Abstract
One dimensional gel electrophoresis was used to separate proteins from the saliva of Rhipicephalus sanguineus female ticks fed on rabbits. Gel slices were subjected to tryptic digestion and analyzed by reversed-phase HPLC followed by MS/MS analysis. The data were compared to a database of salivary proteins of the same tick and to the predicted proteins of the host. Saliva was obtained by either pilocarpine or dopamine stimulation of partially-fed ticks. Electrophoretic separations of both yielded products that were identified by mass spectrometry, although the pilocarpine-derived sample was of much better quality. The majority of identified proteins were of rabbit origin, indicating the recycling of the host proteins in the tick saliva, including hemoglobin, albumin, haptoglobin, transferring, and a plasma serpin. The few proteins found that were previously associated with parasitism and blood feeding include 2 glycine-rich, cement-like proteins, 2 lipocalins, and a thyropin protease inhibitor. Among other of the 19 tick proteins identified, albeit with undefined roles, were SPARC and cyclophilin A. This catalog provides a resource that can be mined for secreted molecules that play a role in tick-host interactions.
Keywords: Proteome, Tick saliva, Rhipicephalus sanguineus, Dopamine, Pilocarpine
Introduction
Rhipicephalus sanguineus, the brown dog tick, is an ubiquitous ectoparasite associated with pet dogs, cats, rabbits, rodents, pigeons, and occasionally found infesting humans. It is also a vector of canine babesiosis and Rocky Mountain spotted fever, among other diseases (Beugnet and Marie, 2009; Dantas-Torres, 2008; Dantas-Torres and Figueredo, 2006; Demma et al., 2005, 2006; Mariotte et al., 1944).
In order to suck blood, ticks use their chelicerae to lacerate the skin and then insert hypostome and chelicerae into the host's epidermis. Concomitantly, a salivary secretion builds a cement cone composed of many proteins, some of which are rich in the amino acid glycine. This structure maintains the tick mouthparts firmly attached, and acarologists believe that it also creates a gasket that facilitates blood sucking (Parola and Raoult, 2001; Sonenshine, 1992).
Tick saliva also assists blood feeding by means of a complex mixture of compounds. It contains vasodilators, inhibitors of blood clotting and platelet aggregation, and modulators of immunity and inflammation (Francischetti et al., 2009a; Hovius et al., 2008; Sá-Nunes and Oliveira, 2010).
Many salivary components are genus-specific proteins indicating a fast evolution of these proteins, perhaps due to different homeostatic pressures posed by the hosts (Francischetti et al., 2009b). Recently, the sialotranscriptome (from the Greek sialo=saliva) of females of R. sanguineus was described (Anatriello et al., 2010). This allows for identification of salivary proteins from tick saliva, which can be obtained by stimulation of partially-fed ticks with the secretagogues dopamine (DA) or pilocarpine (PC) (Kaufman, 1976; Sauer et al., 1995). Tick saliva also contains host proteins (Valenzuela et al., 2002), and the predicted proteomes of several mammals are also available in public databases. Such databases have now made it feasible to identify in greater detail the proteins secreted in tick saliva. In the present work, we describe the proteome of saliva from female R. sanguineus ticks partially fed on rabbits and obtained by stimulating the ticks with DA and PC.
Material and methods
Saliva collection
R sanguineus ticks were laboratory-reared, as previously described (Ferreira and Silva, 1998). To obtain engorged ticks for saliva collection, rabbits (n=4) were infested with 70 pairs of adult R sanguineus ticks restricted by plastic feeding chambers fixed to their backs (Ferreira et al., 1998). The experiments with rabbits are in agreement with the ethical principles in animal research adopted by the Brazilian College of Animal Experimentation (COBEA) in line with the Guidelines for Animal Users as issued by the National Institute of Health, and the animal protocol was approved by the School of Medicine of Ribeirão Preto of the University of São Paulo, Institutional Animal Care and Use Committee (IACUC) under protocol number 144/2010. The procedure for collection of saliva was performed on partially-engorged (after 5–7 days of feeding) female ticks rinsed in distilled water and dried with filter paper. A solution of DA (10 μL at 0.2% in phosphate-buffered saline, pH 7.4) was inoculated into each tick's hemocoel using a micro-fine 29 Gauge (12.7×0.33 mm) needle (BD Biosciences, San Diego, CA). Alternatively, 2 μL of PC hydrochloride (5% solution in 0.7 M NaCl) was inoculated near the border of the dorsal scutum together with a topical application of 5 μL of PC hydrochloride (5% solution in methanol) to their dorsal scutum. Tick saliva was harvested from tick mouthparts using a micropipette, kept on ice, pooled, filtered through a 0.22-μm pore filter (Costar-Corning Inc., Cambridge, MA), and stored at −70°C until further use.
Gel electrophoresis studies
Tick saliva samples (50 μL) collected with DA or PC (0.97 mg/mL and 1.21 mg/mL of protein, respectively) were resolved by one-dimensional (1D) sodium dodecylsulfate polyacrylamide gel electrophoresis (4–12% gradient gels) and visualized with Coomassie blue staining (Pierce, Rockford, IL). Excised gel bands were destained using 50% acetonitrile in 25 mM NH4HCO3, pH 8.4, and vacuum dried. Trypsin (20 μg/mL in 25 mM NH4HCO3, pH 8.4) was added, and the mixture was incubated on ice for one hour. The supernatant was removed, and the gel bands were covered with 25 mM NH4HCO3, pH 8.4. After overnight incubation at 37°C, the tryptic peptides were extracted using 70% acetonitrile, 5% formic acid, and the peptide solution was lyophilized and desalted using ZipTips (Millipore, Bedford, MA).
Nanoflow reversed-phase liquid chromatography tandem mass spectrometry (nanoRPLC -MS/MS)
Tryptic peptides were analyzed using nanoRPLC-MS/MS. A 75-μm i.d.× 360 μm o.d. × 10 cm long fused silica capillary column (Polymicro Technologies, Phoenix, AZ) was packed with 3 μm, 300 Å pore size C-18 silica-bonded stationary RP particles (Vydac/Grace, Deerfield, IL). The column was connected to an Agilent 1100 nanoLC system (Agilent Technologies, Santa Clara, CA) that was coupled online with a linear ion-trap mass spectrometer (LTQ; ThermoElectron, Waltham, MA). Peptides were separated using a gradient consisting of mobile phase A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The peptide samples were injected, and gradient elution was performed under the following conditions: 2% B at 500 nL/min for 30 min; a linear increase of 2–42% B at 250 nL/min for 110 min; 42–98% for 30 min including the first 15 min at 250 nL/min and then 15 min at 500 nL/min; 98% at 500 nL/min for 10 min. The linear ion-trap mass spectrometer was operated in a data-dependent tandem MS (MS/MS) mode in which the 5 most abundant peptide molecular ions in every MS scan were selected for collision-induced dissociation using a normalized collision energy of 35%. Dynamic exclusion was applied to minimize repeated selection of previously analyzed peptides. The capillary temperature and electrospray voltage were set to 160°C and 1.5 kV, respectively. Tandem MS spectra from the nanoRPLC-MS/MS analyses were searched against a protein FASTA database derived from the tick transcriptome previously reported (Anatriello et al., 2010), or from Ixodes scapularis-predicted proteins (obtained from www.vectorbase.org/), or from human and rabbit proteins (obtained from the NCBI sites ftp://ftp.ncbi.nih.gov/genomes/Oryctolagus_cuniculus/protein/ and ftp://ftp.ncbi.nih.gov/genomes/H_sapiens/protein/). These searches used the SEQUEST software operating on an 18-node Beowulf cluster. For a peptide to be considered legitimately identified, it had to achieve stringent charge state and proteolytic cleavage-dependent cross correlation (Xcorr) and a minimum correlation (ΔCn) score of 0.08.
