Summary
The recycling endosome localizes to a pericentrosomal region via microtubule-dependent transport. We previously showed that Sec15, an effector of the recycling endosome component, Rab11-GTPase, interacts with the mother centriole appendage protein, centriolin, suggesting an interaction between endosomes and centrosomes (1, 2). Here we show that the recycling endosome associates with the appendages of the mother (older) centriole. We show that the mother centriole appendage proteins, centriolin and cenexin/ODF2, regulate association of the endosome components Rab11, the Rab11 GTP-activating protein Evi5, and the exocyst at the mother centriole. Development of an in vitro method for reconstituting endosome protein complexes onto isolated membrane-free centrosomes demonstrates that purified GTP-Rab11 but not GDP-Rab11 binds to mother centriole appendages in the absence of membranes. Moreover, centriolin depletion displaces the centrosomal Rab11 GAP, Evi5, and increases mother-centriole-associated Rab11; depletion of Evi5 also increases centrosomal Rab11. This indicates that centriolin localizes Evi5 to centriolar appendages to turn off centrosomal Rab11 activity. Finally, centriolin depletion disrupts recycling endosome organization and function suggesting a role for mother centriole proteins in the regulation of Rab11 localization and activity at the mother centriole.
Results and Discussion
The exocyst, Evi5, and Rab11 localize to mother centriole appendages
The mother centriole protein, centriolin, interacts with the exocyst subunit Sec15 (1), a known effector of the recycling-endosome GTPase, Rab11 (2, 3). Because centriolin localizes to subdistal appendages of the mother centriole (1, 4), we asked if recycling endosome components co-fractionated with isolated centrosomes (see methods, (5)). Sucrose fractions prepared for immunoblotting showed that the endosome components Sec15, Rab11, and the Rab11 GTPase, Evi5 (6-8) were enriched in fractions defined by the centrosome components γ-tubulin, centriolin, and pericentrin (Figure S1A, (5)). The ability of these endosomal proteins to associate with isolated centrosomes demonstrated that they were bona fide centrosome components.
Centrosome localization of endosome-associated proteins was initially observed in cells (e.g. Sec15, Sec6, Rab11, and the Rab11 GTPase, Evi5, Figure S1D). To more precisely test for mother centriole localization, we examined centrosome fractions from sucrose gradients that were spun onto coverslips and prepared for immunofluorescence. Quantitative analysis showed a significant fraction of Rab11 (90%), Sec15 (90%), Exo84 (50%) and Evi5 (20%) at the mother centriole (Figure 1A, 1B). These results are likely an under-representation, as only centrosomes with exclusive localization to the mother centriole were scored. In contrast, neither Rab8, another GTPase with recycling endosome localization (9), nor the Rab11 effector, FIP3 (10, 11) showed significant localization to isolated centrosomes (Figure 1B). It is possible that these proteins may associate, but are less integral and lost in the purification process.
Figure 1. Recycling endosome components associate with appendages of the mother centriole.
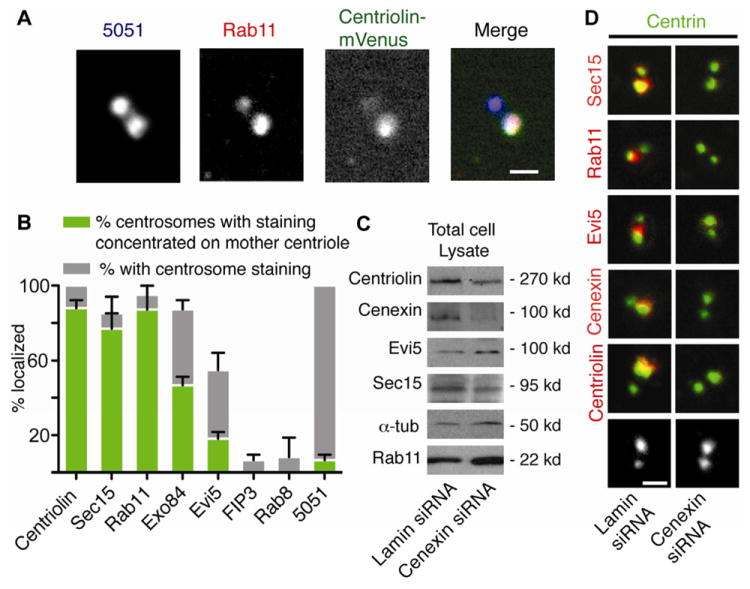
(A) Membrane-free centrosomes were isolated from cells expressing centriolin-mVenus (green), spun onto glass coverslips (31) and stained for centrosomes (5051, blue) and Rab11 (red). Rab11 co-stained with centriolin-mVenus, which was concentrated on the mother centriole (4). Merge of 5051, centriolin-mVenus, and Rab11 signals shown in white. Bar, 1 μm.
