SUMMARY
The immune response in the tumor microenvironment is complex, consisting of cells from both the adaptive and innate immune systems. The phenotype and function of these cells are dictated by cytokines present in the microenvironment, as well as by the interactions of these cells with the tumor cells and each other. Technological advances have allowed investigators to better identify the specific immune cells present and immune-related gene signatures overexpressed in the tumor microenvironment. Increased knowledge of tumor immunology has allowed us to better understand how these cells and the developing tumor interact. Together, these advances have prompted the conduct of numerous studies investigating the prognostic and predictive significance of immune infiltrates.
Virtually all solid tumors contain immune cells at various densities, ranging from gross inflammation apparent by standard staining techniques to subtle infiltration requiring specific antibodies for identification [1]. Depending on the context, the different cell types involved in the immune response may promote or inhibit tumor progression [2]. The immune system therefore plays a critical role in carcinogenesis. Recognizing this, in the 2011 update of the ‘Hallmarks of cancer’, Hanahan and Weinberg included ‘evading immune destruction’ as a new hallmark and identified inflammation as an enabling characteristic for the acquisition of this and other hallmark capabilities [3].
Although breast cancer has not traditionally been considered to be an immunogenic tumor, recent molecular profiling data showed that all breast cancers have an inflammatory gene signature [4]. That same study demonstrated that immune signatures may be prognostic, with a gene signature favoring a high CD8+ cytotoxic T lymphocyte (CTL) and CD4+ T helper (Th) type 1 response being a strong predictor of good outcome relative to a predominant Th type 2 response. In addition, there are data showing that the presence of tumor-infiltrating lymphocytes (TILs) or the high expression of immune gene signatures may predict response to therapy [5,6]. Therefore, there is growing interest in characterizing the immune aspects of the breast tumor microenvironment.
In this review, we will discuss the available data regarding the immune response within the breast tumor microenvironment, highlighting studies that have shown that the immune response has predictive or prognostic significance.
Prognostic impact of intratumoral immune response
Pathologic evaluation of inflammation
Early studies evaluating the prognostic impact of an inflammatory infiltrate in breast cancer reported conflicting results, with some showing an association with improved survival and others showing an association with worse outcome (reviewed by Mohammed et al. [7]). These studies were heterogeneous with respect to the methodology used to identify inflammation, patient population and length of follow-up. In a recently published study, Rakha and colleagues correlated the degree of tumor-associated inflammation with known prognostic characteristics, as well as survival outcomes [8]. The study included 1597 patients treated with definitive surgery between 1974 and 1988. No patients received adjuvant chemotherapy or endocrine therapy, allowing investigators to evaluate the effects of inflammation on the natural history of disease. Pathologic evaluation included determination of histology, the presence of lymphovascular invasion, tumor grade and hormone receptor status. The intensity of inflammation determined on hematoxylin and eosin (H&E) sections was absent or minimal in 72%, mild in 18%, moderate in 8% and marked in 2% of cases. For survival analyses, moderate and marked inflammation were categorized together as ‘prominent’, and on both univariate and multivariate analyses, prominent inflammation correlated with improved overall survival (OS) and recurrence-free survival (RFS). Grade 3 carcinomas with prominent inflammation had improved OS compared with those without. Interestingly, patients with grade 2 carcinomas with absent or mild inflammation had worse OS than grade 3 carcinomas with prominent inflammation [8]. These data are consistent with other studies showing that a strong lymphocytic infiltrate is associated with good clinical outcomes in various other solid tumor types [9].