MS results from legitimately identified peptides as described above were mapped to the Excel spreadsheets using a homemade program. The following example illustrates the convention for interpreting the data: The hit F_2 → 6 indicates that a particular protein had 6 MS/MS peptide hits in gel fraction 2.
Results
To obtain the salivary proteome of R. sanguineus females, we induced tick salivation by 2 different methods, using either DA or PC. Although DA is a commonly used and efficient secretagogue for obtaining tick saliva (Fezza et al., 2003; Kaufman, 1978; Ribeiro et al., 1992; Zhu et al., 1998), we observed that DA-induced saliva was brown in color and produced a precipitate following centrifugation at 14,000 g for 5 min. On the other hand, PC-induced saliva was clear and did not produce a precipitate. Comparison of the 1D gels for saliva induced by these 2 secretagogues showed that the DA-derived saliva had diffuse bands while the sample stimulated by PC had better defined bands (Figs. 1 and 2, respectively). The diffuse bands could derive from protein aggregation/polymerization due to presence of DA or due to the prophenoloxidase-activating system which participates in immunity of invertebrates causing melanization reactions and clotting of fluid proteins (Söderhäll and Cerenius, 1998). Important to highlight that we cannot exclude other forms of protein degradation, as it is caused by free radicals (Dean et al., 1997) during oxidation of DA (Chen et al., 2008)
Fig. 1.
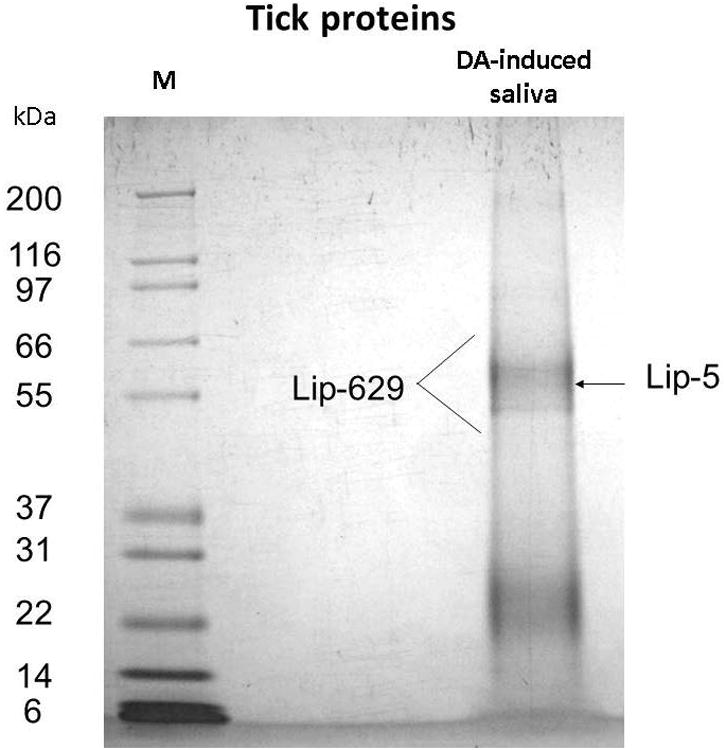
Gel electrophoresis, tryptic digestion, and tick protein identification by MS analysis of digested peptides from R. sanguineus saliva induced by DA. Left lane: Molecular weight standards (M). Right lane, DA-induced tick saliva. Lip stands for lipocalin, and the number refers to the protein numbers shown in Supplemental Spreadsheet S2, which is an updated version of a previously published file (Anatriello et al., 2010).
Fig. 2.
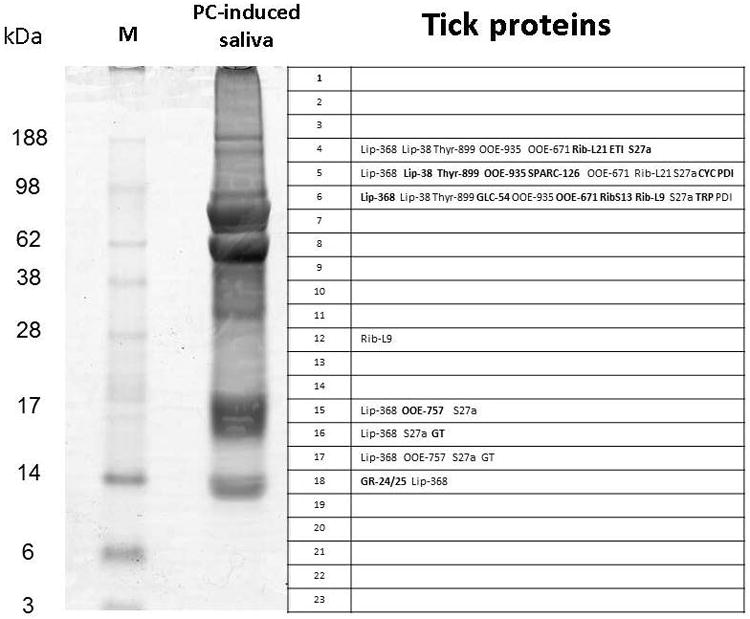
Gel electrophoresis, tryptic digestion, and tick protein identification by MS analysis of digested peptides from R. sanguineus saliva induced by PC. Left lane: Molecular weight standards (M). Right lane, PC-induced tick saliva. The grid numbered 1–23 corresponds to the fractions subjected to tryptic digest and mass spectrometric detection of peptidic fragments matching the tick sialotranscriptome, identified to the right side of the fraction number. For the description of protein symbols see Table 1.