(B) Percentage of Sec15, Rab11, Exo84, Evi5, FIP3, Rab8, centriolin, and 5051 that localize to isolated centrosomes (both centrioles) and concentrate on the mother centriole (as in A), (n=3 independent experiments, Bar is SE, 150 centrosomes/bar).
(C) Cell lysates from cells treated with lamin (control) or cenexin siRNAs show cenexin depletion and no significant changes in the levels of other proteins.
(D) Isolated centrosomes (as in A) from cells depleted of cenexin or lamin were stained for centrin (green), Sec15 (red), Rab11 (red), Evi5 (red), centriolin (red), and cenexin (red). Bottom images show centrin.
We examined mother centriole appendages role in anchoring endosome-associated components. Cenexin/Odf2 is a mother centriole appendage protein, whose depletion specifically disrupts the integrity of appendages, but does not affect overall centrosome structure ((12), Figure 1D, and S1C, D). Cenexin depletion (Figure 1C) mislocalized the appendage protein centriolin, ((12, 13), Figure 1D) as well as Rab11, Evi5, and the exocyst components Sec15 and Sec6 (Figure 1D, quantification in S1B, C). This phenotype was observed in both isolated centrosomes (Figure 1D) and centrosomes in situ (Figure S1D). In parallel studies, centriolin depletion (1) did not affect cenexin localization (Figure S1C). We conclude that several endosome-associated components (Rab11, Evi5, and the exocyst subunits Sec15 and Sec6) require appendages for their localization to the mother centriole.
Centriolin regulates exocyst and Evi5 mother centriole localization
To understand the molecular organization of endosome components at mother centriole appendages we tested centriole-appendage-localization following depletion of appendage or endosome proteins. Centriolin depletion diminished the centrosome localization of the exocyst subunits Sec15, Sec6, and Exo84, as well as Evi5 (Figure 2A). In contrast, depletion of Evi5 (Figure 2A, S2C), Rab11 (Figure 2A, S2A) or Sec15 (data not shown) had no effect on centrosome localization of centriolin, confirming centriolin-anchoring of these molecules (Figure 2A).
Figure 2. Centriolin regulates the centrosomal localization of the exocyst and Evi5.
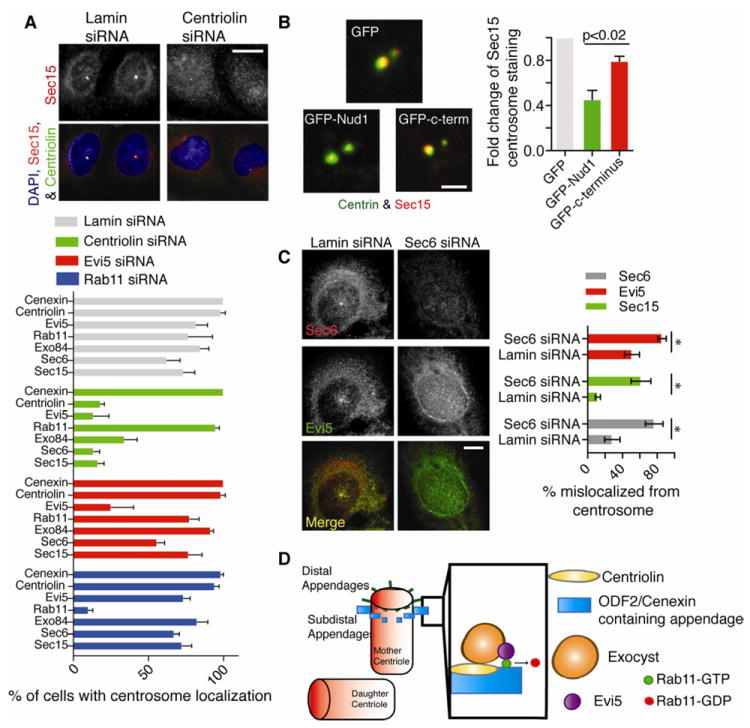
(A) Cells stably expressing centriolin-mVenus (green) were stained for Sec15 (red). Bar, 10 μm. Below, the percent of cells with centrosome (5051) localized Sec15, Sec6, Exo84, Rab11, Evi5, Centriolin, or Cenexin was calculated in cells treated with siRNAs targeting lamin, Rab11, centriolin, or Evi5. (n=3 experiments, n=50 centrosomes/treatment/experiment. Bar is SE.)
