Abstract
Improved medical care could have altered the clinical presentation and survival of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) complicated by autoimmune cytopenia (AID cytopenia). We reviewed the clinical characteristics, treatment, and outcome of AID cytopenia that was diagnosed in 75 (4.3%) of 1750 CLL patients seen at a single institution over 10 years. Compared to historical reported data, our study shows a lower rate of autoimmune hemolytic anemia (2.3%), and similar rates of immune thrombocytopenia (2.0%) and pure red blood cell aplasia (0.5%). AID cytopenia occurred at all stages of CLL, responded well to treatment, did not alter overall survival, and contributed to death in only 6 (12%) patients. We propose that more sensitive and accurate diagnostic methods for CLL have decreased the perceived prevalence of AID cytopenia and that improvements in management could have increased the survival of these patients.
Introduction
Cytopenia in patients with chronic lymphocytic anemia/small lymphocytic lymphoma (CLL) can be caused by progression of disease (bone marrow failure), complicating autoimmune disease (AID cytopenia), hypersplenism, treatment of CLL, or non-CLL related causes. Accurate diagnosis of the cause of cytopenia is crucial for determining prognosis and treatment1. Cytopenia caused by bone marrow failure is clearly a poor prognostic factor1-3 and an indication for treatment in CLL4, 5. Autoimmune disease in CLL most commonly affects hematopoietic tissue causing autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP), pure red blood cell aplasia (PRBCA) and autoimmune agranulocytosis (AIG)6. In contrast to bone marrow failure, AID cytopenias can be more difficult to diagnose, do not have the same adverse prognostic implications as bone marrow failure, and can occur at any time in the course of CLL1, 7, 8. AID cytopenia can also complicate therapy for progressive CLL, particularly when patients are treated with single agent purine analogues or alkylating agents9, 10. AID cytopenia is thus a discrete complication of CLL requiring specific evaluation and management.
Earlier and more accurate diagnosis of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) has had a profound effect on the presentation, demographics, and perceived natural history of patients with CLL. Thus CLL can now be accurately differentiated from other mature B-lymphocyte malignancies and especially the leukemic phase of mantle cell lymphoma11,12. CLL is currently most often diagnosed in an early asymptomatic stage because of the incidental finding of lymphocytosis on routine automated white blood cell differential counts and immunophenotyping of the lymphocytes by flow cytometry13. The prognosis of these patients with earlier stage CLL can be more accurately predicted with biological markers including somatic hypermutation of the immunoglobulin gene heavy chain variable region (IgVH)14, 15, chromosomal abnormalities detected by interphase FISH analysis16, 17, and expression of ZAP-7018 and CD3819, 20. In addition, there have also been major improvements in the efficacy of initial treatment of CLL21-23. These changes could have altered the clinical presentation and implications of AID cytopenia in CLL.
In a previously published study of the same patient cohort we reported the prognostic significance of cytopenia in CLL1. The most common cause of cytopenia in the study population was bone marrow failure with a minority of patients (75, 19%) having AID cytopenia. We now report the clinical presentation and course of the 75 patients with AID cytopenia seen in the Division of Hematology at Mayo Clinic Rochester (MCR) over a period of 10 years with a subsequent 3.9 years of follow up.
Material and Methods
This observational study was conducted at MCR with the approval of the Institutional Review Board. The study population was all CLL patients seen at least once in the Division of Hematology at MCR from 1 January 1995 to 31 December 2004. Patients with CLL and AID cytopenia were identified using the Mayo Clinic CLL database and cross referenced with the MCR Clinic records as previously described1. The entire study population was then followed until 11 November 2008. Prognostic data collected included IgVH mutation status, chromosomal abnormalities detected by interphase FISH, and ZAP-70 and CD38 expression. The date of the diagnosis of AID was compared to the date of diagnosis of CLL and the AID was determined to have been diagnosed before, simultaneously (within 1 month), or after the diagnosis of CLL for each patient. Treatment details recorded for each patient were specific indication for therapy, drugs used, response, duration of response and complications. Response to therapy with or without maintenance therapy was classified according to the criteria detailed in Table 1. Response duration for each patient was measured from the date of achieving remission.
Table 1.