After tryptic digestion of gel plugs from saliva induced by DA, only 2 lipocalins (Lip-629 and Lip-5) were identified in a region of the gel corresponding to the 55 kDa marker, even though these proteins have a predicted mass of 22–23 kDa (Fig. 1 and Supplemental Table S1). More proteins were identified in gel plugs deriving from saliva induced by PC, although far less than predicted from the transcriptome (Anatriello et al., 2010): Lipocalin RS-368 was found in several gel fractions, including at fraction 15 near the 17-kDa marker, compatible with its expected mature molecular mass of 19 kDa, but also in fractions 4, 5, and 6, which span from below the 188-kDa marker to below the 98-kDa marker. Thyropin (RS-899), containing 2 thyropin domains, as indicated by its comparison to the Pfam database, with predicted mass of 23 kDa, was also found in bands 4, 5, and 6, far above its predicted molecular mass. Similarly, RS-54, belonging to a protein family exclusive of ticks, named GLC, was found in fraction 6, just below the 98-kDa marker; the mature protein is predicted to have a mass of 25 kDa, suggesting these proteins may have further post-translation modifications. Two members of the “Metastriate one-of-each family ”, RS-935 and RS-757, were also identified; RS-935, with predicted molecular mass of ∼20 kDa, in fractions 5 and 6, near the 98-kDa marker, while RS-757 was found at fractions 15 and 17, near the markers for 17 and 14 kDa. The glycine-rich proteins RS-24 and RS-25 were found in fraction 18, near the 14-kDa marker. Because we do not have the full-length sequences of these proteins, it is not possible to correlate predicted masses with the gel fraction where they were found. Surprisingly, the lipocalins Lip-629 and Lip-5 found in the DA-induced saliva were not found in the saliva induced by PC. Other proteins matching the R. sanguineus tick sialotranscriptome (n=19) are listed in Table 1 and shown in Fig. 2, including ribosomal proteins, cyclophilin A, thioredoxin peroxidase, and a glycosyltransferase. Interestingly, although they are among the most abundant transcripts in the transcriptome of R. sanguineus (Anatriello et al., 2010), only 2 glycine-rich proteins were identified in the secreted proteome. This finding is compatible with the possibility that most glycine-rich proteins are indeed fixed in the cement cone and are not secreted into the host via saliva.
Table 1.
Tick proteins identified in R. sanguineus saliva induced by pilocarpine. See Fig. 2 legend for more details.
| Tick protein | MW (kDa) | Description | Symbol in Fig. 2 | Fraction → number of ionsa |
|---|---|---|---|---|
| RS-899 | 23.5 | Putative thyropin precursor | Thyr-899 | F_5 → 10| F_4 → 4| F_6 → 3| |
| RS-126 | 22.9 | Matricellular protein osteonectin/SPARC/BM-40 | Sparc-126 | F_5 → 3| |
| RS-54 | 29.3 | GLC family member | GLC-54 | F_6 → 3| |
| RS-935 | 17.2 | One of each family member 1 | OOE-935 | F_6 → 6| F_5 → 4| F_4 → 2| F_15 → 2| F_16 → 2| |
| RS-757 | 18.3 | One of each family member 2 | OOE-757 | F_15 → 2| F_17 → 2| |
| RS-671 | 17.2 | One of each family member 3 | OOE-671 | F_6 → 4| F_4 → 2| F_5 → 2| |
| RS-38 | 18.3 | Lipocalin | Lip-38 | F_5 → 4| |
| RS-368 | 21.3 | Lipocalin | Lip-368 | F_6 → 11| F_5 → 6| F_16 → 4| F_4 → 3| F_15 → 3| F_17 → 2| F_18 → 2| |
| RS-25 | 19.9 | Glycine-rich protein | GR-24/25 | F_18 → 2| |
| RS-24 | 32.0 | Glycine-rich protein | GR-24/25 | F_18 → 2| |
| RS-104 | 17.3 | 40S ribosomal protein S13 | Rib-S13 | F_6 → 3| F_15 → 2| |
| RS-67 | 21.8 | 60S ribosomal protein L9 | Rib-L9 | F_6 → 3| F_12 → 2| |
| RS-78 | 18.8 | Ribosomal protein L21 | Rib-L21 | F_4 → 4| F_5 → 4| |
| RS-815 | 20.9 | Eukaryotic translation initiation factor 3 subunit 2 beta | ETI | F_4 → 2| |
| RS-766 | 17.1 | Ubiquitin/ribosomal protein S27a fusion protein | S27a | F_4 → 4| F_5 → 3| F_6 → 3| F_15 → 2| F_16 → 2| F_17 → 2| |
| RS-279 | 22.3 | Thioredoxin peroxidase - truncated at 5′ | TRP | F_6 → 2| |
| RS-310 | 17.5 | Cyclophilin A | Cyc | F_5 → 3| |
| RS-878 | 26.8 | Protein disulfide isomerase | PDI | F_6 → 17| F_5 → 15| |
| RS-99 | 17.7 | Glycosyltransferase, putative | GT | F_16 → 2| F_17 → 2| |
F_5 → 10 indicates 10 ions were found for the protein in fraction 5.
Previous work has reported the presence of host blood proteins in the saliva of I. scapularis (Valenzuela et al., 2002) and Hyalomma marginatum rufipes (Francischetti et al., 2011). In fact; here we are also documenting 56 rabbit proteins in R. sanguineus saliva induced by PC. We used the predicted proteins of the rabbit (available from the NCBI) as identification targets for the electrophoresis MS/MS study of PC-induced saliva. This led to the identification of many rabbit proteins, with much larger peptide hit intensity than the identified tick proteins themselves (Table 2 and Fig. 3). The precursor of rabbit serum albumin (SA), for example, was found with high intensity (measured by the number of peptide ions matching SA) on bands spanning fractions 8 (188 ions detected), 9 (84 ions), 12 (82 ions), 7 (70 ions), 11 (60 ions), and 5 (54 ions), to name only the 6 most intense bands. These MS-MS-derived ions were found to produce over four-fold coverage of the protein on fraction 8, located near the 62-kDa marker, compatible with its predicted MW, and indicative of its large abundance. In comparison, the most abundant tick salivary protein identified, a protein disulfide isomerase, had only 17 ions detected in band 6, and the thyropin (RS-899) putative cysteine proteinase inhibitor had only 10 ions detected in band 5.
Table 2.
Rabbit proteins identified in R. sanguineus saliva induced by pilocarpine. Some of these proteins may have closely related tick protein homologs. See Table 3 for rabbit proteins without known tick homologs. Some proteins are isoforms of the same gene or closely related gene products. See Fig. 3 legend for more details.