Additional studies have evaluated the prognostic significance of lymphocytic infiltration in particular subtypes of breast cancer. A study from Liu et al. evaluated TILs on a tissue microarray constructed from over 3990 breast tumors [10]. On multivariate analysis, the presence of TILs was an independent prognostic factor associated with improved breast-cancer-specific survival for patients with basal-like breast cancer, which they defined as being estrogen receptor (ER)-, progesterone receptor- and HER2-negative, and either CK5/6 or EGF receptor-positive. A study from Alexe et al. evaluated patients with node-negative, HER2-overexpressing breast cancer, and found that patients with tumors with a marked lymphocytic infiltrate had significantly improved rates of distant metastasis-free survival [11]. Taken together, these data suggest that the immune response in the tumor microenvironment of both HER2+ and basal tumors is an important prognostic factor that could potentially be used to stratify high- or low-risk patients within these subgroups for inclusion in future clinical trials, or to identify patients to enroll into trials evaluating immunotherapeutic agents.
Gene-expression profiles
Improvements in microarrays have allowed investigators to identify gene-expression profiles that provide prognostic information in breast cancer [12,13]. Several investigators have evaluated prognostic gene signatures that include immune markers. Desmedt and colleagues performed a comprehensive meta-analysis integrating previously published gene-expression data with clinicopathologic data focusing on defined molecular subtypes [14]. They developed gene-expression modules related to key biologic processes, including proliferation, angiogenesis, apoptosis, tumor invasion, ER and HER2 signaling, and immune response. For the ER+/HER2− subgroup, histologic grade and the proliferation gene module were prognostic. In HER2+ tumors, tumor invasion and immune response gene modules were associated with survival, a finding confirmed by Staaf et al. [15]. In the ER−/HER2− subgroup, only the immune response module was associated with clinical outcome. Teschendorff et al. evaluated gene-expression profiles in ER− tumors and found a seven-gene module related to immune response that, when downregulated, conferred a greater risk for distant metastasis [16]. The prognostic significance of the immune response module was independent of lymph node status and the presence of TIL. In a subsequent study evaluating 430 ER− tumors, these investigators showed that in ER− tumors (both basal and HER2+), high activation of a gene-expression module reflecting a Th1 cell type response and low activation of a module reflecting a Th2 response defined a subtype with a good prognosis [17]. Th1 versus Th2 responses will be discussed further below. These data confirm that the immune response in the microenvironment of tumors that are highly proliferative is an important prognostic factor.
Intratumoral immune response as a predictive marker for response to chemotherapy
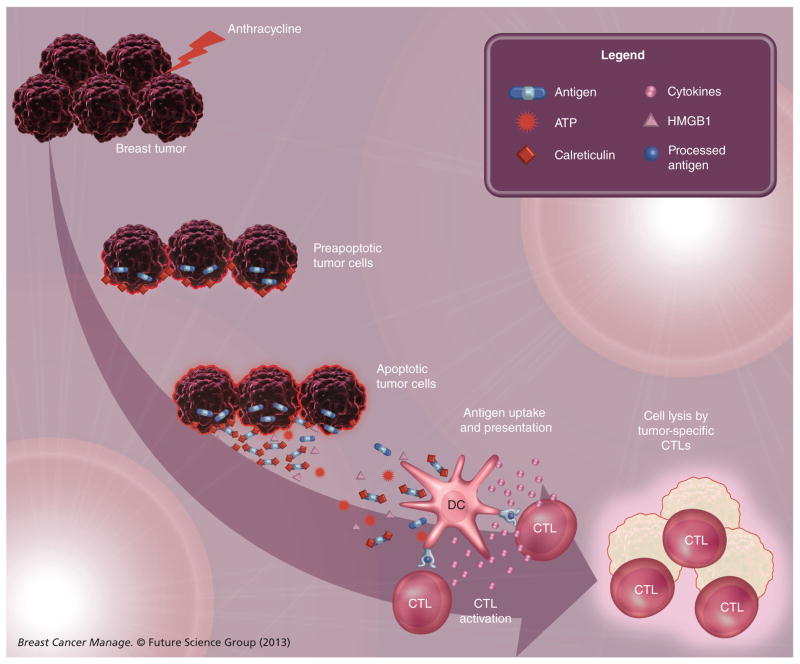
One of the most interesting findings regarding inflammation and immune response pathways in breast cancer is the ability to use these markers to predict response to therapy. In a study investigating the hypothesis that the presence of TILs in breast tumors predicts response to neoadjuvant chemotherapy, Denkert and colleagues investigated over 1000 pretreatment biopsies from patients enrolled in two neoadjuvant chemotherapy trials investigating anthracycline/taxane-based regimens [5]. The presence of TIL was determined on H&E sections and patients with a prominent lymphocytic infiltrate were identified. The presence of TILs was an independent predictor