Treatment response criteria for autoimmune cytopenia
| AIHA and PRCA |
| CR = normal hemoglobin |
| PR = hemoglobin > 10 g/dL |
| NR = hemoglobin < 10 g/dL or no improvement |
| ITP |
| CR = normal platelet count |
| PR = platelet count ≥ 30 × 109/L |
| NR = platelet count < 30 × 109/L or no improvement |
| AIG |
| CR = normal neutrophil count |
| PR = ANC ≥ 1 × 109/L |
| NR = ANC < 1 × 109/L or no improvement |
CR – complete response
PR – partial response
NR – no response
Statistical Analysis
Descriptive counts and frequency distributions were computed and either the Wilcoxon rank test or chi-square test was used to compare difference between demographic variables. Survival curves were calculated using the Kaplan-Meier method. Log-rank tests or Cox proportional hazard regression analysis was used for statistical comparisons, with death or censoring (as of 11 November 2008) as the event. When needed, we modeled our binary covariates (such as AID cytopenia status) as a time-dependent covariate in the Cox regression model. All analyses were done using S-plus (Insightful, Seattle, WA) or SAS (SAS Institute, Inc., Cary, NC). Statistical significance was set at a 5% threshold.
Results
One thousand seven hundred and fifty patients with CLL were seen in the Division of Hematology at MCR over the 10 year period from 1 January 1995 to 31 December 2004. Of these 75 (4.3%) had AID cytopenia. The median follow up for the entire CLL population was 6.5 years and for patients with AID cytopenia was 7.8 years.
Clinical Features
The median age at diagnosis of the first episode of AID cytopenia was 66.7 years (range 30 – 85) with a male predominance (79%). The majority of patients (n = 54, 72%) had their AID cytopenia diagnosed after their CLL diagnosis, 14 (19%) patients had AID cytopenia and CLL diagnosed within one month of each other, and 7 (9%) patients had AID cytopenia diagnosed at least one month before their CLL (median difference in time between diagnosis of CLL and AID was 1.7 years). For patients with AID cytopenia diagnosed after the diagnosis of CLL, the median interval between the diagnosis of CLL and AID cytopenia was 3.3 years (range 0.2 – 17.4). Ten patients (13%) had more than one type of AID cytopenia. Of the patients with AID cytopenia, 62 (82%) had lymphocytosis > 5 × 109/L and the other 13 (18%) had the SLL variant of CLL. Thirty two (43%) patients were considered regional (non-referred) because they lived within 120 miles of Rochester MN (excluding the Minneapolis – St. Paul metropolitan area). A comparison of the clinical features of patients with AID cytopenia, bone marrow failure and the entire CLL cohort has been previously published1.
AIHA
(n = 41, 55%) was the most common presentation of AID cytopenia and presented with symptomatic anemia in 74% of cases (median hemoglobin (Hgb) 7.3 g/dL, standard deviation (SD) 1.7, range 4.7 - 10.9, normal range 13.5 – 17.5 in males and 12.0 – 15.5 in females). The absolute reticulocyte count was increased in 25 out of 39 (67%) patients with a median count of 209 × 109/L (SD 90.5, range 110 to 436, normal range 29.5 -87.3). The total bilirubin was elevated in 37 out of 39 (95%) patients (median 2.2 mg/dL, SD 1.2, range 1.1 – 5.3, normal range 0.1 – 1.0). The indirect bilirubin was elevated in 34 of 37 (92%) patients (median 1.70 mg/dL, SD 0.98, range 0.7 – 4.5, normal range 0.0 – 0.3). LDH was increased in 32 out of 39 (82%) of patients (median 358 U/L, SD 192, range 232 – 1155, normal range 122 – 222). The direct antiglobulin test (DAT) was positive in 35 (92%) patients and negative in 3 (8%) patients with no data available for 3 patients.