(B) Centrosomes isolated from cells expressing GFP, GFP-Nud1, or GFP-C-terminus were stained for centrin (green) and Sec15 (Red). Right, fold-change of Sec15 on isolated centrosomes was calculated (n=3 experiments, p<0.02, n>25 centrosomes/treatment. Bar is SE).
(C) Left, exocyst disruption (Sec6 depletion) diminishes Evi5 and Sec15 signal at centrosome. Right, quantification of percent of cells with no centrosome localization (n=3 experiments, * p-values <0.02, bar is SE). Lamin depletion was used as control.
(D) Proposed structural model for the hierarchy of molecular anchoring at the mother centriole: 1) cenexin anchors centriolin at the appendages of the mother centriole 2) centriolin anchors its binding partner the exocyst 3) the exocyst anchors Evi5 which is a known binding partner of Rab11-GTP and its proposed GAP.
We next tested the requirement of centriolin for Sec15 mother centrosome localization using a competition assay (Figure 2B, S2B). When expressed in cells, the centriolin Nud1 domain, previously shown to interact with Sec15 (1), decreased mother-centriole-associated Sec15 in isolated centrosome preparations (2-fold, Figure 2B). In contrast, the centriolin C-terminus, which does not interact with Sec15 (1), had no significant effect. Taken together, these findings suggest a hierarchy of centrosome organization, where cenexin and appendages anchor centriolin, which, in turn, anchors Sec15 and Evi5 (Figure 2A).
We confirmed that myc-tagged-Evi5 coimmunoprecipitated Rab11(7, 8), as well as the exocyst subunit, Exo84 and centriolin (Figure S2D). Thus, the molecular organization of this subcomplex at the centrosome was examined. Sec6 depletion caused the expected decrease in centrosome-associated Sec6 and Sec15, suggesting exocyst complex disruption (Figure 2C). Moreover, exocyst disruption mislocalized Evi5 from centrosomes (Figure 2C). In contrast, Evi5 depletion had no effect on centrosome association of the exocyst (Figure 2A) suggesting that the centriolin-bound-exocyst anchors Evi5 at centrosomes (Figure 2D). We conclude that centriolar-appendage-bound centriolin anchors the exocyst and Evi5.
Rab11 activity governs its association with mother centriole appendages
The dependency of Rab11 on cenexin (Figure 1D) for its localization to the mother centriole, suggested that these proteins might interact. This idea was supported by related data showing that a cenexin splice variant, cenexin-3, which localizes to the primary cilium, interacts with Rab8-GTP (14). Rab8 and Rab11 share certain effectors that include the exocyst subunit Sec15 (2), and they may share others. Immunoprecipitated Rab11 pulled down full-length endogenous cenexin. In a reciprocal experiment, cenexin immunoprecipitation pulled down Rab11 (Figure S3A). In addition, full-length cenexin expressed in cells and a C-terminal cenexin domain (GFP-T6) interacted with Rab11, but an N-terminal domain did not (GFP-T3), (Figure S3B). To our knowledge, this is the first demonstration of an interaction between the endosome protein, Rab11, and the centrosome appendage protein, cenexin. This interaction may provide the structural and functional link between the endosome and centrosome.
We tested whether the GTP/GDP state of Rab11 plays a role in its ability to associate with cenexin (Figure S3C) and more importantly, the mother centriole (Figure 3A, S3D). Cenexin showed a significant preference for the constitutively-active Rab11 (Q70L) compared to the dominant negative form (Rab11-S25N), (Figure S3D). Immunoflourescence staining of isolated centrosomes from cells expressing Rab11 (Q70L) and wild-type Rab11 showed colocalization with the mother centriole ~80% of the time, compared with ~20% for the dominant-negative Rab11 (S25N, Figure S3D).
Figure 3. Membrane-free Rab11-GTP specifically associates with cenexin at mother centrioles.
