Abstract
Touch sensation is essential for behaviours ranging from environmental exploration to social interaction; however, the underlying mechanisms are largely unknown1. In Drosophila larvae, two types of sensory neurons, class III and class IV dendritic arborization neurons, tile the body wall. The mechanotransduction channel PIEZO in class IV neurons is essential for sensing noxious mechanical stimuli but is not involved in gentle touch2. On the basis of electrophysiological-recording, calcium-imaging and behavioural studies, here we report that class III dendritic arborization neurons are touch sensitive and contribute to gentle-touch sensation. We further identify NOMPC (No mechanoreceptor potential C), a member of the transient receptor potential (TRP) family of ion channels, as a mechanotransduction channel for gentle touch. NOMPC is highly expressed in class III neurons and is required for their mechanotransduction. Moreover, ectopic NOMPC expression confers touch sensitivity to the normally touch-insensitive class IV neurons. In addition to the critical role of NOMPC in eliciting gentle-touch-mediated behavioural responses, expression of this protein in the Drosophila S2 cell line also gives rise to mechanosensitive channels in which ion selectivity can be altered by NOMPC mutation, indicating that NOMPC is a pore-forming subunit of a mechanotransduction channel. Our study establishes NOMPC as a bona fide mechanotransduction channel that satisfies all four criteria proposed for a channel to qualify as a transducer of mechanical stimuli3 and mediates gentle-touch sensation. Our study also suggests that different mechanosensitive channels may be used to sense gentle touch versus noxious mechanical stimuli.
Similar to vertebrates, Drosophila displays mechanosensation such as gravity sensing, hearing, proprioception, mechanical nociception and gentle-touch sensation4–11. To identify sensory neurons for gentle touch we used a previously established behavioural assay12. In brief, one side of the larval thoracic segments was gently touched with an eyelash and the behavioural responses were scored (Supplementary Fig. 1 and Supplementary Methods). By expressing the tetanus toxin light chain (TNT) in different classes of dendritic arborization neurons to interfere with synaptic transmission, we found that the behavioural response to gentle touch was reduced by TNT expression in class III (Fig. 1a, b) but not class IV dendritic arborization neurons (Supplementary Fig. 2). In all of the behavioural studies we used a single copy of upstream activation sequence (UAS)-TNT and a single copy of the yeast Gal4 driver, which may not have been sufficient to completely eliminate the synaptic output of class III dendritic arborization neurons. Alternatively, the residual larval behavioural response to gentle touch may indicate the involvement of additional sensory neurons.
Figure 1. NOMPC is required for mechanotransduction of class III dendritic arborization neurons that function in gentle-touch sensation of larvae.
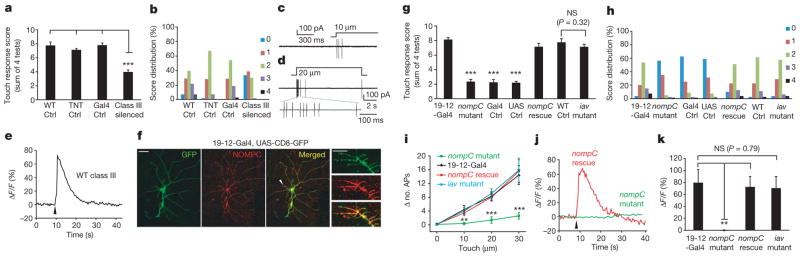
a, Silencing class III neurons with TNT expression impaired larval behavioural responses to gentle touch (n =24–26). WT, wild type. b, Score distribution of different genotypes. 