ITP
(n = 35, 47%) was an incidental finding in most patients (68%). The median platelet count at diagnosis of ITP was 15.5 × 109/L (SD 31, range 1 – 100, normal range 150 - 450). In patients presenting with bleeding/bruising (32%) the median platelet count at diagnosis was 7.0 × 109/L (SD 4.6, range 1-15) compared to a median of 46.2 × 109/L (SD 30.5, range 6-100) in patients without bleeding. Bone marrow studies were done on all ITP patients and showed that megakaryocytes were either increased (n = 16, 46%) or normal in number (n = 18, 51%) (no specific comment was available in 1 patient whose bone marrow material was not available for review). DAT tests were done at diagnosis in 15 out of 33 (49%) ITP patients and were positive in 7 (47%) patients of whom 5 also had AIHA.
PRBCA
(n = 9, 12%) patients all presented with symptomatic anemia. Data on Hgb (median 5.7 g/dL, SD 1.9, range 3.8 – 9.0) and absolute reticulocyte count (median 5.0 × 109/L, SD 10.2, range 2.2 – 31.2) at presentation with anemia was available for 7 patients. Parvovirus serology was positive for IgM in 3 out of the 6 patients who were tested and polymerase chain reaction (PCR) for parvovirus was not done on any of the specimens. All patients had a bone marrow examination and none showed the characteristic features of parvovirus infection.
AIG
(n = 3, 4%) patients all presented with serious neutropenic infections.
Most of the AID patient cohort reported in this study underwent their initial evaluation prior to the widespread availability of molecular prognostic factors and these tests were only done on a limited number of patients (Table 2). The available data suggests that CLL patients with AID cytopenia are more likely to have unmutated IGVH, high risk FISH abnormalities and ZAP-70 expression. However, because this data is not available for most patients, the results cannot be analyzed for statistical significance.
Table 2.
Prognostic Factors in patients with AID cytopenia (n = 75)
| Prognostic Factor | |
|---|---|
| IgVH | Unmutated = 13 (65%) |
| Mutated = 7 (35%) | |
| No data = 55 | |
| FISH | High risk = 12 (57%) |
| Not high risk = 16 (43%) | |
| No data = 47 | |
| ZAP-70 | Positive = 8 (89%) |
| Negative = 1 (11%) | |
| No data = 66 | |
| CD38 | Positive = 18 (43%) |
| Negative = 24 (57%) | |
| No data = 33 | |
High risk FISH – 17p13- and/or 11q22-
Treatment
AIHA
There was information on the management of AIHA in 37 (90%) patients. Three of these patients met the 1996 National Cancer Institute Working Group (NCI-WG 1996)4 criteria other than their cytopenia for concomitant treatment for their CLL at the time of their first treatment of their AID cytopenia and were treated for both progressive CLL and AIHA. The other 34 patients had initial treatment specifically directed at their AIHA. Sixteen patients (43%) required only one treatment regimen and 21 (51%) patients required multiple treatment regimens (range 2- 7). There was detailed information on the first treatment of AIHA for 35 patients. Of these patients, most (n = 30, 86%) received corticosteroids and for 8 (23%) patients this was the only therapy used. Among the 34 patients who did not require concomitant treatment for CLL, 16 (47%) received red blood cell (RBC) transfusions, 2 underwent splenectomy, 3 received rituximab, 2 received cyclophosphamide, and 1 received intravenous immunoglobulin as part of their therapy. Thirteen (35%) of the treated patients achieved a complete response (CR) to therapy with 14 (38%) achieving a partial response (PR). The median duration of response to initial therapy among the 27 responding patients was 0.62 years.
ITP
Thirty one (89%) patients with ITP were treated including 8 patients who also had AIHA. Two of the patients with AIHA needing treatment for progressive CLL also had ITP. Thirteen (42%) patients required only one treatment regimen and 18 (58%) patients required multiple treatment regimens (range 2 – 7). Most patients (n = 27, 87%) received corticosteroids for the initial treatment of ITP and this was the only treatment used in 11 (35%) patients. Five (16%) patients received rituximab either as monotherapy (n = 3) or in combination with other drugs. Eight (26%) patients received platelet transfusions. Nine (29%) patients achieved a CR and 11 (35%) had a PR. The median duration of response to initial therapy among the 19 responding patients was 1.9 years.