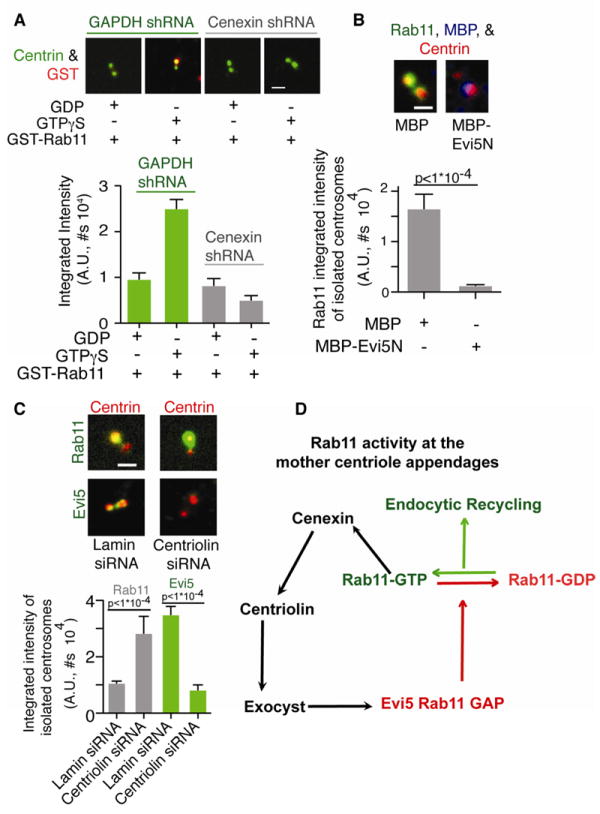
(A) Isolated centrosomes from GAPDH- or cenexin-depleted cells were incubated with purified GST-Rab11-GTPγS or GST-Rab11-GDP, spun onto glass coverslips and stained for centrin (green) and GST (red). Below, GST intensity at centrosome was calculated. Representative of n=3 experiments, n>100 centrosomes/condition. P-value is p<1*10-4 between Rab11-GDP and Rab11-GTPγS in control cells, Bar is SE.
(B) Isolated centrosomes were incubated with purified MBP or MBP-Evi5N, spun onto glass coverslips, stained for Rab11 (green), MBP (blue), or centrin. Below, Rab11 intensity at centrosome was calculated. Representative of n=3 experiments, n>30 centrosomes/condition. P-value is p<1*10-4. Bar is SE.
(C) Isolated centrosomes from cells depleted of centriolin were stained for centrin (red), Evi5 (green), and Rab11 (green). Bar, 1 μm. Below, Rab11 (grey) or Evi5 (green) intensity at the centrosome was calculated. Representative of n=3 experiments with p-value <1×10-4, n>50 centrosomes/treatment/experiment, Bar is SE.
(D) Model for regulating Rab11 activity at the mother centriole. Cenexin organizes centriolin, Exocyst, and Evi5. The mother centriole localization of Evi5 can then act on Rab11-GTP and convert it to inactive Rab11-GDP.
Based on these findings, we developed an in vitro assay for reconstituting endosome protein complexes on isolated centrosomes to test for direct binding of purified bacterially-expressed GST-Rab11 to centrosomes in the absence of microtubules and membranes. We found that GST-Rab11 coupled to the GTP analog, GTPγS, showed increased binding to the mother centriole compared to GST-Rab11 coupled to GDP (2.5-fold, Figure 3A). Importantly, binding of GST-Rab11-GTPγS to isolated centrosomes was inhibited in cells depleted of cenexin (Figure 3A). These biochemical and morphological data show that GTP-bound Rab11 preferentially associates with the mother centriole, through cenexin.
To assess the role of Rab11 activity in mother centriole association, a centrosome-localized Rab11 GAP, Evi5 (6-8), was depleted from cells ((15), Figure S2C). Evi5 depletion significantly increased Rab11 (Rab11-GTP) at the mother centriole (Figure S3E). We showed that this was due to Evi5 GAP activity by comparing the amount of active-Rab11 in Evi5 depleted cells compared to control (Figure S4D). In a reciprocal experiment, an MBP-tagged bacterially expressed Evi5 N-terminal GAP domain (MBP-Evi5N) ((16, 17), Figure 3B) was purified and added to isolated centrosomes. This induced a decrease in endogenous mother centriole-associated Rab11 (vs., MBP alone, Figure 3B). Taken together, the increase in active Rab11 at the mother centriole: 1) in the in vitro Rab11 centrosome binding experiments (Figure 3A), 2) in cells depleted of Evi5 (Figure S3E), and 3) in cells expressing Rab11 mutants (Figure S3D) provides strong evidence for a model in which the association of Rab11 with the mother centriole appendages is specific and enhanced when the GTPase is in its active, GTP-bound state.