0, no response; 1, pause; 2, recoil; 3, single reverse contractile wave; 4, multiple reverse contractions. c, Touching the dendrite field of a class III dendritic arborization neuron to cause a 10-μm displacement induced a burst of action potentials. d, Class III dendritic arborization neurons responded to prolonged mechanical stimulation, with an on response displaying rapid adaptation as well as an off response. e, Internal calcium increase in a class III dendritic arborization neuron in response to a 40-μm touch displacement lasting 300 ms. Arrowhead indicates stimulus onset. f, Immunostaining of NOMPC in ddaF and ddaA class III dendritic arborization neurons (labelled by class III-neuron-specific 19-12-Gal4-driven GFP). Arrowhead marks a dendritic segment with NOMPC expression in dendritic spikes, as shown at high magnification on the right. Scale bars: left, 50 μm; right, 5 μm. g, nompC mutant was defective in gentle-touch sensation; this behavioural phenotype could be rescued by expressing NOMPC in class III dendritic arborization neurons (nompC rescue), but not by UAS-NOMPC (UAS Ctrl) or Gal4 (Gal4 Ctrl) alone. nompC mutant, Gal4 Ctrl and UAS Ctrl were compared to both 19-12-Gal4 and nompC rescue for the significance test. iav mutant showed a normal behavioural response to gentle touch compared with wild-type control (n =20–24). h, Score distribution of different genotypes. i, Summary of action potential firing of class III dendritic arborization neurons in the nompC mutant, 19-12-Gal4, nompC rescue, and iav mutant in response to increasing mechanical stimuli. y axis denotes increase in the number of action potentials (APs) in 1 s after stimulus onset compared to 1 s before stimulus onset. nompC mutant showed markedly reduced responses compared to 19-12-Gal4 and nompC rescue, whereas the iav mutant showed no difference from 19-12-Gal4 (P >0.2; n =7–10). j, The calcium response of class III dendritic arborization neurons in nompC mutant (green) and nompC rescue (red). Arrowhead indicates the onset of the stimulus (40-μm displacement lasting for 300 ms). k, Group data of calcium response of class III dendritic arborization neurons with different genotypes (n =7–10). We used unpaired t-test for comparison between two groups, and one-way analysis of variance followed by Tukey’s comparison for comparison among three or four groups. NS, not significant. **P <0.01, ***P <0.001. All error bars denote ±s.e.m. Genotypes are as follows: for a, b, wild-type control: w1118. TNT control: UAS-TNT/+. Gal4 control: repo-GAL80/+; 19-12-GAL4/+. Class III silenced: repo-GAL80/UAS-TNT; 19-12-GAL4/+. For g, h, 19-12-Gal4: repo-GAL80/+; 19-12-GAL4/+, nompC mutant: nompC1/nompC3; +/+. Gal4 control: nompC1/nompC3; 19-12-GAL4/+. UAS control: nompC1/nompC3, UAS-nompC; +/+. nompC rescue: nompC1/nompC3, UAS-nompC; 19-12-GAL4/+. Wild-type control: w1118. iav mutant: iav1. For i, 19-12-Gal4: 19-12-GAL4, UAS-CD8-GFP/+. nompC mutant: nompC1/nompC3; 19-12-GAL4, UAS-CD8-GFP/+. nompC rescue: nompC1/nompC3, UAS-nompC; 19-12-GAL4, UAS-CD8-GFP/+. iav mutant: iav1; +/+; 19-12-GAL4, UAS-CD8-GFP/+. For j, k, 19-12-Gal4: 19-12-GAL4/UAS-GCaMP5. nompC mutant: nompC1/nompC3; 19-12-GAL4/UAS-GCaMP5. nompC rescue: nompC1/nompC3, UAS-nompC; 19-12-GAL4/UAS-GCaMP5. iav mutant: iav1/y; +/+; 19-12-GAL4/UAS-GCaMP5.
As dendrites of five class III dendritic arborization neurons tile 70–80% of each abdominal hemisegment13 (Supplementary Fig. 3), a Drosophila larva could use these neurons to detect gentle touch on most of its body. Extracellular recordings of class III neurons in abdominal segments of fillet preparations (Supplementary Fig. 4) revealed that a single touch causing a displacement of the body wall by as little as 10 μm—about one-hundredth of the width of a third-instar larva—induced a burst of action potentials (Fig. 1c). Progressively larger displacements elicited greater numbers of action potentials in a graded fashion (Fig. 1d, i). In addition, a single touch causing a 40-μm displacement induced a large calcium response in class III neurons (Fig. 1e). Similar to the five class III dendritic arborization neurons in each abdominal hemisegment, class III neurons in thoracic segments showed touch responses (Supplementary Fig. 5). By contrast, the same touch stimulation had no effect on class IV dendritic arborization neurons that are mechanical nociceptive neurons2,14 (Supplementary Fig. 6).