PRBCA
All 9 patients required treatment with 3 requiring only 1 treatment regimen and 6 patients requiring 2-9 treatment regimens. The initial treatment for PRBCA included corticosteroids in 7 patients and in 5 patients this was the only therapy used. One patient was treated with corticosteroids and cyclosporine and 1 patient received corticosteroids and rituximab. One patient required concomitant treatment for CLL. Five patients required RBC transfusions. Three patients had a CR and 4 had a PR. The median duration of the initial response to therapy was 0.24 years.
AIG
All patients were treated, 1 with G-CSF and 2 with immunosuppressive therapy, with no responders.
Complications of treatment
The major complication of treatment of AID cytopenia was serious infection which occurred in 3 patients and included Klebsiella pneumonia and Pneumocystis pneumonia. One patient had a pulmonary embolism on treatment and one patient with PRBCA developed clinically significant iron overload because of the requirement for repeated red blood cell transfusions.
Treatment of CLL in Patients with AID
Nineteen (25%) of the 75 patients were never treated for their CLL. Twenty two (29%) were treated for CLL for the first time after they had developed AID. Of the 34 (45%) patients who were treated for CLL prior to developing AID cytopenia, 9 (12%) received regimens containing a purine analogue. Among these 9 patients, 4 subsequently developed AIHA, 2 ITP, 2 PRCA and 1 AIG. The median time from the start of treatment with purine analogues to the diagnosis of AID cytopenia was 0.68 years (range 0.04 – 2.69).
Survival
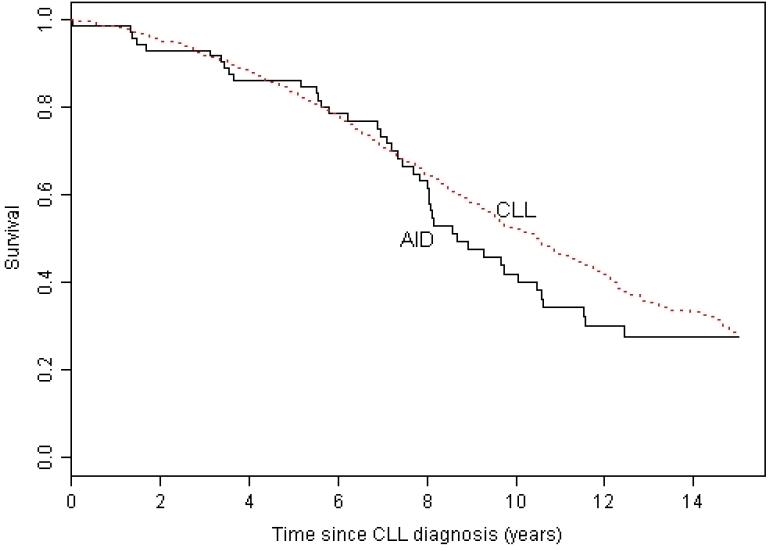
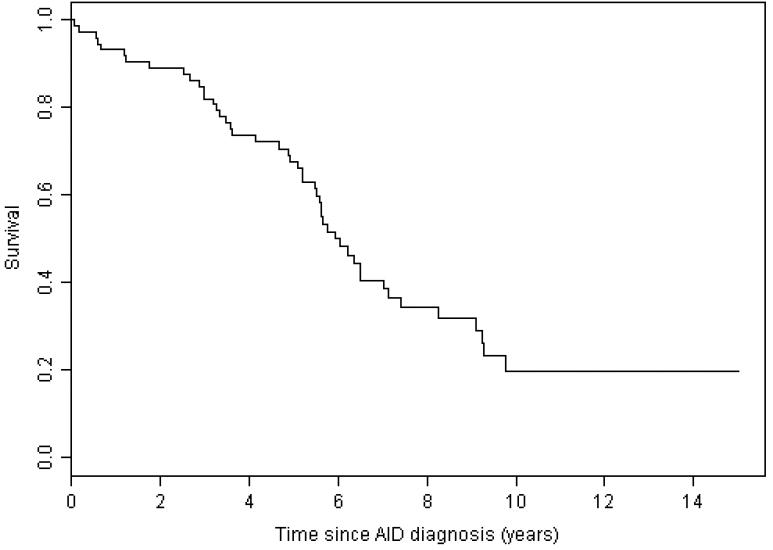
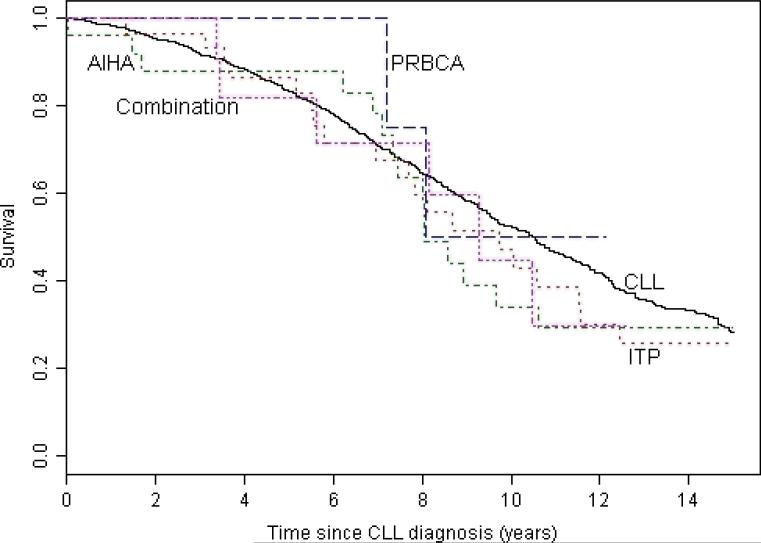
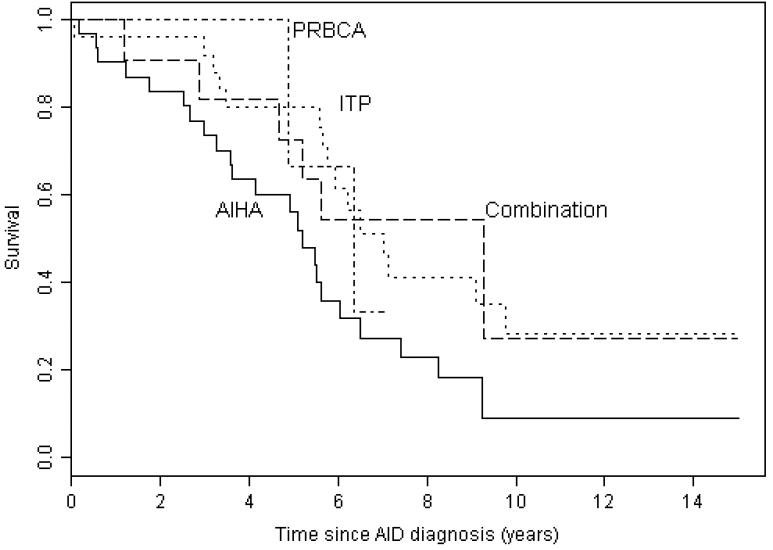