We examined the relationship between Rab11 and Evi5. Earlier, we showed that Evi5 is mislocalized from mother centriole appendages upon cenexin- or centriolin-depletion (Figure 1D, 2A). In contrast, Rab11 is mislocalized from appendages only in cenexin-depleted cells (Figure 1D, 2A). When isolated centrosomes were prepared from centriolin-depleted cells lacking Evi5 (Figure 3C), there was a significant mother-centriole-specific increase in Rab11 levels (Figure 3C). We propose a model where Evi5 bound to cenexin through centriolin regulates the activity of cenexin-bound Rab11 at the mother centriole and possibly endocytic recycling (Figure 3D).
Endosomes are organized around mother centriole appendages and their recycling activity requires centrosome-associated endosome proteins
We have shown that endosome proteins localize to mother centriole appendages. We next asked if centrosome-anchored endosome components regulate endosome organization and function. We examined endosome organization at the centrosome by labeling endosomal compartments with transferrin (Tfn)-HRP (see methods) and examined the electron-dense Tfn-HRP reaction product by transmission electron microscopy (TEM). We found that endosomes were primarily localized to one centriole (Figure 4A). Moreover, most were organized into multiple discrete elongated periodic structures around the distal aspect of one centriole, ultrastructural features highly reminiscent of subdistal appendages (Figure 4A, white arrows), (4, 18, 19). In fact, the majority of microtubules emanating from this centriole was consistent with the unique microtubule anchoring function of mother centriole subdistal appendages (Figure 4A), (19, 20). Other Tfn-labeled compartments were organized into linear assemblies of vesicles and tubules in the pericentrosomal area (Figure 4A, B black arrowheads), suggestive of vectorial transport of endosomes to and from the mother centriole.
Figure 4. Mother centriole appendage protein depletion disrupts recycling endosome function.
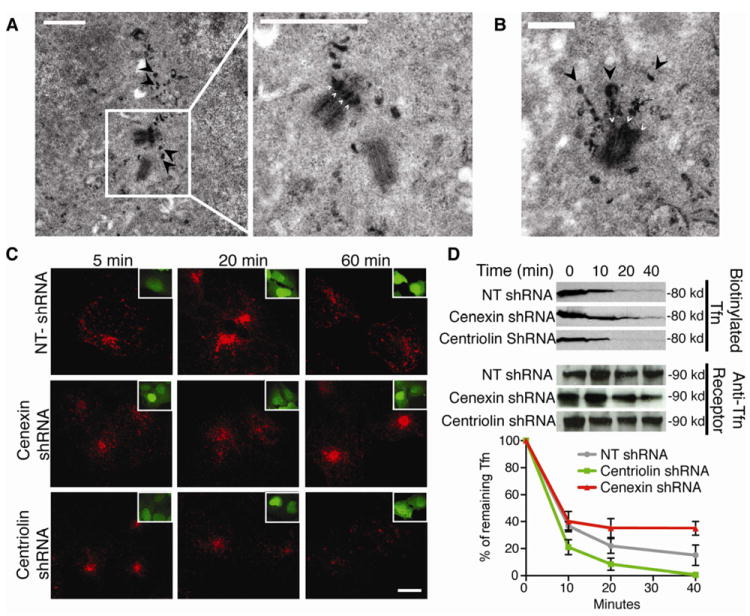
(A) TEM of cells after endocytosis of Tfn-HRP-DAB, showing reaction product around the mother centriole and concentrated near appendages (4 small white arrows right panel). Arrowheads (black) depict “trails” of Tfn in left and right panels. Bar, 1 μm.
(B) Image of a single centriole with electron-dense Tfn-HRP-DAB filled endosomes emanating from what appear to be centriole appendages (small white arrows indicate appendages; large black arrowheads indicate “endosome trails” of Tfn). Bar, 500 nm.
(C-D) Cells were treated with centriolin shRNA, non-targeting (NT) shRNA (control), or cenexin shRNA.
