be different in diverse developmental contexts.
The hierarchy model is supported by genetic evidence as well as several pieces of biochemical data. SYD-1 contains a PDZ domain and SYG-1 has a PDZ-binding motif at the C terminus. Thus, SYG-1 might directly interact with SYD-1, thereby potentially linking layer 1 and layer 2. Second, vertebrate liprin-α has been shown using a variety of in vitro binding assays to bind to presynaptic proteins such as GIT, ERC and RIM, which might explain the essential role of SYD-2 (liprin-α) in assembling these presynaptic proteins. Notably, in vitro experiments showed that GIT, ERC and RIM bound to different domains of SYD-2 (liprin-α), indicating that there may be a parallel assembly pathway downstream of SYD-2 (liprin-α). The hierarchy of organization implies that synaptogenesis in vivo is initiated by the recruitment of general scaffolding molecules by specific adhesion molecules, triggering the construction of the presynaptic apparatus.
Roles of other presynaptic proteins on presynaptic assembly
The relative importance of individual proteins to presynaptic development may be deduced from our marker-mutant analysis. SAD-1 kinase specifically affects vesicle accumulation and GIT localization, without affecting ELKS-1. Localization studies showed that SAD-1 failed to localize in syd-1 or syd-2 mutants, whereas SYD-1 and SYD-2 (liprin-α) localized normally in sad-1 mutants. Therefore, it is conceivable that SAD-1 is one of the downstream effectors of SYD-1 and SYD-2 (liprin-α) that is involved in certain aspects of synaptic assembly. sad-1 mutants exhibited different synaptic phenotypes in DD and VD neurons than in HSNL39, indicating that the molecular mechanism of synaptic assembly might vary between different types of synapses.
Notably, presynaptic development is more or less intact in a large number of mutants defective in synaptic function, including unc-10, unc-13 and unc-18. The α subunit of calcium channels has been shown to bind to basal lamina protein laminin-β2 and trigger presynaptic assembly in the vertebrate neuromuscular junction9. We tested this hypothesis using two mutations in different calcium channel subunits, unc-2 and unc-36. We were intrigued to find that the accumulation of all presynaptic markers appeared to be normal in both mutants. One possible explanation is that HSNL synapses do not contain obvious basal lamina structures; therefore, calcium channels do not have essential roles in presynaptic assembly in HSNL. It is also possible that subtle developmental defects that are beyond our detection threshold might exist in these mutants. However, not only did all the presynaptic markers examined accumulate at appropriate locations in these mutants, the appearance of individual markers was indistinguishable from wild-type controls. Therefore, despite the severe functional defects in synaptic transmission, these mutants appear to have relatively normal presynaptic development. The lack of phenotypes in many mutants for important presynaptic proteins further emphasizes the unique importance of SYD-1 and SYD-2 (liprin-α) in presynaptic assembly in HSNL.
Methods
Strains and genetics
Wild-type worms were of Bristol variety N2 strain. Strains were maintained using standard methods48 at 20 °C. Some strains were provided by the Caenorhabditis Genetics Center.
Transgenic lines
kyIs235 [Punc-86::snb-1::yfp; Punc-4::lin-10::dsred; Podr-1::dsred], kyIs288 [Punc-86::syg-1::gfp; Podr-1::dsred], kyEx672 [Pegl-17::syg-2; Podr-1::gfp], wyIs22 [Punc-86::gfp::rab-3; Podr-1::dsred], wyIs12 [Punc-86::gfp::syd-2; Podr-1::gfp], wyEx12 [Punc-86::syd-2; Podr-1::dsred], wyEx118 [Punc-86::gfp::syd-1; Podr-1::dsred], wyEx122 [Punc-86::gfp::snn-1; Podr-1::dsred], wyEx146 [Punc-86::F14F3.2(git)::yfp; Podr-1::dsred], wyEx163 [Punc-86::sad-1::yfp; Podr-1::dsred], wyEx196 [Punc-86::elks-1::yfp; Podr-1::dsred], wyEx200 [Punc-86::unc-57::yfp; Podr-1::dsred], wyEx314 [Punc-86::syd-1; Podr-1::gfp], wyEx410 [Punc-86::mcherry::rab-3; Punc-86::gfp::syd-2; Podr-1::gfp], wyEx420 [Punc-86::mcherry::rab-3; Punc-86::gfp::syd-1; Podr-1::gfp], wyEx431 [Punc-86::mcherry::rab-3; Podr-1::dsred], wyEx434 [Punc-86::gfp::syd-1; Punc-86::mcherry::syd-2; Podr-1::dsred].