The median survival for patients with AID cytopenia complicating CLL was 8.7 years from the diagnosis of CLL (Figure 1) and 5.9 years from the onset of cytopenia (Figure 2). At the end of the study 7 (9%) of the 75 patients were alive with no evidence of active disease (neither CLL nor AID cytopenia), 20 (27%) were alive with evidence of disease, and 48 (64%) had died. The cause of death was progressive CLL in 14 (29%), CLL with active AID cytopenia in 2 (4%), AID cytopenia in 4 (8%), Richters transformation to diffuse large B cell lymphoma in 1 (2%), a second non-hematological malignancy in 7 (15%), unrelated to CLL or AID cytopenia in 11 (23%), and unknown in 9 (19%) patients. We have previously shown that the patients in this study with AID cytopenia had significantly better survival than the 228 CLL patients from this patient cohort with cytopenia caused by bone marrow failure, and similar survival compared to patients without cytopenia1. In this analysis with an additional 1.9 years of follow up, we confirm that patients with CLL do not have a significantly shorter survival if their disease is complicated by AID cytopenia (Figure 1). There was no significant difference in survival from the time of diagnosis of CLL for patients with the different forms of AID cytopenia (Figure 3) with median survival of 9.7 years (range 0.04 – 20.1) for AIHA, 8.1 years (range 0.02 – 25.3) for ITP, not yet reached for PRCA, and 9.3 years (range 0.07 – 12.6) for patients with multiple forms of AID cytopenia (p =0.716). There was also no significant difference in survival from the first diagnosis of AID cytopenia (Figure 4) with a median survival of 5.2 years (range 0.07 – 17.7) for AIHA, 7.0 years (range 0.07 – 16.7) for ITP, 6.3 years (3.42 – 7.13) for PRBCA, and 9.3 years (range 0.07 – 12.6) for patients with multiple forms of AID cytopenia (p = 0.081).