(C) Cells were incubated with Alexa Fluor 594-conjugated Tfn (red) and chased for up to 60 minutes with non-conjugated Tfn. Cells were fixed at the indicated times. Insets show cells with GFP-shRNA expression.
(D) Biotinylated-Tfn filled cells were chased with non-conjugated Tfn for indicated times. Biotinylated-Tfn levels were quantified by densitometry (The difference between NT-shRNA and centriolin shRNA or cenexin shRNA at 40 minutes is statistically significant, p-value <0.05, n=3 experiments, bar is SE).
To test the role of centrosome-anchored endosome components in endosome recycling, we first examined Tfn-recycling in a pulse-chase experiment ((21), Figure 4C, D). Depletion of centriolin had no detectable effect on microtubule organization or centrosome integrity (Figure S4A, (4)), but increased the rate of Tfn recycling (Figure 4C, 4D). Initially, there was no observable difference in the amount of Tfn in cells (0 to 10 minutes), but from 20 to 40 minutes centriolin depleted cells contained less Tfn (Figure 4D). In contrast, cenexin depletion decreased Tfn recycling and caused Tfn to accumulate in a juxta-centrosome recycling endosome.
Inspection of centriolin-depleted cells revealed an increase in Rab11 immunofluorescence intensity at centrosomes compared with controls (Figure S4B). There were no gross defects in early endosome (Figure S4A) or transferrin receptor organization (Figure S4A), suggesting that centriolin depletion selectively disrupted Rab11 localization in cells. To test for Rab11 activity changes we used two approaches. We isolated a membrane fraction as a readout of GTP-bound Rab11 (22). We also immunoprecipitated active-Rab11 using a cell line expressing its effector GFP-FIP3 (10). In both cases, centriolin depletion increased Rab11 (~2-fold, Figure S4C, D), consistent with an increased amount of active-GTP-bound Rab11.
We propose that centriolin depleted cells lose Evi5 causing active Rab11 to be retained on centrosomes (Figure 3C) causing an increase in centrosome-bound Rab11-GTP (Figure 3C, S4B). We suggest that the enhanced recycling (Figure 4D) is due to an increase in the ability of the centrosome-bound fraction of Rab11 to recycle cargo from the recycling endosome to the plasma membrane at a faster rate. In contrast, cenexin depletion inhibits the ability of Rab11 to recycle endosomes (Figure 4D) due to loss of appendage-bound Rab11 (Figure 1D). These findings support and add to our in vitro binding studies by illustrating that the association of Rab11 with the mother centriole is important for efficient recycling through the juxta-centrosomal recycling endosome.
In conclusion, it has been known for some time that recycling endosomes are found in the pericentrosomal area and are influenced by Rab11 activity. However, little is known about the relationship between these juxtaposed organelles at the structural, molecular or functional level. Here we show that the appendages of the mother centriole and recycling endosomes are in intimate contact (Figure 4A, B). We show that Rab11 and its effector, Evi5, are linked to the centriolar appendages through the centrosome proteins cenexin and centriolin (Figure 1 and 2). This is the first evidence for a novel centrosome-anchored molecular pathway for the inactivation of Rab11 and regulation of endosome recycling through a complex series of protein interactions and activities.
The discovery of a structural association between the endosome and the centrosome with complex molecular underpinnings has new and unexpected implications for recycling endosome function. This new liaison has additional implications for a variety of biological processes including cilia formation and function (9, 23), asymmetric cell division (24-27) and cell polarity (28, 29). It is also likely that centrosome function will itself be influenced through its association with the endosome, although this has yet to be explored. The latter brings up an intriguing question: Do recycling endosomes bound to centrosomes impart properties to centrosome activities, known and new? One result that suggests this might be the case is the dual role of Dynamin-2 in both vesicle formation and centrosome cohesion (30). Thus, it is also possible that Rab11 and other centrosome-bound endosome associated molecules may play dual roles in endosome and centrosome function. This observation and the endosome-centrosome connection, lead us to speculate that the centrosome may directly associate with other (membranous) organelles, such as the Golgi apparatus or endoplasmic reticulum, generating a new repertoire of previously unappreciated combined functions for both organelles. Future investigations will be required to test these ideas.
Supplementary Material
Highlights.
Endosome-proteins localize to centrosomes independent of membranes.
Rab11 and its modulators localize to mother centriole appendages.
Mother centriole appendages regulate membrane recycling through Rab11.