Class III neurons adapted quickly to a prolonged stimulus with a time constant of 153 ms, and showed an off response upon the removal of the stimulus in most cases (Fig. 1d and Supplementary Fig. 7), similar to touch-sensitive mechanoreceptors in Caenorhabditis elegans and mice1,15–17. Thus, class III dendritic arborization neurons are low-threshold mechanoreceptors for sensing gentle touch.
To identify the mechanotransduction channel that mediates gentle touch, we examined the role of NOMPC, which is important for hearing and mechanotransduction in adult Drosophila and larval locomotion12,18–21. Our immunostaining of Drosophila larvae confirmed high levels of NOMPC expression in the ciliate tips of type I sensory neurons10,18,22 (Supplementary Fig. 8b), and we found high levels of NOMPC throughout the soma and dendrites of class III dendritic arborization neurons of wild-type but not nompC null mutant larvae (Fig. 1f and Supplementary Fig. 8a, c). We further uncovered severe defects of nompC null mutant larvae in the behavioural response to gentle touch, which could be rescued by expressing NOMPC in class III neurons (Fig. 1g, h), indicating that NOMPC functions cell autonomously in these neurons to mediate gentle-touch sensation. Moreover, class III neurons of the nompC mutant larvae failed to respond to touch stimuli with action potential firing (Fig. 1i) or calcium response (Fig. 1j, k) despite their normal morphology (Supplementary Fig. 9), a defect that could also be rescued by class III neuron expression of NOMPC (Fig. 1i–k). Two Drosophila TRP channels, Inactive (IAV) and Nanchung (NAN), are interdependent in their contribution to the hearing of adult Drosophila4. We found normal mechanosensitivity of class III dendritic arborization neurons in iav null mutant larvae (Fig. 1i, k), which showed a normal behavioural response to gentle touch (Fig. 1g, h). Thus, NOMPC, but not IAV, is required for mechanotransduction of larval class III dendritic arborization neurons that sense touch.
We next tested whether touch sensitivity can be conferred to class IV dendritic arborization neurons—which normally do not express NOMPC (Fig. 2a and Supplementary Fig. 10)—by expressing NOMPC ectopically (Fig. 2b). Although class IV neurons with the ppk-Gal4 driver alone were insensitive to touch, class IV neurons with NOMPC expression responded to the touch stimulation with a burst of action potentials (Fig. 2c–e) and an increase in internal calcium level (Fig. 2f–h). Thus, not only is NOMPC necessary for mechanosensitivity of class III neurons, but its ectopic expression is sufficient to enable the normally touch-insensitive class IV neurons to respond to touch. Overexpression of NOMPC also conferred touch sensitivity to the normally touch-insensitive class I dendritic arborization neurons (Supplementary Fig. 11).
Figure 2. Ectopic NOMPC expression confers mechanosensitivity to the normally touch-insensitive class IV dendritic arborization neurons.
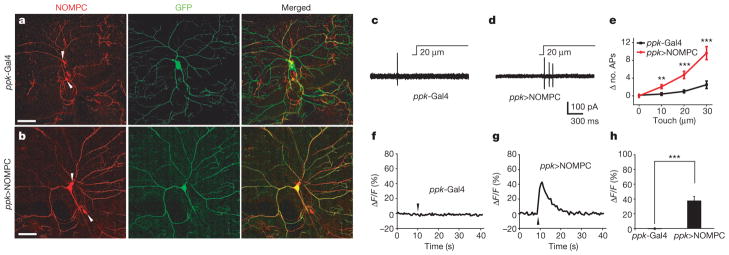
a, Class IV neurons showed no detectable NOMPC expression, whereas class III neurons nearby displayed NOMPC staining (indicated by arrowheads). b, Class IV neurons with ectopic expression of NOMPC showed strong NOMPC staining. Scale bars in a and b, 50 μm. c, f, Class IV neurons did not show detectable electrophysiological (c) or calcium (f) response to mechanical stimuli. d, g, Class IV neurons with NOMPC expression displayed touch-induced action potential firing (d) and calcium rise upon 40-μm touch displacement lasting 300 ms (g). The same mechanical stimuli were used in f and g. Arrowheads indicate stimulus onset. e, h, Group data of electrophysiological (e) and calcium (h) response to mechanical stimulation of class IV neurons from control larvae and larvae with NOMPC expression. ppk>NOMPC denotes the UAS-NOMPC/+; ppk-Gal4/+genotype. All error bars denote ±s.e.m. n =8–10. **P <0.01, ***P <0.001, unpaired t-test.