Figure 1. Survival from diagnosis of CLL.
The median survival from the time of diagnosis of the 75 CLL in patients who had cytopenia caused by autoimmune disease (AID) was 8.68 years (range 0.02 – 25.33) which was not significantly different from the 10.5 years (range 0 – 45.9) for the 1675 CLL patients without AID cytopenia (p = 0.178).
Figure 2. Survival from diagnosis of cytopenia caused by autoimmune disease (AID).
The median survival from the time of diagnosis of AID cytopenia was 5.94 years (range 0.07 – 23.14).
Figure 3. Survival from diagnosis of CLL.
There was no significant difference in survival from the time of diagnosis of CLL for patients with autoimmune hemolytic anemia (AIHA) (median 9.7 years, range 0.04 – 20.1), immune thrombocytopenia (ITP) (median 8.1 years, 0.02 – 25.3), pure red blood cell aplasia (PRBCA) (median not reached), and multiple types of autoimmune cytopenias (combination) (9.3 years, range 0.07 – 12.62) compared to patients with CLL that was not complicated by AID cytopenia (median 10.5 years, range 0.00 – 45.8) (p = 0.716).
Figure 4. Survival from diagnosis of cytopenia caused by autoimmune disease (AID).
There was no significant difference in survival from the time of diagnosis of AID cytopenia for patients with autoimmune hemolytic anemia (AIHA) (median 5.2 years, range 0.07 – 17.07), immune thrombocytopenia (ITP) (median 7.0 years, range 0.07 – 16.71), pure red blood cell aplasia (PRBCA) (6.3 years, range 3.42 – 7.13), and more than one manifestation of AID cytopenia (combined) (median 9.3 years, 0.07 – 23.14) (p = 0.081).
Discussion
This study reports the clinical features of AID cytopenia complicating CLL in 75 (4.3%) of 1750 patients seen at a single institution over 10 years. We show that the presentation and outcome for CLL patients with individual AID cytopenias (AIHA, ITP and PRBCA) differs from the original reports as reviewed by Hamblin in 20016. CLL patients in this study had a lower rate of AID cytopenia with a higher proportion of patients having ITP (47%). AID cytopenia was diagnosed throughout the course of CLL rather than being predominantly a complication of advanced stage CLL and usually responded well to treatment. Although many required long term maintenance therapy to prevent relapse, AID cytopenia caused or contributed to mortality in only 12% of deaths in patients with this complication of their CLL. In this analysis with a longer follow up and higher mortality rate (64%) we confirm our previous finding that AID cytopenia complicating CLL did not significantly decrease survival from the time of diagnosis of CLL and show in addition that there was no significant difference in survival from the onset of CLL or AID cytopenia for patients with different types of AID cytopenia (AIHA vs. ITP vs. PRBCA vs. a combination of these). These data suggest that the clinical presentation and prognosis of AID cytopenia has changed compared to historical reports.
The apparent decrease in the prevalence of AID cytopenia in patients with CLL could be caused in part by changes in the demographics of the CLL population. Earlier diagnosis of CLL13 and better treatment outcomes in CLL patients with progressive disease21, 22 have increased the duration of time that patients are monitored for cytopenias without necessarily increasing the risk for the development of AID cytopenia. The 2.3% rate of AIHA in our study is lower but comparable to the 4.3% rate reported from a study of 1203 patients from an Italian referral center7 and the 5.1% (10 of 195 patients)24 and 4.5% (6 of 132 patients)8 reported from single center studies with well characterized and stable patient populations. The lower rate in our study could be due to the larger patient population, high percentage of patients with early stage at diagnosis, and the data loss inherent in a largely retrospective study. In contrast, the rate of AIHA in our study is considerable less than the 10% reported recently in a population of patients following initial treatment for progressive CLL10 which could reflect the decrease in immune tolerance caused by therapy. The 2% rate of ITP in this study is similar to the 2.1 – 5% rate reported in other comparable studies8, 24, 25. PRBCA occurred in 0.5% of patients which is comparable to the rate of 1% previously reported6. The lower risk of AID cytopenia in our study population (4.3%) compared to older historical data is thus due to the lower rate of AIHA.