for a pathologic complete response (pCR) in the discovery cohort (n = 218) and the validation cohort (n = 840). For patients with a prominent lymphocytic infiltrate, the pCR rates were 42% in the discovery cohort and 40% in the validation cohort. By contrast, for patients without TILs, the pCR rates were 3% (discovery) and 7% (validation). In a study evaluating ER− patients enrolled in the EORTC 10994/BIG 00-01 anthracycline-based neoadjuvant chemotherapy trial, West et al. confirmed that the presence of TILs was associated with a pCR [18]. These investigators demonstrated that the predominant cell type associated with anthracycline sensitivity were CTLs. These findings are provocative in light of an emerging body of literature demonstrating that some chemotherapeutic agents, including anthracyclines, mediate an ‘immunogenic cell death’ that will activate the immune system (Figure 1; reviewed by Kroemer et al. [19]). Immunogenic cell death involves a series of events that alter the cell surface composition and result in the release of soluble factors that augment the ability of dendritic cells (DCs) to present tumor antigens to T cells. Briefly, certain chemotherapeutic agents, including anthracyclines, promote a stress response that results in translocation of calreticulin (CRT) from the lumen of the endoplasmic reticulum to the cell surface. On the cell surface, CRT functions as an ‘eat me’ signal that promotes the uptake of CRT-exposing cells by DCs and macrophages [20]. In addition, CRT promotes the production of proinflammatory cytokines by these antigen presenting cells [21]. In addition, ATP is secreted during anthracycline-induced apoptosis. ATP is a prominent ‘find me’ signal for macrophage and DC precursors, hence, in response to anthracycline-based chemotherapy, dying tumor cells attract these immune cells to the microenvironment. Finally, dying tumor cells secrete HMGB1 protein, which interacts with Toll-like receptor 4 expressed by DCs, promoting optimal antigen presentation by these DCs and activating the release of proinflammatory cytokines [22]. Taken together, these events promote an antitumor T-cell response. Anthracycline-mediated lysis of breast cancer cells may therefore function such that the tumor itself may act as a vaccine. This suggests possible strategies for immunotherapy where, after administration of an anthracycline, patients could be given anti-CTL antigen (CTLA)-4 or anti-PD1 antibodies (discussed further below) to potentiate the antitumor T-cell response that is generated.
Figure 1. Anthracycline-killed tumor cells undergo immunogenic cell death.
As a result of endoplasmic reticulum stress, cancer cells expose calreticulin on their cell surface at a preapoptotic stage. During apoptosis, the tumor sheds antigens, releases ATP and secretes HMGB1 protein. Together, these substances facilitate recruitment of DCs to the tumor microenvironment (ATP), uptake of antigens by the DCs (calreticulin) and optimize antigen presentation to CTLs (HMGB1). In turn, these tumor-specific CTLs can lyze additional tumor cells.
CTL: Cytotoxic T lymphocyte; DC: Dendritic cell.
Immune module scores assessed by gene-expression arrays have also been shown to predict response to neoadjuvant chemotherapy. In a meta-analysis of 996 patients treated with anthracycline-based neoadjuvant chemotherapy (with or without a taxane), high immune module scores were associated with increased probability of pCR in all subtypes [6]. When added to a model that included standard clinicopathologic factors, the immune module significantly improved the predictive accuracy for pCR in the HER2+ and ER−/HER2− subtypes. No patients included in this analysis had received trastuzumab as part of their neoadjuvant regimen.