Mutants
LG1, unc-13(e450); LGII, syd-1(ju82), syd-1(wy18), syd-1(wy19), unc-104(e1265); LGIII, fsn-1(hp1), unc-36(e251); LGIV, elks-1(tm1233), unc-26(e345); LGV, rpm-1(ju41); LGX, sad-1(ky330), syd-2(ju37), syd-2(ky292), syd-2(wy5), syd-2(wy11), syg-1(ky652), syg-2(ky671), unc-10(e102), unc-18(e81), unc-2(e55).
Genetic screens
The ky292 allele of syd-2 was isolated from a secondary visual screen of 10,000 F2s for mutants with defects in SNB-1::GFP clustering in the ASI neuron39. An independent F2 semiclonal screen covering ∼6,000 haploid genomes was performed for mutants with abnormal clustering of SNB-1::YFP in the HSNL neuron (transgenic line kyIs235). The wy5 and wy11 alleles of syd-2, as well as the wy18 and wy19 allele of syd-1, were isolated from this visual screen. In addition, three alleles of syg-1 and eight alleles of syg-2 were isolated from the HSNL screen (D. Chao and K.S., unpublished data). Worms were mutagenized with EMS in both screens.
Molecular biology
The unc-86::gfp::syd-2 construct was created by replacing a NotI (blunted)-NcoI fragment in pJH23 (ref. 49) with a SphI (blunted)-NcoI fragment containing the unc-86 promoter from the N-terminal GFP pSM vector7. The gfp-containing fragment was cut out and the plasmid religated to generate the rescuing construct unc-86::syd-2. To generate unc-86::unc-57::yfp, UNC-57 coding region was amplified using PCR reactions from N2 genomic DNA and ligated between NheI and KpnI in the C-terminal YFP pSM vector. To make unc-86::gfp::rab-3, we amplified a plasmid fragment containing gfp::rab-3 (see Acknowledgments) using PCR and ligated it between NheI and ApaI in pSM. unc-86::mcherry::rab-3 was made by amplifying mcherry (see Acknowledgments) and cloning it into the XmaI and NheI sites of pSM vector. rab-3 was amplified and cloned downstream of mcherry between NheI and ApaI. unc-86::mcherry::syd-2 was generated by cloning mcherry between XmaI and NheI, and syd-2 between NheI and KpnI. N-terminal GFP pSM vector and C-terminal YFP vector were converted into Gateway Destination vectors by ligating the attR containing Gateway Cassette Rfa between NheI and KpnI. cDNAs in attL containing pDONR201 vector (from OpenBiosystems) encoding SAD-1 (F15A2.6), ELKS-1 (YK5760) or GIT (F14F3.2) were recombined into the C-terminal YFP pSM Gateway Destination vector with LR clonase (Invitrogen). CDNAs encoding SYD-1 and SNN-1 were similarly recombined into the N-terminal GFP pSM Gateway Destination vector.
Transgenic lines and fluorescence microscopy
Plasmids were injected into the gonad of adult worms at concentrations of 0.5–1ng μl−1 with 10–20 ng μl−1 of either odr-1::dsred or odr-1::gfp as a coinjection marker. Multiple transgenic lines of each transgene were examined for fluorescence expression and localization patterns. Fluorescence images were obtained on either a Bio-Rad MRC-1024 or a Zeiss LSM 510 META laser scanning confocal imaging system.
Quantification
To quantify fluorescence intensities, fluorescence images of synapses in young adults were captured with a Zeiss AxioCam MRm camera on a Zeiss Axioplan 2 Imaging System with a ×63 objective. For the quantification of syg-1, syd-1 and syd-2 mutants, the vulval segment was defined as the most dorsally defasciculated part of HSNL (∼ 10 μm). This segment of axon contacts the 1° vulval epithelial cells at the L4 stage. The anterior ectopic synaptic region was defined as the 30 μm of HSNL immediately anterior to the vulval region. The fluorescence intensity of particular fusion proteins at the synapses was obtained by integrating pixel intensity across the specific segments of axons. Error bars represent 2 s.e.m. This, which is a more stringent value than 1 s.e.m., corresponds to a P value of 0.05.