Acknowledgments
We thank Mary Munson and David Lambright (UMMS), Alison Bright and Tse-Chun Kuo (UMMS, Doxsey Lab), and Charles Yeaman (University of Iowa) for reading versions of this manuscript. The following grants supported this work S10RR027897 (TEM, UMMS), 2R01 GM051994-15A2 (SD), and F32 GM095161-01 (HH).
Abbreviations
- MTOC
microtubule organizing center
- GAP
GTPase-activating protein
- Tfn
Transferrin
- TfR
Transferrin Receptor
- PCM
pericentrosome material
- DAB
3,3’-diaminsobenzidine-HCl
- TEM
Transmission Electron Microscope
- GEF
Guanine Nucleotide Exchange Factor
- Nuf
Nuclear Fallout
- FIP3
Family of Rab11-interacting proteins 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gromley A, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nature structural & molecular biology. 2005;12:879–85. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X-M, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. The Journal of biological chemistry. 2004;279:43027–34. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 4.Gromley A, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. The Journal of cell biology. 2003;161:535–45. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchison T, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 312:232–7. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- 6.Faitar SL, Dabbeekeh JTS, Ranalli Ta, Cowell JK. EVI5 is a novel centrosomal protein that binds to alpha- and gamma-tubulin. Genomics. 2005;86:594–605. doi: 10.1016/j.ygeno.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Dabbeekeh JTS, Faitar SL, Dufresne CP, Cowell JK. The EVI5 TBC domain provides the GTPase-activating protein motif for RAB11. Oncogene. 2007;26:2804–8. doi: 10.1038/sj.onc.1210081. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme C, et al. Evi5 promotes collective cell migration through its Rab-GAP activity. The Journal of cell biology. 2012;198:57–67. doi: 10.1083/jcb.201112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6346–51. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson GM, et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Molecular biology of the cell. 2005;16:849–60. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. The EMBO journal. 2005;24:3389–99. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nature cell biology. 2005;7:517–24. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 13.Soung N-K, et al. Plk1-dependent and -independent roles of an ODF2 splice variant, hCenexin1, at the centrosome of somatic cells. Developmental cell. 2009;16:539–50. doi: 10.1016/j.devcel.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura S-I, Egerer J, Fuchs E, Haas AK, Barr Fa. Functional dissection of Rab GTPases involved in primary cilium formation. The Journal of cell biology. 2007;178:363–9. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faitar SL, Sossey-Alaoui K, Ranalli TA, Cowell JK. EVI5 protein associates with the INCENP-aurora B kinase-survivin chromosomal passenger complex and is involved in the completion of cytokinesis. Experimental cell research. 2006;312:2325–35. doi: 10.1016/j.yexcr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Eldridge AG, et al. The evi5 oncogene regulates cyclin accumulation by stabilizing the anaphase-promoting complex inhibitor emi1. Cell. 2006;124:367–80. doi: 10.1016/j.cell.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Westlake CJ, et al. Identification of Rab11 as a small GTPase binding protein for the Evi5 oncogene. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1236–41. doi: 10.1073/pnas.0610500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–50. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 19.Bornens M. The Centrosome in Cells and Organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- 20.Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. Journal of cell science. 2005;118:1565–75. doi: 10.1242/jcs.02302. [DOI] [PubMed] [Google Scholar]
- 21.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. The Journal of cell biology. 1999;145:123–39. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews Molecular cell biology. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 23.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2759–64. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emery G, et al. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–73. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nature communications. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–55. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science (New York, N Y) 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman JL, Priess JR. A Role for the Centrosome and PAR-3 in the Hand-Off of MTOC Function during Epithelial Polarization. Current biology: CB. 2012;22:575–82. doi: 10.1016/j.cub.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hehnly H, Doxsey S. Polarity sets the stage for cytokinesis. Molecular biology of the cell. 2012;23:7–11. doi: 10.1091/mbc.E11-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson HM, Cao H, Chen J, Euteneuer U, McNiven MA. Dynamin 2 binds gamma-tubulin and participates in centrosome cohesion. Nature cell biology. 2004;6:335–42. doi: 10.1038/ncb1112. [DOI] [PubMed] [Google Scholar]
- 31.Blomberg-Wirschell M, Doxsey SJ. Rapid isolation of centrosomes. Methods in enzymology. 1998;298:228–38. doi: 10.1016/s0076-6879(98)98022-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