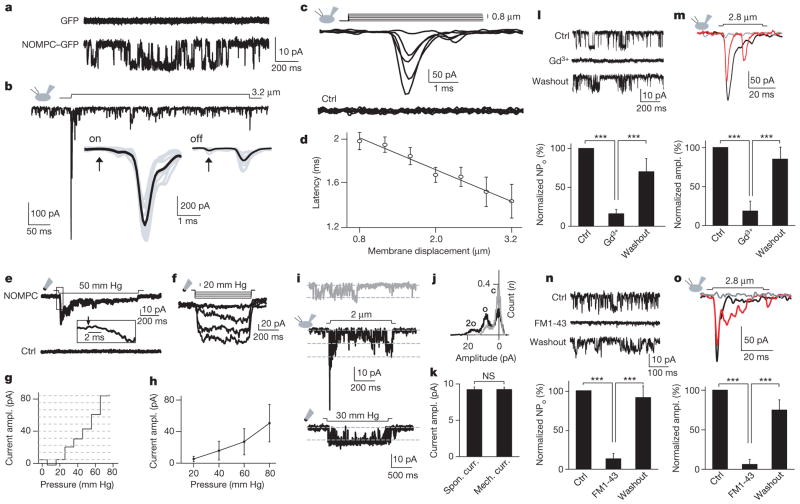
Next, we tested whether NOMPC could form mechanosensitive channels in a heterologous expression system. Although there is strong evidence supporting the notion that TRP-4, the C. elegans homologue of Drosophila NOMPC, is a pore-forming subunit of a native mechanotransduction channel23, it remains to be tested whether heterologous expression of these channels gives rise to mechanosensitive channels23. We found that Drosophila S2 cells transfected with NOMPC displayed spontaneous channel openings in whole-cell recordings (Fig. 3a and Supplementary Fig. 12). This current could be blocked reversibly by gadolinium (Gd3+) and SKF-96365 (Fig. 3l and Supplementary Fig. 13a, b), which are known to block many TRP channels. The current could also be blocked by FM1-43, a blocker for mechanotransduction channels on hair cells24 (Fig. 3n).
Figure 3. Heterologous expression of NOMPC in S2 cells yields mechanosensitive channels.
a, Representative traces of spontaneous channel activities in control and NOMPC–GFP-expressing S2 cells at −60 mV. b, Mechanosensitive current triggered by the piezo-actuator-driven probe causing a 3.2-μm displacement of the cell membrane. Insets, the latency is less than 2 ms (arrows indicate the onset and the end of the stimulation for the on and off response, respectively). c, The current amplitude (at −60 mV) varied with the size of the displacement (5 trials with 0.4-μm increment, from 0 to 1.6 μm). d, The latency of the current response as a function of the mechanical displacement (n =7). Error bars denote ± s.e.m. e, Mechanosensitive current of an outside-out patch in response to negative pressure applied with a high-speed pressure clamp. Arrow indicates the pressure onset. f, The mechanosensitive current amplitude varied with the pressure intensity (at −60 mV). g, Surface plot showing the current amplitude on a single patch increased progressively with increasing negative pressure at −60 mV. h, Group data of the dose-dependent curve of pressure-induced current (n =7). Error bars denote ± s.e.m. i, Single-channel current (at −60 mV) from spontaneously active channels (top trace) and channels activated by mechanical stimulation via probe displacement (middle trace) and negative pressure on excised patch (bottom trace). j, Histogram showing the increase of channel open probability after stimulation. Black denotes displacement-triggered channel activity and grey denotes spontaneous channel activity. c, closed; o, open. k, Comparison of the single-channel current amplitude of spontaneously active channels and pressure-induced channels on a patch from the same cell at −60 mV. mech. curr., mechanicosensitive current; spon. curr., spontaneous current. n =7; paired t-test; error bars denote ± s.e.m. l, Spontaneously active channels in S2 cells expressing NOMPC–GFP were sensitive to 100 μM Gd3+ and the block was reversible upon washout (n =6). NPo indicates open probability. m, The displacement-induced mechanosensitive current was blocked by 100 μM Gd3+ and then recovered after washout (black, control; grey, Gd3+; red, washout; n =4). n, Spontaneously active channels in S2 cells expressing NOMPC–GFP were sensitive to 3 μM FM1-43 and the block was reversible upon washout (n =6). o, The displacement-induced mechanosensitive current was blocked by 3 μM FM1-43 and then recovered after washout. Black, control; grey, FM1-43; red, washout; n =5. ***P <0.001, paired t-test. All error bars denote ± s.e.m.