| Rabbit protein | MW (kDa) | Description | Symbol in Fig. 3 | Fraction → number of ionsa |
|---|---|---|---|---|
| gi|126723746 | 69 | Serum albumin precursor | SA | F_8 → 188| F_9 → 84| F_12 → 82| F_7 → 70| F_11 → 60| F_5 → 54| |
| gi|156119356 | 77 | Serotransferrin | STF | F_7 → 72| F_12 → 36| F_9 → 36| F_10 → 28| F_8 → 24| F_11 → 22| |
| gi|291409423 | 50 | Tubulin, beta-like | TB | F_9 → 56| F_12 → 4| F_10 → 4| |
| gi|156119364 | 42 | Actin, cytoplasmic 1 | ACT | F_10 → 48| F_11 → 36| F_12 → 22| F_13 → 20| F_9 → 15| F_5 → 13| |
| gi|291415548 | 51 | Tubulin, beta 4 | TB | F_9 → 44| F_10 → 2| |
| gi| 126722957 | 46 | Alpha-1-antiproteinase S-1 precursor | α-1-AT | F_9 → 43| F_8 → 10| F_7 → 6| F_10 → 4| |
| gi| 126722876 | 46 | Alpha-1-antiproteinase E | α-1-AT | F_9 → 42| F_8 → 8| F_10 → 4| F_7 → 4| |
| gi| 126722912 | 46 | Alpha-1-antiproteinase F precursor | α-1-AT | F_9 → 34| F_8 → 10| F_7 → 6| F_10 → 4| |
| gi| 126723457 | 16 | Hemoglobin subunit beta-1/2 | Hb | F_12 → 30| F_13 → 30| F_4 → 30| F_10 → 28| F_3 → 28| F_5 → 26| |
| gi| 126723668 | 53 | Vitamin D-binding protein precursor | VDb | F_9 → 28| F_8 → 4| |
| gi|291400273 | 61 | Histidine-rich glycoprotein | HRGP | F_7 → 28| F_12 → 14| F_10 → 12| F_9 → 12| F_6 → 10| F_11 → 8| |
| gi|283806600 | 46 | Alpha-1-antitrypsin | α-1-AT | F_9 → 25| F_8 → 6| F_10 → 4| F_7 → 4| |
| gi|291392526 | 37 | L-lactate dehydrogenase B | LacDeh | F_12 → 25| |
| gi|291388359 | 71 | Poly A-binding protein, cytoplasmic 4 | PolA | F_14 → 24| F_17 → 24| F_16 → 20| F_18 → 18| F_15 → 16| F_21 → 4| F_18 → 23| F_16 → 23| F_14 → 23| F_19 → 23| F_17 → 23| F_15 → |
| gi|291393283 | 33 | Ribosomal protein SA | RibSA | 20| |
| gi|291415360 | 39 | Hypothetical protein LOC100009097 | HypP | F_2 → 22| F_3 → 20| F_9 → 16| F_11 → 10| F_5 → 10| F_4 → 8| F_18 → 21| F_16 → 21| F_14 → 21| F_19 → 21| F_17 → 21| F_15 → |
| gi|291402382 | 33 | Ribosomal protein SA-like | RibSA | 18| |
| gi|291395394 | 42 | Beta actin | ACT | F_11 → 21| F_10 → 20| F_12 → 9| F_13 → 4| F_17 → 4| F_9 → 3| |
| gi|291406576 | 70 | Heat shock 70 kDa protein 2 | HS70 | F_7 → 20| F_8 → 10| F_9 → 4| |
| gi|291389089 | 50 | Tubulin, alpha 4a | TB | F_9 → 18| F_11 → 8| F_10 → 6| F_12 → 4| |
| gi|291402475 | 52 | GL12416-like | GL | F_9 → 18| F_11 → 8| F_10 → 6| F_12 → 4| |
| gi|291408513 | 36 | Delta-aminolevulinic acid dehydratase | DALD | F_12 → 18 F_11 → 4| |
| gi|283837861 | 39 | Annexin A1 | Annex | F_11 → 18 F_2 → 16| |
| gi|156119416 | 37 | Serine/threonine-protein phosphatase PP1-beta catalytic subunit | PPh | F_12 → 18 F_11 → 8| |
| gi|291400199 | 71 | Heat shock 70 kDa protein 8 | HS70 | F_7 → 17| F_8 → 8| F_9 → 4| F_18 → 16| F_16 → 16| F_17 → 16| F_15 → 14| F_14 → 14| F_22 → |
| gi|291395508 | 30 | Ribosomal protein S4, X-linked X-like | RibS4 | 12| |
| gi|291407056 | 34 | Acidic ribosomal phosphoprotein P0 | RibP0 | F_17 → 16| F_18 → 12| F_16 → 12| F_14 → 12| F_15 → 10| |
| gi|291408341 | 72 | Heat shock 70 kDa protein 5 | HS70 | F_7 → 16| F_8 → 4| F_9 → 2| |
| gi|291410975 | 84 | Heat shock 90 kDa protein 1, beta | HS90 | F_7 → 16| F_6 → 6| F_9 → 4| F_4 → 4| |
| gi|291389085 | 50 | Tubulin, alpha 4a | TB | F_9 → 16| F_11 → 4| F_12 → 2| F_10 → 2| |
| gi|291389091 | 73 | mCG18413-like | mC | F_9 → 16| F_11 → 4| F_12 → 2| F_10 → 2| |
| gi|291411146 | 50 | Tubulin, beta 1-like | TB | F_9 → 15| |
| gi|291391538 | 42 | Beta actin | ACT | F_10 → 15| F_12 → 10| F_13 → 6| F_4 → 6| F_11 → 5| F_9 → 5| F_18 → 14| F_16 → 14| F_17 → 14| F_15 → 12| F_14 → 12| F_22 → |
| gi|291407691 | 26 | Ribosomal protein S4, X-linked X-like isoform 3 | RibSA | 10| |
| gi| 126723309 | 82 | Complement C3 alpha chain | C3 | F_5 → 14| F_4 → 12| F_11 → 8| F_2 → 6| F_10 → 4| F_3 → 4| |
| gi|291403445 | 32 | Nucleoside phosphorylase | NPp | F_13 → 14| F_12 → 8| |
| gi|291413837 | 34 | Serine protease inhibitor A3N-like, partial tyrosine 3/tryptophan 5 -monooxygenase Activation protein, | SPI | F_10 → 14| F_9 → 14| |
| gi|291405401 | 29 | epsilon polypeptide | Tmox | F_13 → 12| F_12 → 10| |
| gi|291388220 | 33 | Carbonic anhydrase I | CA | F_12 → 12| F_13 → 12| |
| gi|156119384 | 36 | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | PPh | F_12 → 12 F_11 → 8| |
| gi| 126723706 | 39 | Haptoglobin precursor | Hapto | F_10 → 12| F_11 → 8| F_9 → 6| |
| gi|291400837 | 30 | Ribosomal protein S4, X-linked X-like | RbSA | F_18 → 10| F_16 → 10| F_17 → 10| F_22 → 8| F_15 → 8| F_14 → 8| |
| gi|291398505 | 34 | Ribosomal protein L5 | RibL5 | F_17 → 10| F_18 → 8| F_15 → 8| F_14 → 8| F_16 → 6| F_20 → 4| |
| gi|291395123 | 39 | G-protein beta subunit-like protein | Gptn | F_18 → 10| F_15 → 10| F_14 → 10| F_17 → 10| F_16 → 8| F_22 → 6| |
| gi|291406239 | 102 | Solute carrier family 4, anion exchanger | SC | F_11 → 10| F_12 → 6| F_2 → 6| |
| gi|291385496 | 31 | Protein phosphatase 1, catalytic subunit, alpha | PPh | F_12 → 10 F_11 → 6| |
| gi|291397052 | 53 | Heat shock 70 kDa protein 8 | HS70 | F_7 → 9| F_8 → 6| F_9 → 4| |
| gi|291405719 | 81 | Myeloperoxidase-like | Mpox | F_9 → 8| |
| gi| 126723487 | 34 | Heterogeneous nuclear ribonucleoprotein C | NRNP | F_18 → 8| F_14 → 8| F_15 → 6| F_16 → 6| F_17 → 6| F_19 → 4| |
| gi|291394305 | 35 | DNA-binding protein B-like | DNABp | F_17 → 8| F_15 → 6| F_16 → 6| F_14 → 6| |
| gi|130488167 | 36 | Nuclease-sensitive element-binding protein 1 | NSEBP | F_17 → 8| F_15 → 6| F_16 → 6| F_14 → 6| |
| gi|291383209 | 29 | Ribosomal protein S6-like | RbS6 | F_17 → 8| F_18 → 6| F_15 → 6| F_21 → 6| F_16 → 6| F_14 → 6| |
| gi|291386387 | 34 | Ribosomal protein L5-like | RibL5 | F_17 → 8| F_18 → 6| F_15 → 6| F_16 → 6| F_14 → 6| F_20 → 4| |
| gi|291410580 | 32 | Ribosomal protein L7a-like | RibL7 | F_17 → 8| F_22 → 6| F_21 → 6| F_16 → 6| F_19 → 6| F_18 → 4| |
| gi|291396282 | 84 | Heat shock 90 kDa protein 1, beta | HSP90 | F_7 → 8| F_6 → 6| F_4 → 4| F_9 → 2| |
| gi|130500366 | 52 | Hemopexin precursor | HEMOP | F_8 → 6| F_9 → 4| |
F_8 → 188 indicates 188 ions were found for the protein in fraction 8.