Cytopenia has long been recognized as a predictor of advanced stage disease and poor prognosis in CLL2, 3. In our study population only 7% of patients had cytopenia at diagnosis1 compared to the 28% reported by Rai et al in 19752 and 16% by Binet et al in 19813. Consequently, more patients with earlier stages are being observed for longer periods before needing treatment for progressive disease. In this study 71% of patients who developed AID cytopenia had already been diagnosed with CLL and ITP was an asymptomatic complication in 68% of patients. Our findings support the regular follow up of patients with asymptomatic untreated CLL and reinforce the need to include in the differential diagnosis in all patients with CLL who develop cytopenia.
Investigation of the etiology of cytopenia in CLL requires both clinical evaluation and appropriate laboratory testing. As shown in this study, most patients with CLL complicated by AIHA present with symptomatic anemia, have evidence of increased erythropoiesis with an elevated absolute reticulocyte counts, markers of active hemolysis and evidence of anti-RBC antibodies. A bone marrow study is not always required to diagnose AIHA but is important for determining both the extent of involvement by CLL and the bone marrow reserve which are critical for making appropriate decisions on treatment. In patients with CLL who develop anemia without clinical evidence of AIHA, a bone marrow examination is required to distinguish between bone marrow failure and PRBCA. In contrast, ITP complicating CLL is often asymptomatic and the diagnosis requires a bone marrow study to show normal-increased production of platelets. Based on these data we recommend that patients with CLL who have cytopenia should be evaluated for increased blood cell destruction and have a bone marrow study to evaluate hematopoiesis study prior to initiation of treatment.
There is no standard treatment for AID cytopenia in CLL. In this patient population patients without progressive CLL usually responded well to AID cytopenia targeted therapy. However, initial response duration was often short and most patients required long term maintenance therapy. In patients who required treatment for both progressive CLL and AID cytopenia, purine analogue containing regimens were usually avoided because of the concern about exacerbating the autoimmune blood cell destruction. These patients were usually treated with combinations of corticosteroids, alkylating agents and rituximab26. However, because of the small number of patients in each treatment group, we cannot make any specific treatment recommendations.
AID cytopenia contributed to the death of only 12% of the patients in this study. The most common cause of death was progressive CLL (29%) followed by non-CLL related causes (23%). However the high rate of deaths from a second malignancy (n = 8, 17%), of which only one was lymphoid, is of concern. Although CLL is known to increase the risk of second non hematological malignancies27, 28, this finding does suggest that the immune defects leading to AID cytopenia and the further suppression of immune function by treatment of AID cytopenia could increase the risk of second non-hematological malignancies. Determining the relationship between AID cytopenia and the risk of a second malignancy in CLL will require further investigation. In the interim, patients with CLL and AID cytopenia should be monitored carefully for evidence of complicating second malignancies.
The applicability of the findings in this study to other CLL patient populations could be limited by the retrospective collection of data collection prior to 2003. However, the loss of data was minimized by the availability of comprehensive electronic records for all patients. Because MCR is a large tertiary care center, referral bias could affect the applicability of our findings to the general population. However, 43% of the AID cytopenia patients were considered regional suggesting that this population should be representative. Many patients in this study were initially evaluated before the currently used prognostic factors were available and we have insufficient data for this patient population to formally analyze the correlation between these biological risk factors and the risk of AID cytopenia. However, our finding that most of the analyzed samples showed use of unmutated IGVH is compatible with previously reported studies25. We are currently investigating the relationship between molecular prognostic factors and the risk of AID cytopenia in CLL in an ongoing prospective study.