Trastuzumab works through multiple mechanisms, including immune-mediated mechanisms. Through its Fc receptor, which can be recognized by natural killer (NK) cells, trastuzumab mediates antibody-dependent cellular cytotoxicity. A study from Arnould et al. confirmed this in vivo [23]. Surgical specimens from patients treated preoperatively with docetaxel-based chemotherapy with or without trastuzumab were evaluated by immunohistochemistry. Specimens from patients treated with trastuzumab had increased numbers of NK cells and increased expression of granzyme B, suggesting T-cell activation. This T-cell activation may be due in part to the potent lysis of breast cancer cells by trastuzumab, which provides an antigen source that can be taken up and presented by DCs to elicit an adaptive immune response. Based on these data, one may hypothesize that TILs, specifically T cells, could predict response to trastuzumab therapy. The predictive value of TILs was evaluated by Loi et al. in 935 patients enrolled in the FinHer adjuvant therapy trial that randomized patients to chemotherapy with or without trastuzumab [24]. In data that have only been reported in abstract form, these investigators found that TILs identified on H&E-stained sections were associated with trastuzumab efficacy. In patients with TILs, trastuzumab was associated with a significant decrease in risk of relapse (hazard ratio: 0.16; 95% CI: 0.031–0.81; p = 0.013). By contrast, in patients without TIL, trastuzumab was not associated with a decreased relapse risk (hazard ratio: 1.0; 95% CI: 0.55–1.75; p = 0.99) [24].
Lymphocyte composition in the breast tumor microenvironment
Immune infiltrates in tumors are heterogeneous and the immune outcome may vary depending on the predominant cell type. In order to make the observations regarding the prognostic and predictive significance of inflammatory infiltrates more clinically relevant, it is important to further characterize the specific immune cell types comprising the infiltrate. A study from Ruffell et al. used polychromatic flow cytometry in combination with confocal immunofluorescence and immunohistochemical analysis of breast tumor and adjacent normal specimens to further evaluate the inflammatory infiltrate composition [25]. They found that breast tumor tissue contained infiltrates dominated by T lymphocytes (CD3+), including both CD8+ T cells and CD4+ T cells, with minor populations of B lymphocytes. These cell types are important components of the adaptive immune system (Table 1). Broadly speaking, the immune system can be divided into adaptive or innate immune responses. Adaptive immune responses are slow to develop but they are antigen-specific, long-lasting and have the feature of immunologic memory, enabling a more efficient response to previously encountered antigens. Adaptive immunity can be further divided into cellular (mediated by T cells) or humoral (mediated by B cells) immune responses. The role of cellular versus humoral immunity in tumor development, progression and therapy has been debated; however, as will be discussed further below, there is evidence that both aspects of the adaptive immune response are relevant [26].
Table 1.
Immune cells present in the breast tumor microenvironment.