Fig. 3.
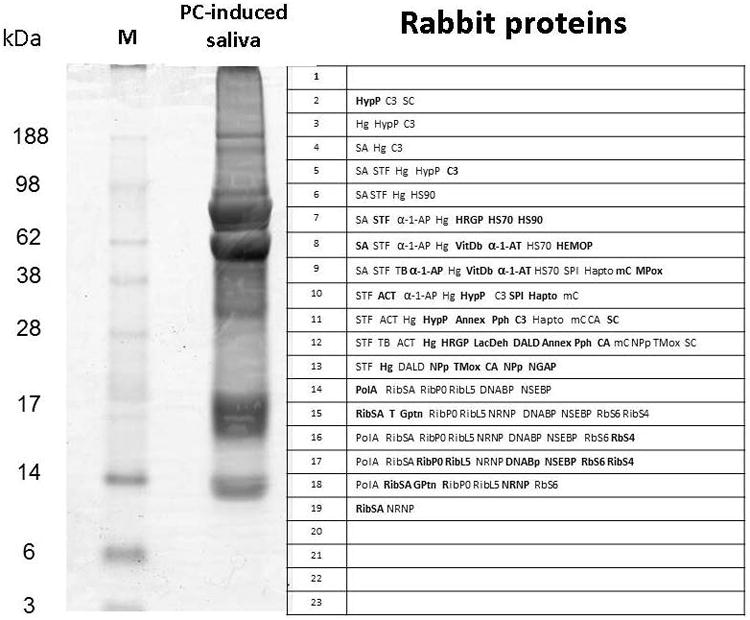
Gel electrophoresis, tryptic digestion, and rabbit protein identification by MS analysis of digested peptides from R. sanguineus saliva induced by PC. Left lane: Molecular weight standards (M). Right lane, PC-induced tick saliva. The grid numbered 1–23 corresponds to the fractions subjected to tryptic digest and MS detection of peptidic fragments matching the tick rabbit proteome, identified to the right side of the fraction number. For the description of protein symbols see Table 2.
Several other abundant rabbit blood proteins were identified, such as serotransferrin, haptoglobin, alpha-1 proteinase inhibitor, vitamin D-binding protein histidine-rich glycoprotein, erythrocyte band 3, an anion carrier, complement C3, carbonic anhydrase, and hemoglobin. Cytoskeletal proteins such as actin and tubulin were also found with large signals. Both of these latter proteins are largely conserved, and these signals could have been derived from tick proteins instead. Many hits to rabbit ribosomal proteins and enzymes were also found, as indicated in Tables 2 and 3 and in Supplemental Spreadsheet S2 and in Fig. 3. Interestingly, rabbit immunoglobulins were not identified in this study, although they are among the 3 most abundant in mammalian serum (Adkins et al., 2002; Pieper et al., 2003).
Some of the rabbit proteins identified in Tables 2 and 3 and Fig. 3 could actually be artifacts due to conservation of protein sequences among species. Because we do not have the full repertoire of proteins from R. sanguineus, these matches could be derived from nearly identical tick proteins and not host proteins. Accordingly, to further control the origin of the putative rabbit proteins identified in the tick salivary sample, in Supplemental Spreadsheet S2 we show the comparisons of these host protein sequences with proteins derived from the tick I. scapularis (Ribeiro et al., 2006). Indeed, a great part of the rabbit proteins identified is highly conserved. For example, mammalian tubulin is 97% identical to the I. scapularis tubulin while actin is 98% identical to the tick protein. Supplemental Spreadsheet S2 shows several such comparisons, with many proteins having >90% similarity to tick proteins, and thus their origin cannot be determined. They could have originated from R. sanguineus and not from host proteins, with several significant exceptions, as the proteins named in the previous paragraph and indicated in Table 3, listing proteins with no closely related tick homologs, and in Supplemental Spreadsheet S2.
Table 3.
Rabbit proteins without similarity with tick proteins identified in R. sanguineus saliva.