We then tested whether NOMPC channels could be gated by mechanical force. First, by using a glass probe driven by a piezo actuator to deliver mechanical stimuli to the cell membrane during whole-cell recording, we observed both on and off responses (Fig. 3b). As expected for mechanically gated channels with fast activation (Fig. 3b), with increasing stimulation strength the current amplitude increased progressively (Fig. 3c) whereas the latency decreased to less than 1.5 ms (Fig. 3d). Gd3+and FM1-43 blocked these mechanosensitive channels, and the channel activity recovered after washout (Fig. 3m, o).
Next, we applied negative pressure through the recording pipette with a high-speed pressure clamp to stretch the membrane of an outside-out excised patch, and found that the NOMPC channel was activated within 2 ms of the pressure application (Fig. 3e). As the pressure was gradually increased, the current amplitude increased progressively (Fig. 3f–h). The pressure-induced NOMPC current was also sensitive to Gd3+ and FM1-43 (Supplementary Fig. 13c, d).
By resolving the single-channel current amplitude for mechanosensitive channels (Fig. 3i, j), we found that the single-channel current amplitude for channels activated by negative pressure is identical to that for the spontaneously active channels (Fig. 3i, k). Reversal potential measurements under bi-ionic conditions further revealed that NOMPC channels are permeable not only to monovalent cations such as Na+, K+ and Cs+, but also the divalent cation Ca2+ (Supplementary Fig. 14).
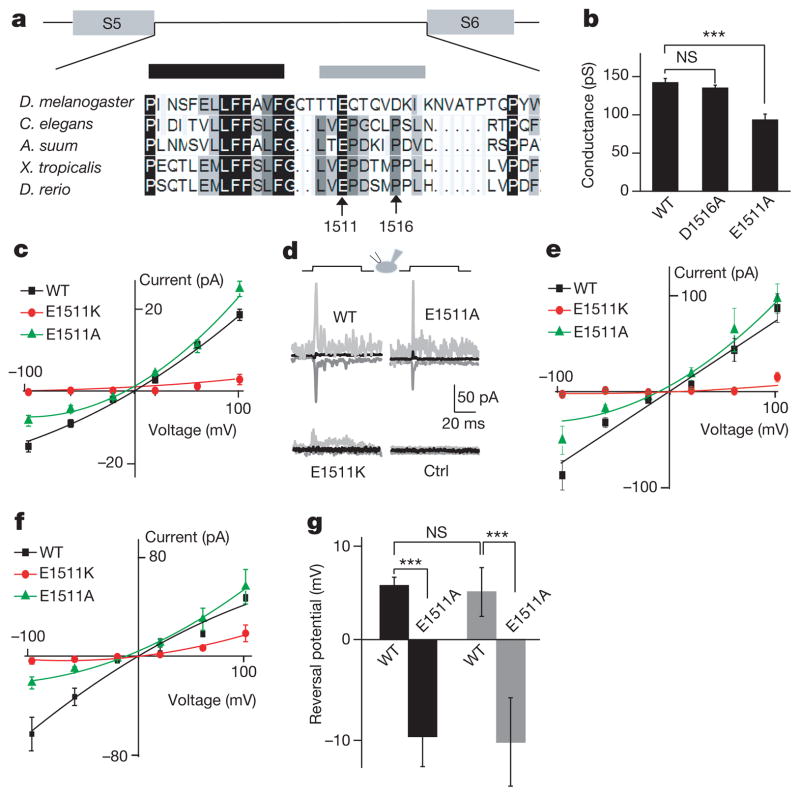
A previous study has shown that mutations in several residues of the selective filter of the TRP-4 channel in C. elegans change the ion selectivity of the channel23. On the basis of the sequence homology between NOMPC and TRP-4 channels, we mutated acidic residues near the putative pore region of NOMPC23,25,26 (Fig. 4a). Whereas alanine substitution of Asp 1516 had no detectable effects on channel properties including the single-channel conductance (Fig. 4b), replacing Glu 1511 with the basic residue lysine considerably reduced channel activity (Fig. 4c). Alanine substitution of Glu 1511 resulted in a reduction of single-channel conductance measured at −60 mV in Na+/Cs+ solutions (Fig. 4b and Supplementary Fig. 15) and altered the shape of current–voltage relationship (I–V) curves (Fig. 4c and Supplementary Fig. 16). These mutant NOMPC channels showed similar distributions on the cell membrane as wild-type NOMPC (Supplementary Fig. 17). Notably, these mutations had similar effects on the spontaneously active channels and the channels activated by mechanical stimulation. The current response to piezo displacement at −100 mV was reduced in the E1511A mutant, whereas the response was largely eliminated in the E1511K mutant (Fig. 4d, e). The