Egg-laying assay
The rate of egg-laying was measured as described before50. Briefly, staged L4 worms were allowed to develop for 29 h at 20 °C, and 20 adults were transferred to a fresh Petri plate at 20 °C. After 30 min, the developmental stage of each freshly laid egg was identified as being in one of three categories: one- to eight-cell stage, nine-cell stage to comma stage, or postcomma stage. Differences between strains measured by this assay reflect differences in rates of egg laying rather than differences in rates of egg development or in rates of egg production.
Supplementary Material
Acknowledgments
We thank D. Chao (Stanford University) for contributing alleles of syd-1 and syd-2; the Caenorhabditis Genetics Centre, Y. Jin (University of California, Santa Cruz), the C. elegans Gene Knockout Consortium and the Japanese NBPR for strains; Y. Kohara (National Institute of Genetics, Japan) for EST cDNAs; M. Nonet (Washington University, St. Louis) for the rab-3 cDNA construct and J. Audhya (University of California, San Diego) for the mcherry cDNA; Y Jin for communicating unpublished results; C. Gao and F. Chen for technical support; C. Garner for critical comments on the manuscript. This work was funded by the following grants to K.S.: US National Institutes of Health 1R01NS048392, the McKnight Endowment Fund, Sloan research fellowship and Whitehall Foundation fellowship.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Author Contributions: M.R.P. and K.S. designed the experiments, analyzed the data and wrote the paper. M.R.P, E.K.L. and V.Y.P performed the experiments. J.G.C. and C.I.B. designed the ASI synapse screen. J.G.C. isolated syd-2(ky292) and M.Z. contributed the GFP::SYD-2 construct.
Competing Interests Statement: The authors declare that they have no competing financial interests.
References
- 1.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- 2.Ackley BD, Jin Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 2004;27:540–547. doi: 10.1016/j.tins.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 4.Dean C, et al. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 6.Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- 7.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 8.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 9.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 10.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KG, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Dick O, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 14.Bamji SX, et al. Role of β-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-α and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 17.Ackley BD, et al. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci. 2005;25:7517–7528. doi: 10.1523/JNEUROSCI.2010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kittel RJ, et al. Bruchpilot promotes active zone assembly, Ca2+-channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 19.Wagh DA, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Liao EH, Hung W, Abrams B, Zhen M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 2004;430:345–350. doi: 10.1038/nature02647. [DOI] [PubMed] [Google Scholar]
- 21.Nakata K, et al. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 2000;26:331–343. doi: 10.1016/s0896-6273(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 24.Wan HI, et al. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 25.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Marie B, et al. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Sieburth D, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- 28.Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 30.Olsen O, et al. Neurotransmitter release regulated by a MALS-liprin-α presynaptic complex. J Cell Biol. 2005;170:1127–1134. doi: 10.1083/jcb.200503011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serra-Pages C, et al. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoch S, et al. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 33.Ko J, et al. Interaction between liprin-α and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003a;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-α family of multidomain proteins. J Biol Chem. 2003b;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T, et al. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J Cell Biol. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takao-Rikitsu E, et al. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 38.Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, Jahn R, Sudhof TC. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA. 1990;87:1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29:115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 40.Hallam SJ, Goncharov A, McEwen J, Baran R, Jin Y. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat Neurosci. 2002;5:1137–1146. doi: 10.1038/nn959. [DOI] [PubMed] [Google Scholar]
- 41.Inoue E, et al. SAD: a presynaptic kinase associated with synaptic vesicles and the active zone cytomatrix that regulates neurotransmitter release. Neuron. 2006;50:261–275. doi: 10.1016/j.neuron.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Miller KE, et al. Direct observation demonstrates that liprin-α is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–689. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 43.Wyszynski M, et al. Interaction between GRIP and liprin-α/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- 44.Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- 45.Shin H, et al. Association of the kinesin motor KIF1A with the multimodular protein Liprin-α. J Biol Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- 46.Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 47.Zhai RG, et al. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 48.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh E, Kawano T, Weimer RM, Bessereau JL, Zhen M. Identification of genes involved in synaptogenesis using a fluorescent active zone marker in Caenorhabditis elegans. J Neurosci. 2005;25:3833–3841. doi: 10.1523/JNEUROSCI.4978-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