| Seq name | Description | Symbol in Fig. 3 |
|---|---|---|
| gi|126723746 | Serum albumin precursor | SA |
| gi|156119356 | Serotransferrin | STF |
| gi|126722957 | Alpha-1-antiproteinase | α-1-AT |
| gi|126723457 | Hemoglobin subunit beta-1/2 | Hb |
| gi|126723668 | Vitamin D-binding protein precursor | VDb |
| gi|291400273 | Histidine-rich glycoprotein | HRGP |
| gi|291408513 | Delta-aminolevulinic acid dehydratase | DALD |
| gi|283837861 | Annexin A1 | Annex |
| gi|126723309 | Complement C3 alpha chain | C3 |
| gi|291403445 | Nucleoside phosphorylase | NPp |
| gi|291388224 | Carbonic anhydrase | CA |
| gi|126723706 | Haptoglobin precursor | Hapto |
| gi|291406239 | Band 3 anion exchanger | SC |
| gi|291405719 | Myeloperoxidase-like | Mpox |
| gi|291413525 | Neutrophil gelatinase-associated lipocalin | NGAP |
| gi|130500366 | Hemopexin precursor | HEMOP |
Discussion
Apart from leakage of intracellular components from degenerated or damaged salivary gland cells, the presence of ‘housekeeping’ components in tick saliva might have functions in parasitism and is supported by previous observations of apocrine and merocrine secretion in tick salivary glands (Coons and Roshdy, 1981). In this regard, among the tick salivary proteins identified by this work in saliva induced by PC, only a few have known functions in parasitism by ticks, i.e. glycine-rich, cement-like proteins, lipocalins, and thyropin protease inhibitor (Anatriello et al., 2010; Francischetti et al., 2009b). On the other hand, the other tick proteins identified in this proteomic study have undefined roles. Two such proteins are SPARC and cyclophilin A; in our previous work describing the transcriptome of female salivary glands, their transcript sequences had in silico evidence of a signal peptide indicating that the tick can secrete these expressed proteins into saliva. In mammals, SPARC functions as a matricellular glycoprotein and modulates cellular interactions within the extracellular matrix, inhibits angiogenesis (Bhoopathi et al., 2010), regulates the expression of plasminogen activator inhibitor-1 by fibrotic fibroblasts (Chang et al., 2010), organizes the assembly of collagen fibrils (Giudici et al., 2008), and participates of Th17 differentiation and germinal centre formation (Piconese et al., 2011). Given the intimate relationship of ticks with their hosts' extracellular matrix, their SPARC protein may interfere in this structure to the tick's advantage. Tick SPARC must be further studied. In vertebrates, cyclophilin A is a ubiquitous, abundant protein of the cytosol. During inflammatory responses, it is released into extracellular spaces by live and dying cells and binds to cell surface heparans and to its main signaling receptor, CD147, displaying potent chemotactic activity for monocytes, neutrophils, eosinophils, and T cells in the periphery (Xu et al., 1992; Yurchenko et al., 2002). In addition, secretion of cyclophilin A by thymocytes is able to modulate bone marrow cell migration (Khromykh et al., 2007). Through its peptidyl-prolyl cis-trans isomerase activity, it assists the folding of proteins by catalyzing cis-trans isomerization of proline imidic peptide bonds. In bacterial pathogens, cyclophilin A is a virulence factor that promotes colonization of mucosa by Streptococcus pneumoniae, although the exact mechanism is still unknown (Hermans et al., 2006). In Legionella pneumophila, a surface-exposed peptidyl-prolyl cis-trans isomerase called Mip promotes secretion or activation of p-nitrophenol phosphorylcholine hydrolase beyond the outer membrane of the pathogen (Debroy et al., 2006). In Toxoplasma gondii, a secreted cyclophilin stimulates interleukin-12 from dendritic cells and has a high affinity for the CCR5 chemokine receptor (Aliberti et al., 2003). Whether the peptidyl-prolyl cis-trans isomerase property of cyclophilin A could affect the activity of parasitic and/or host proteins or the chemotaxis and activation of host leukocytes, remains to be elucidated.
Regarding the large amount of host proteins present in R. sanguineus tick saliva, this apparently unusual finding merits some speculation. We suggest that these ectoparasites recycle these molecules from the hemolymph. It is widely demonstrated that ticks as well as other kinds of blood-feeding and non-blood-feeding insects, after their meal, move intact proteins across the digestive system to the hemolymph (reviewed by Jeffers and Roe, 2008). Once in the hemolymph, it is suggested that these proteins may cross the digestive system and are transported to the salivary glands where they are secreted back into the host. This hypothesis has been supported by different groups that demonstrated the presence of host proteins in tick saliva (Valenzuela et al., 2002; Madden et al., 2002; Wang and Nuttall, 1994; Francischetti et al., 2011). Hence, looking at our own results, several data support our suggestion that ticks are able to recycle host protein from the hemolymph: First, prior to collection of saliva, the tick mouthparts were carefully washed, but the amount of host protein in the proteome is too high to explain contamination from such a source; some of the host proteins described in this proteome (e.g. hemoglobin, transferrin, albumin) have been found in the tick hemolymph; host proteins can be found in the salivary glands of R. microplus nymphs and adults (I.K. de Miranda Santos, pers. communication); host proteins (e.g. albumin and hemoglobin) were found only selectively in the saliva because IgG, an abundant protein of the blood, was not detected. We also exclude regurgitation because in this case the saliva would be red in color, which was not the case. In addition, the strongly stained bands in the gel contained mostly host proteins. Indeed, this feature was correlated with the abundance of peptide ions for the respective proteins.
The intense bands near the 62-kDa marker (Fig. 3) correspond to the strong recovery of peptide ions for the abundant plasma proteins rabbit SA and serum transferrin, which should appear in this region of the gel (Tirumalai et al., 2003). Hemoglobin, band 3 anion exchanger, and carbonic anhydrase, important red cell constituents were also found. Leukocyte products such as myeloperoxidase and the abundant neutrophil gelatinase-associated lipocalin (NGAL) were found, as were the C3 protein of the complement system, a haptoglobin precursor and a plasma serpin that inhibits elastase and is the most abundant serine protease inhibitor in the circulation of mammals (Ranes and Stoller, 2005).
Why would ticks waste precious host proteins while feeding on them? Perhaps they do not need such proteins. Indeed, tick excretions are very rich in protein, and SDS gel electrophoresis of I. scapularis feces has the same Coomassie-stained bands as a sample of rabbit blood (Ribeiro, unpublished). Over 70 years ago, a classical study showed that the amounts of blood taken by ticks in the first days of feeding is much larger than the tick's increase in weight, leading to exsanguination and death of their rabbit hosts (Jellison and Kohls, 1938). It has been suggested that in the first days of feeding, ticks are skimming the blood for oligonutrients and/or lipids, while excreting the unnecessary or excessive components via saliva or fecal material (Ribeiro, 1988), similar to feeding aphids that excrete a sugary liquid when feeding on sugar-rich but amino acid-poor diet (Klingauf, 1987). Another explanation is that ticks are, instead, recycling pivotal host proteins in order to subvert their role in the host. We have previously shown that tick infestations induce systemic acute-phase reaction proteins, including transferrin and haptoglobin (Carvalho et al., 2010). The presence of a host haptoglobin precursor and transferrin in the tick salivary proteome indicates that it probably is present locally in the blood feeding pool. Among plasmatic proteins, haptoglobin has the highest binding affinity for hemoglobin. It defends the host's vasculature against hemoglobin and heme by removing them with the CD163 scavenger receptor of the monocyte/macrophage system. CD163 is specific for the haptoglobin-hemoglobin complex. Once it binds to this complex, it signals anti-inflammatory responses that culminate in the release of IL-10 and the induction of heme oxygenase-1 (Abraham and Drummond, 2006), an enzyme that generates the immunosuppressive metabolites CO, Fe++, and biliverdin from heme. Haptoglobin might thus establish a favorable milieu for the tick, and the possibility that ticks recirculate host haptoglobin through their salivary glands for this exact purpose is intriguing. Transferrin is a negative acute-phase protein and a regulator of levels of free iron. It affects the immune response by regulating the amount of iron available to cells of the immune system and ticks may be recirculating or sequestering host transferrin in order to regulate the host immune and/or the levels of a potentially toxic ion.