In conclusion our study suggests that improvements in the diagnosis and management of CLL have changed the clinical presentation and course of AID cytopenia. We recommend that physicians should consider AID in the differential diagnosis of cytopenia in patients with CLL and that a bone marrow study should be part of the evaluation of cytopenia in all CLL patients. AID cytopenia does not appear to be an independent prognostic factor in CLL patients, but prospective studies including biological risk factor analysis will be needed to resolve this question.
Acknowledgements
This study was supported by research funding from the Mayo Clinic Hematological Malignancies Fund, University of Iowa/Mayo Clinic NIH SPORE Grant CA97274, and Bayer Health Care Pharmaceuticals.
References
- 1.Zent CS, Ding W, Schwager SM, Reinalda MS, Hoyer JD, Jelinek DF, et al. The prognostic significance of cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) Br J Haematol. 2008;141:615–621. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 3.Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–205. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 5.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) updating the National Cancer Institute-Working Group (NCI-WG) 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamblin T. Autoimmune disease and its management in chronic lymphocytic leukemia. In: Cheson B, editor. Chronic lymphocytic leukemias. 2 ed. Marcel Dekker; New York: 2001. pp. 435–458. [Google Scholar]
- 7.Mauro F, Foa R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, et al. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–2792. [PubMed] [Google Scholar]
- 8.Kyasa MJ, Parrish RS, Schichman SA, Zent CS. Autoimmune cytopenia does not predict poor prognosis in chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Hematol. 2003;74:1–8. doi: 10.1002/ajh.10369. [DOI] [PubMed] [Google Scholar]
- 9.Myint H, Copplestone J, Orchard J, Craig V, Curtis D, Prentice A, et al. Fludarabine-related autoimmune hemolytic anaemia in patients with chronic lymphocytic leukaemia. Br J Haematol. 1995;91:341–344. doi: 10.1111/j.1365-2141.1995.tb05300.x. [DOI] [PubMed] [Google Scholar]
- 10.Dearden C, Wade R, Else M, Richards S, Milligan D, Hamblin T, et al. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: a beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood. 2008 Feb 15;111(4):1820–1826. doi: 10.1182/blood-2007-07-101303. [DOI] [PubMed] [Google Scholar]
- 11.Harris N, Jaffe E, Stein H, Banks P, Chan J, Cleary M, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 12.Muller-Hermelink HK, Catovsky D, Montserrat E, Harris NL. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. Tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon: 2001. pp. 127–130. [Google Scholar]
- 13.Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 15.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 16.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 17.Dewald GW, Brockman SR, Paternoster SF, Bone ND, O'Fallon JR, Allmer C, et al. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia. Br J Haematol. 2003;121:287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 18.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim S, Keating M, Do KA, O'Brien S, Huh YO, Jilani I, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98:181–186. doi: 10.1182/blood.v98.1.181. [DOI] [PubMed] [Google Scholar]
- 20.Zent CS, Call TG, Hogan WJ, Shanafelt TD, Kay NE. Update on risk-stratified management for chronic lymphocytic leukemia. Leuk Lymphoma. 2006;47:1738–1746. doi: 10.1080/10428190600634036. [DOI] [PubMed] [Google Scholar]
- 21.Keating MJ, O'brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 24.Hamblin T, Oscier D, Young B. Autoimmunity in chronic lymphocytic leukaemia. J Clin Pathol. 1986;39:713–716. doi: 10.1136/jcp.39.7.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visco C, Ruggeri M, Laura Evangelista M, Stasi R, Zanotti R, Giaretta I, et al. Impact of immune thrombocytopenia on the clinical course of chronic lymphocytic leukemia. Blood. 2008;111:1110–1116. doi: 10.1182/blood-2007-09-111492. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Kavuru S, Patel D, Janson D, Driscoll N, Ahmed S, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia. 2002 Oct;16:2092–2095. doi: 10.1038/sj.leu.2402676. [DOI] [PubMed] [Google Scholar]
- 27.Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45:507–513. doi: 10.1080/10428190310001612939. [DOI] [PubMed] [Google Scholar]
- 28.Tsimberidou AM, Wen S, McLaughlin P, O'Brien S, Wierda WG, Lerner S, et al. Other Malignancies in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. J Clin Oncol. 2008 Dec 29; doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]