| Cell type | Immune arm | Function† | Phenotypic marker‡ | Expected outcomes§ |
|---|---|---|---|---|
| CTLs | Adaptive | Direct tumor lysis | CD3, CD8 | Favorable, directly eliminate the tumor cells |
|
| ||||
| Helper T cells | ||||
| – Th1 | Adaptive | Promote CTL response | CD3, CD4 | Favorable, promote a CTL immune response |
| – Th2 | Adaptive | Promote B-cell response | CD3, CD4 | Favorable, promote antibody production Detrimental, inhibit CTL response |
| – Th17 | Adaptive | Recruit other inflammatory cells via production of IL-17 | CD3, CD4, intracellular IL-17 | Further study required, but initial study suggests favorable |
|
| ||||
| T-regs | Adaptive | Inhibit immune responses | CD3, CD4, CD25, FOXP3 | Detrimental, inhibit immune responses |
|
| ||||
| B cells | Adaptive | Produce antibodies | CD19 | Favorable, produce antitumor antibodies |
|
| ||||
| Natural killer cells | Innate | Direct tumor lysis | CD16, CD56 | Favorable, directly eliminate the tumor cells |
|
| ||||
| Dendritic cells | Innate; link between adaptive and innate | Present antigens to T and B cells and produce cytokines | CD11, CD141, CD303 | Favorable, generate immune responses |
|
| ||||
| Macrophages | Innate | Antigen presentation and phagocytosis | CD14, CD16, CD68, MAC-1/MAC-3 | Favorable, direct cytotoxicity and promoting CTL responses Detrimental, inhibit CTL response and promote angiogenesis |
|
| ||||
| Neutrophils | Innate | Phagocytosis and promote inflammation | CD16, CD66b | No studies have looked at the prognostic significance of neutrophils. Neutrophil elastase is detrimental, possibly due to degradation of the extracellular matrix |
The immune cells perform numerous functions. The major function is listed.
Cells are identified based on the presence and absence of numerous CD surface markers. Listed are the principal CD markers associated with each cell type.
Although we list the main outcome associated with each cell type, the true outcomes are dependent on the summation of different functions of the immune cells infiltrating the tumor.
CTL: Cytotoxic T lymphocyte.
T cells
T cells, which mediate cellular immune responses, are stimulated when their T-cell receptor (TCR) recognizes foreign antigens, including tumor antigens. There are several types of T cells, including CD8+ CTLs and CD4+ Th cells.
CD8+ CTLs
CTLs confer cytolytic activity by releasing perforin and other cytotoxins that induce apoptosis. Tumor-associated CTLs have been demonstrated to be independently associated with improved survival in a variety of epithelial-derived tumors [27–30]. A study by Mahmoud et al. evaluated the prognostic significance of CD8+ T cells in over 1300 breast cancer patients diagnosed between 1987 and 1998 [31]. All patients underwent mastectomy or lumpectomy with radiation. Patients treated before 1988 did not receive systemic therapy. Patients treated from 1988 forward with a low Nottingham Prognostic Index were not treated; patients with a Nottingham Prognostic Index >3.4 received tamoxifen if they were ER+ or chemotherapy (cyclophosphamide, methotrexate and fluorouracil) if they were ER−. Tissue microarrays were obtained from these patients’ tumors and stained with an anti-CD8 antibody. The number of CD8+ T cells correlated with a higher tumor grade and inversely correlated with hormone receptor expression. In a multivariate model that included tumor size, stage, grade, vascular invasion, HER2 and ER status, age and adjuvant treatment, the number of CD8+ T cells was independently associated with improved disease-specific survival (DSS; p = 0.001). When investigators analyzed specific breast cancer subtypes, the number of infiltrating CD8+ T cells was associated with improved DSS in ER− tumors but not in ER+ tumors, which is consistent with the gene-expression data from Teschendorff et al. [16]. The prognostic significance of CD8+ T cells was also demonstrated in a recent study of 179 treatment-naive breast cancer patients, where low CD8+ T-cell density correlated with reduced OS [32].