We can also speculate that if the host proteins pass through the intracellular tick compartment, these proteins could be subjected to the tick glycosylation machinery and thus immunogenic ‘tick’ sugar-based antigens would be presented to the host, possibly to function as decoys that divert host immunity away from tick proteins, or the host self epitopes associated with the tick glycosyl-epitopes may lead to immune suppression towards tick epitopes. Indeed, bovine beta chain hemoglobin shown to be present in saliva from females of the cattle tick, Rhipicephalus microplus, is recognized by IgG antibodies from tick-infested bovines and, furthermore, the reactive protein is acidic (pI 6.09) in comparison to native bovine hemoglobin, indicating that the native hemoglobin was modified in the tick (I.K. de Miranda Santos, unpublished).
It is also interesting to note that the lipocalin marked as Lip-368 (Fig. 2) is identified in 4 gel fractions where it is expected (15, 16, 17, and 18 in Fig. 2, near the 14-kDa marker), as well as on gel fractions of much higher MW, in particular in fraction 6, by the 98-kDa marker. The tick lipocalin Lip-38 (Fig. 2), although having a MW near 20 kDa as most lipocalins, is found in fractions corresponding to 100 kDa and above (fractions 4 and 5 in Fig. 2). The same occurs with some host proteins. Rabbit hemoglobin appears in fraction 13, above the 17-kDa marker, but also on bands 5 and 6, near the 98-kDa marker (Fig. 3). This increased molecular weight could, again, reflect glycosylation of part of the proteins, other post-translation modifications, or strong binding of tick salivary proteins with host proteins. The possible modification of host proteins by ticks is indeed a subject worth of consideration.
While this putative modification of host proteins by ticks needs further confirmation, there is much evidence showing that the binding of host proteins by tick salivary components is strong enough to survive to SDS denaturation. Sá-Nunes and colleagues (Sa-Nunes et al., 2009) demonstrated that sialostatin L, a tight-binding cysteine protease inhibitor from I. scapularis saliva, binds to cathepsin S and that the complex is stable under SDS conditions. Unbound cathepsin S was detected only upon boiling the sample. In fact, sialostatin L presents a nanomolar affinity to its major targets (Kotsyfakis et al., 2006), and high-affinity binding was also demonstrated for other tick salivary-binding molecules, such as salivary anti-complement proteins (Nunn et al., 2005; Valenzuela et al., 2000) and disintegrins (Mans et al., 2002). Modification of host proteins by ticks can have yet another biological role. It can obviously involve proteolysis, but with consequences far-reaching those that facilitate absorption of amino acids: Hemoglobin degradation leads to formation of hemorphins, opioid peptides active in the immune system and in pain reception and hemoglobin-derived peptides also have antimicrobial activities (Nyberg et al., 1997).
Tick saliva induced by either DA or PC has been employed in different experimental approaches in order to identify its immunopharmacological activities. A few years ago, the concentration of PC present in PC-induced saliva was determined, and it ranged from 3 to 50 mM (Ribeiro et al., 2004). Because pilocarpine presents effects on different cell types, appropriate controls are required when employing tick saliva in biological assays, in order to avoid experimental artifacts. At least for activation assays of dendritic cells in the presence of I. scapularis and R. sanguineus saliva, the fraction containing PC has not induced significant changes of cytokine production (Sa-Nunes et al., 2007). In the same manner, DA does not affect the biological activities of saliva obtained from R. microplus (Carvalho et al., 2010). However, our experience with tick salivation employing DA or PC suggests that researchers using dopamine to obtain tick saliva should take into consideration the possible melanization of their salivary sample via the tick's prophenoloxidase pathway or spontaneous sample oxidation. Perhaps, it should be advisable that the salivary sample be used immediately, that inhibitors for the prophenoloxidase pathway be employed (e.g. EDTA and DTT), or that the proteins be separated from the dopamine as soon as possible to avoid dopamine oxidation and possible protein cross-linking.
Supplementary Material
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP through grant 2009/53645-3 to IKMS, and scholarships to EA (2007/50869-2) and CJFO (2006/54985-4); by the Brazilian National Science Foundation-CNPq through grants 559603/2009-6 and 471946/2010-9 to IKMS, Fundação de Amparo à Pesquisa do Estado de Minas Gerais- FAPEMIG and the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank the Research Technology Branch under Dr. Robert Hohman for preparing the gel and mass spectrometric data collection and analysis, and to the NIAID intramural editor Brenda Rae Marshall for assistance.
Because JMCR and IMF are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
Footnotes
Conflict of interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NG, Drummond G. CD163-mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- Anatriello E, Ribeiro JM, de Miranda-Santos IK, Brandao LG, Anderson JM, Valenzuela JG, Maruyama SR, Silva JS, Ferreira BR. An insight into the sialotranscriptome of the brown dog tick, Rhipicephalus sanguineus. BMC Genomics. 2010;11:450. doi: 10.1186/1471-2164-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beugnet F, Marie JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163:298–305. doi: 10.1016/j.vetpar.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka SS. The role of MMP-9 in the anti-angiogenic effect of secreted protein acidic and rich in cysteine. Br J Cancer. 2010;102:530–540. doi: 10.1038/sj.bjc.6605538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho WA, Maruyama SR, Franzin AM, Abatepaulo AR, Anderson JM, Ferreira BR, Ribeiro JM, More DD, Augusto Mendes Maia A, Valenzuela JG, Garcia GR, de Miranda Santos IK. Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands. Exp Parasitol. 2010;124:428–435. doi: 10.1016/j.exppara.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ding Y, Cagniard B, Van Laar AD, Mortimer A, Chi W, Hastings TG, Kang UJ, Zhuang X. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons LB, Roshdy MA. Ultrastructure of granule secretion in salivary glands of Argas (Persicargas) arboreus during feeding. Parasitol Res. 1981;65:225–234. [Google Scholar]