mutations also altered the shape of the I–V curves for the current induced by both mechanical displacement (Fig. 4e) and negative pressure (Fig. 4f) and shifted the reversal potential of the mechanosensitive channels in Na+/Cs+ solutions (Fig. 4g). The wild-type NOMPC channels exhibited a slightly higher permeability (P) to Na+ than Cs+ (PNa+/PCs+ = 1.2), whereas the converse was true for the E1511A mutant (PNa+/PCs+ = 0.7). These results support the notion that NOMPC is a pore-forming subunit of the mechanotransduction channel.
Figure 4. NOMPC is likely a pore-forming subunit of the mechanotransduction channel.
a, Sequence alignment of the putative pore region of NOMPC and its homologues, putative pore helix (black bar) and the putative selective filter (grey bar). Arrows mark the negatively charged Glu 1511 and Asp 1516 residues targeted for mutagenesis. A. suum, Ascaris suum; X. tropicalis, Xenopus tropicalis; D. rerio, Danio rerio. b, The E1511A mutation altered the single-channel conductance of the spontaneous current determined at −60 mV from whole-cell recording in extracellular Na+/intracellular Cs+ solution (n =7, 6, 14 from left to right). ***P <0.001, unpaired t-test. c, The E1511A mutation altered the single-channel I–V curve of spontaneously active channels in the same Na+/Cs+ solutions. The E1511A mutant channel displayed outward rectification and the E1511K mutation greatly reduced channel activity. d, Sample traces of wild-type and mutant channels in response to 2.8-μm displacement at −100 (dark grey), 0 (black) and 100 (grey) mV. e, The E1511A mutation altered the I–V curve of displacement (2.8 μm)-triggered currents in the same Na+/Cs+ solutions whereas the E1511K mutation largely eliminated the mechanosensitive current at negative voltage. f, The E1511A mutation altered the I–V curve of pressure-elicited currents in the same Na+/Cs+ solutions whereas the E1511K mutation largely eliminated the mechanosensitive current (50 mm Hg) at negative voltage. g, The E1511A mutation shifted the reversal potential of spontaneously active NOMPC channels (black bars) and NOMPC channels activated by negative pressure (50 mm Hg, grey bars) in the bi-ionic Na+/Cs+ conditions (n =6, 6, 6, 4 from left to right). All error bars denote ± s.e.m.
For a channel to be considered a bona fide transducer of mechanical stimuli, it has to meet four criteria3,27. NOMPC satisfies all of these criteria: first, it is expressed in class III dendritic arborization neurons that respond to touch; second, it is required for touch-induced excitation of class III neurons; third, ectopic expression of NOMPC endows the normally touch-insensitive dendritic arborization neurons with sensitivity to mechanical stimuli; and fourth, heterologous expression of NOMPC in S2 cells generates non-selective cation channels.