Despite the presence of CD8+ T cells infiltrating breast cancers, these tumors do not undergo spontaneous regression. This is probably due to multiple regulatory mechanisms inhibiting T-cell responses within the tumor microenvironment (reviewed in Mittendorf and Sharma [33]). Well-described examples include tumor antigen loss or downregulation of HLA class I molecules, rendering the tumors invisible to the CD8+ T cells [34]. Another example is upregulation of critical components of immune checkpoints. Immune checkpoints refer to inhibitory pathways that are critical for modulating immune responses [35]. Following TCR engagement with antigens, CD8+ T cells upregulate CTLA-4, an inhibitory molecule that counteracts the stimulatory receptor CD28. In the periphery, T cells upregulate another inhibitory receptor, PD-1. One ligand for PD-1 is PD-L1, which can be upregulated by tumor cells. Activation of the PD-1/PD-L1 pathway suppresses T-cell-mediated antitumor immune responses by decreasing T-cell proliferation, survival and cytokine production [35]. Ipilimumab, a monoclonal antibody targeting CTLA-4, has been shown to extend OS in patients with unresectable or metastatic melanoma and has received US FDA approval for this indication [36]. Antibodies targeting PD-1 and PD-L1 have shown benefit in melanoma, renal cell carcinoma and non-small-cell lung cancer in early-stage trials [37,38]. These agents have not been studied extensively in breast cancer. There is an ongoing trial investigating a single preoperative dose of ipilimumab alone or in combination with cryoablation for patients with operable, early-stage breast cancer [101]. The rationale for this clinical trial is that cryoablation will induce tumor cell lysis, with subsequent release of tumor antigens stimulating a CD8+ T-cell response. Ipilimumab will ‘take the brakes off’ the stimulated T cells, allowing for expansion of the immune response.
Additional mechanisms that inhibit effective CD8+ T-cell function include factors extrinsic to the T cells themselves. Soluble suppressive factors elaborated by the tumor or stroma, such as IL-10, TGF-β or indoleamine 2,3-dioxygenase, have been shown to abrogate antitumor immunity [39–41]. In addition, there are inhibitory cells present in the tumor microenvironment, including myeloid-derived suppressor cells and Tregs, which can also have negative effects on the immune response.
Despite the fact that CD8+ T-cell responses do not lead to tumor regression, the data showing that an increased number of CD8+ T cells is prognostically significant suggest that tumors stimulate an adaptive immune response, including an effector–memory T-cell response, which could play a role in preventing tumor recurrence. Based on this observation in colorectal cancer, an immune score that quantifies the density and location of CD8+ T cells, including memory T cells, within the tumors has been proposed [42–44]. This score is able to stratify patients with respect to disease-free survival, DSS and OS, with those showing the most significant T-cell infiltrate having the best clinical outcomes.
CD4+ Th cells
There are several subtypes of Th cells that have been described, including Th1, Th2, Th17 and Tregs. Th1 cells produce cytokines, such as IL-2 and IFN-γ, which play important roles in activating and regulating the development and persistence of CTL responses [45]. Th2 cells secrete cytokines that play a role in B-cell maturation, clonal expansion and class switching, and therefore play a role in humoral immune responses. Among the cytokines secreted by Th2 cells are IL-4 and IL-10, which have been shown to downregulate the proinflammatory state and inhibit the synthesis of Th1 cytokines, suggesting that Th2 responses could have both anti- and pro-tumor effects [46]. There are no cell surface markers that differentiate Th1 CD4+ T cells from Th2 CD4+ T cells. However, investigators have used gene-expression profiles to discriminate Th1 and Th2 responses.
As was discussed above, using a gene-expression data set from over 400 ER− tumors, Teschendorff et al. identified pathways involved in Th1-mediated immune responses (IL-2, IL-12 and IFN-γ) and Th2-mediated immune responses (IL-13 and TGF-β) [17]. The Th1 and Th2 pathways were inversely correlated and Th1 activation was associated with a lower risk for distant metastasis, while Th2 activation was associated with a higher risk. Combining these pathways allowed for better stratification than the analysis of either pathway alone with respect to distant metastasis-free survival.
Th17 cells, which secrete IL-17, play a key role in autoimmune diseases [47]. Their role in cancer is less well studied, although a recent study from Yang et al. identified Th17 cells in the majority of 50 breast tumor specimens analyzed [48]. The expression of Th17 cells was significantly higher in tumor tissue versus normal breast tissue and there was a negative association between the presence of Th17 cells and the stage and number of positive lymph nodes. These data suggest that Th17 cells may be involved in antitumor responses in breast cancer.