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Figueredo LA. Canine babesiosis: a Brazilian perspective. Vet Parasitol. 2006;141:197–203. doi: 10.1016/j.vetpar.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324(Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debroy S, Aragon V, Kurtz S, Cianciotto NP. Legionella pneumophila Mip, a surface-exposed peptidylproline cis-trans-isomerase, promotes the presence of phospholipase C-like activity in culture supernatants. Infect Immun. 2006;74:5152–5160. doi: 10.1128/IAI.00484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma LJ, Traeger M, Blau D, Gordon R, Johnson B, Dickson J, Ethelbah R, Piontkowski S, Levy C, Nicholson WL, Duncan C, Heath K, Cheek J, Swerdlow DL, McQuiston JH. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 2006;6:423–429. doi: 10.1089/vbz.2006.6.423. [DOI] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- Ferreira BR, Silva JS. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-gamma-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:279–293. doi: 10.1016/s0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- Fezza F, Dillwith JW, Bisogno T, Tucker JS, Di Marzo V, Sauer JR. Endocannabinoids and related fatty acid amides, and their regulation, in the salivary glands of the lone star tick. Biochim Biophys Acta. 2003;1633:61–67. doi: 10.1016/s1388-1981(03)00087-8. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Anderson JM, Manoukis N, Pham VM, Ribeiro JM. An insight into the sialotranscriptome and proteome of the coarse bontlegged tick, Hyalomma marginatum rufipes. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.07.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009a;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IMB, Sá-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. 2009b;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudici C, Raynal N, Wiedemann H, Cabral WA, Marini JC, Timpl R, Bächinger HP, Farndale RW, Sasaki T, Tenni R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J Biol Chem. 2008;283:19551–19560. doi: 10.1074/jbc.M710001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans PW, Adrian PV, Albert C, Estevão S, Hoogenboezem T, Luijendijk IH, Kamphausen T, Hammerschmidt S. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem. 2006;281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- Hovius JW, Levi M, Fikrig E. Salivating for knowledge: potential pharmacological agents in tick saliva. PLoS Med. 2008;5:e43. doi: 10.1371/journal.pmed.0050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers LA, Roe MR. The movement of proteins across the insect and tick digestive system. J Insect Physiol. 2008;54:319–332. doi: 10.1016/j.jinsphys.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Jellison WL, Kohls GM. Tick-host anemia: a secondary anemia induced by Dermacentor andersoni Stiles. J Parasitol. 1938;24:143–154. [Google Scholar]
- Kaufman W. The influence of various factors on fluid secretion by in vitro salivary glands of ixodid ticks. J Exp Biol. 1976;64:727–742. doi: 10.1242/jeb.64.3.727. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. Actions of some transmitters and their antagonists on salivary secretion in a tick. Am J Physiol. 1978;235:R76–81. doi: 10.1152/ajpregu.1978.235.1.R76. [DOI] [PubMed] [Google Scholar]
- Khromykh LM, Kulikova NL, Anfalova TV, Muranova TA, Abramov VM, Vasiliev AM, Khlebnikov VS, Kazansky DB. Cyclophilin A produced by thymocytes regulates the migration of murine bone marrow cells. Cell Immunol. 2007;249:46–53. doi: 10.1016/j.cellimm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Klingauf FA. Feeding, adaptation and excretion. In: Minks AK, Harrewijn P, editors. Aphids: Their Biology, Natural Enemies, and Control World Crop Pests. 2A. Elsevier; Amsterdam: 1987. pp. 225–253. [Google Scholar]
- Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Madden RD, Sauer JR, Dillwith JW. A proteomics approach to characterizing tick salivary secretions. Exp Appl Acarol. 2002;28:78–87. [PubMed] [Google Scholar]
- Mans BJ, Louw AI, Neitz AW. Savignygrin, a platelet aggregation inhibitor from the soft tick Ornithodoros savignyi, presents the RGD integrin recognition motif on the Kunitz-BPTI fold. J Biol Chem. 2002;277:21371–21378. doi: 10.1074/jbc.M112060200. [DOI] [PubMed] [Google Scholar]
- Mariotte CO, Bustamante ME, Varela G. Hallazago del Rhipicephalus sanguineus Latreille infectado naturalmente con fiebre manchada de las Montanas Rocosas, en Sonora (Mexico) 1944. Rev Inst Salub Enferm Trop. 1944;5:297–300. [Google Scholar]
- Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174:2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- Nyberg F, Sanderson K, Glämsta EL. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 1997;43:147–156. doi: 10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Piconese S, Costanza M, Tripodo C, Sangaletti S, Musio S, Pittoni P, Poliani PL, Burocchi A, Passafaro AL, Gorzanelli A, Vitali C, Chiodoni C, Barnaba V, Pedotti R, Colombo MP. The matricellular protein SPARC supports follicular dendritic cell networking toward Th17 responses. J Autoimmun. 2011;37:300–310. doi: 10.1016/j.jaut.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, Su Q, McGrath AM, Estock MA, Parmar PP, Zhao M, Huang ST, Zhou J, Wang F, Esquer-Blasco R, Anderson NL, Taylor J, Steiner S. The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics. 2003;3:1345–1364. doi: 10.1002/pmic.200300449. [DOI] [PubMed] [Google Scholar]
- Ranes J, Stoller JK. A review of alpha-1 antitrypsin deficiency. Semin Respir Crit Care Med. 2005;26:154–166. doi: 10.1055/s-2005-869536. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. The midgut hemolysin of Ixodes dammini (Acari: Ixodidae) J Parasitol. 1988;74:532–537. [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Evans PM, MacSwain JL, Sauer J. Amblyomma americanum: characterization of salivary prostaglandins E2 and F2 alpha by RP-HPLC/bioassay and gas chromatography-mass spectrometry. Exp Parasitol. 1992;74:112–116. doi: 10.1016/0014-4894(92)90145-z. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Zeidner NS, Ledin K, Dolan MC, Mather TN. How much pilocarpine contaminates pilocarpine-induced tick saliva? Med Vet Entomol. 2004;18:20–24. doi: 10.1111/j.0269-283x.2003.0469.x. [DOI] [PubMed] [Google Scholar]
- Sa-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IM, Andersen JF, Shi GP, Chavakis T, Ribeiro JM, Kotsyfakis M. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol. 2009;182:7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM, Francischetti IM. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- Sá-Nunes A, Oliveira CJ. Sialogenins and immunomodulators derived from blood feeding parasites. In: Kini RM, Clemetson KJ, Markland FS, McLane MA, Morita T, editors. Toxins and Hemostasis: From Bench to Bedside. Springer; New York: 2010. pp. 131–152. [Google Scholar]
- Söderhäll K, Cerenius L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. [DOI] [PubMed] [Google Scholar]
- Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annu Rev Entomol. 1995;40:245–267. doi: 10.1146/annurev.en.40.010195.001333. [DOI] [PubMed] [Google Scholar]
- Sonenshine D. Biology of Ticks. Oxford University Press; USA: 1992. [Google Scholar]
- Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics. 2003;2:1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IMB, Pham VM, Garfield MK, Mather TN, Ribeiro JMC. Exploring the sialome of the tick, Ixodes scapularis. J Exp Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- Wang H, Nuttall PA. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology. 1994;109(Pt 4):525–530. doi: 10.1017/s0031182000080781. [DOI] [PubMed] [Google Scholar]
- Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267:11968–11971. [PubMed] [Google Scholar]
- Yurchenko V, Zybarth G, O'Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- Zhu K, Bowman AS, Dillwith JW, Sauer JR. Phospholipase A2 activity in salivary glands and saliva of the lone star tick (Acari: Ixodidae) during tick feeding. J Med Entomol. 1998;35:500–504. doi: 10.1093/jmedent/35.4.500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