We show that Drosophila NOMPC is most likely a pore-forming subunit of a channel that can be activated by mechanical force in a heterologous expression system and is required for mechanotransduction of gentle touch in vivo. Together with Drosophila PIEZO, which is gated by mechanical force in a heterologous expression system and is required for sensing noxious mechanical stimuli in vivo2,28, these studies reveal that the class III and class IV dendritic arborization neurons that tile the larval body wall use different molecular mechanisms to sense gentle touch and noxious mechanical stimuli, respectively, raising the question of whether different groups of sensory neurons in the skin of other organisms including mammals might also use different molecular mechanisms for sensing these different stimuli types.
METHODS SUMMARY
Behavioural assay
Animals were raised at 25 °C in an incubator. The thoracic segments of the larvae were touched with an eyelash and the behavioural responses were scored as described in ref. 12. 19-12-Gal4 is a marker for class III dendritic arborization neurons and is not expressed in class I, II or IV neurons29,30.
Electrophysiological recordings
Fillet preparations were made by dissecting third-instar larvae. Action potentials were recorded extracellularly. Whole-cell and outside-out patch recordings of S2 cells were carried out 1–2 days after transfection.
Calcium imaging of dendritic arborization neurons
Calcium imaging of larval dendritic arborization neurons was carried with a newly available genetically coded calcium indicator, GCaMP5.
Mutagenesis of NOMPC channel and S2 cell culture
All point mutations were introduced by site-directed mutagenesis and verified by sequencing of the full-length construct. Drosophila S2 cells were transfected using the product protocol.
Mechanical stimulation
A glass probe was driven by a piezo actuator mounted on a micromanipulator to give mechanical stimulation. The movements were triggered and controlled by the piezo amplifier, which was synchronized with the programmed signals from pClamp software. Negative pressure on excised patch was delivered by high-speed pressure clamp.
Immunohistochemistry
In brief, third-instar larvae were dissected in PBS, fixed and exposed to the primary antibody overnight at 4 °C and then the secondary antibody for 2 h at room temperature (25 °C).
Supplementary Material
Acknowledgments
We thank C. Zuker, U. Heberlein, G. Rubin, T. Lee and R. Bodmer for fly lines, J. Howard for the NOMPC antibody and L. Looger for GCaMP5. We thank D. Minor and S. Bagriantsev for assistance with the high-speed pressure clamp. We thank S. Younger, S. Barbel and T. Cheng for technical support, W. P. Ge, M. P. Klassen, P. Fan, E. K. Unger and C. J. Peters for reading the manuscript, and members of the Jan laboratory for discussion. Z.Y. and Y.X. are recipients of the Long-Term Fellowship from the Human Frontier Science Program. This work was supported by National Institutes of Health grants (R37NS040929 and 5R01MH084234) to Y.N.J. L.Y.J. and Y.N.J. are investigators of the Howard Hughes Medical Institute.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions Z.Y. and W.Z. initiated the project, designed and conducted most experiments; Y.N.J. and L.Y.J. supervised the project and provided guidance throughout. L.E.C. and W.Z. made the NOMPC mutants. Y.H., Y.X. and S.M. assisted with part of the behavioural experiments and immunostaining. D.G. helped set up the piezo actuator system. Z.Y., W.Z., L.Y.J. and Y.N.J. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Árnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 4.Gong Z, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell JC, Eberl DF. Towards a molecular understanding of Drosophila hearing. J Neurobiol. 2002;53:172–189. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamikouchi A, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 7.Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 9.Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X, Madrid J, Saleh HS, Howard J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton. 2011;68:1–7. doi: 10.1002/cm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci USA. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 13.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 14.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geffeney SL, Goodman MB. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron. 2012;74:609–619. doi: 10.1016/j.neuron.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nature Rev Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 18.Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effertz T, Wiek R, Gopfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 20.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE. 2010;5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers JR, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nature Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 28.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumpf S, Lee SB, Jan LY, Jan YN. Neuronal remodeling and apoptosis require VCP-dependent degradation of the apoptosis inhibitor DIAP1. Development. 2011;138:1153–1160. doi: 10.1242/dev.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.