Tregs modulate the immune response by maintaining a balance between an effective cell-mediated attack and the suppression of autoimmunity. Tumors recruit Tregs to the tumor microenvironment where they inhibit antitumor immune responses through their ability to suppress T-cell proliferation and cytokine production [49]. There is evidence that Tregs make up a component of TIL populations in multiple tumor types and that the presence of Tregs correlates inversely with outcome [50–53].
Initial studies assessing the presence of Tregs identified them as a population of CD4+ T cells that expressed the phenotypic marker CD25, a subunit of the receptor for the T-cell-stimulating cytokine IL-2. FOXP3 is a transcription factor that regulates Treg development and adds sensitivity as an identification marker for Tregs [54,55]. In an initial study in breast cancer looking at Tregs defined as FOXP3+ T cells, Bates et al. stained tissue microarrays consisting of normal breast tissue, ductal carcinoma in situ and invasive breast cancer [50]. FOXP3 staining was assessable in 217 patients with invasive disease and complete clinicopathologic data. There was a positive correlation between the presence of FOXP3+ Tregs and positive lymph node status and higher tumor grade. FOXP3+ Tregs were associated with significantly shorter RFS and OS. In a multivariate analysis, the presence of FOXP3+ Tregs remained significantly associated with shorter RFS but not OS. These investigators also showed a negative correlation between FOXP3+ Tregs and ER status. Therefore, to determine whether the correlation between Tregs and survival was a reflection of ER status, they looked only at ER+ tumors and again found that Tregs were associated with significantly shorter RFS and OS. In ER− tumors, Tregs had no significant impact on survival. Another interesting finding in this study was that the median number of FOXP3+ Tregs differed significantly between normal breast tissue (0 Tregs), ductal carcinoma in situ (4) and invasive disease (15), suggesting that Treg accumulation may be a marker of breast cancer progression.
A second study looking at the prognostic significance of FOXP3+ T cells in a series of 1445 cases of invasive breast cancer found that, in a univariate analysis, FOXP3+ T cells were associated with a worse DSS, while in a multivariate analysis, after adjusting for grade, tumor size, lymph node stage and vascular invasion, the number of FOXP3+ T cells was not an independent prognostic factor. In contrast to the study by Bates et al. [50], when these investigators evaluated only ER+ patients, the FOXP3+ T-cell number was not an independent predictor of worse DSS in a multivariate analysis. There were differences between the two studies, including the survival end point evaluated and the methodology used to score the presence of Tregs. Taken together, however, these data confirm the presence of Tregs in the breast tumor microenvironment and suggest that additional studies evaluating the role of Tregs are necessary.
B cells
Humoral immunity is mediated by B cells that originate in the bone marrow and then migrate to secondary lymphoid organs, such as lymph nodes, where they interact with antigens, differentiate into plasma cells and produce antigen-specific antibodies (immunoglobulins). Antibodies are an important tool in cancer immunotherapy, as evidenced by the positive impact of trastuzumab on the treatment of HER2-overexpressing breast cancer [56].
Although most of the focus in TIL populations has been on T cells, it is important to note that the TIL population in breast tumors is heterogeneous and includes TIL-B cells [25,57,58]. These TIL-B cells are predominantly IgG+ in contrast to the low level of predominantly IgA+ B cells in healthy breast tissue [59,60]. The presence of IgG+ B cells in a tumor indicates that these B cells have undergone class switching, which is critical in the development and maturation of B cells, and suggests a potential role of B cells in mediating an antitumor humoral immune response. In a study evaluating the histology, IgG repertoire and antibody specificity of TIL-B cells from three invasive ductal carcinoma tumors, Coronella et al. found a preponderance of clonal B cells and provided evidence of antigen-driven oligoclonal B-cell expansion [61]. This is an important observation suggesting that infiltrating B cells represent a tumor antigen-specific immune response as opposed to nonspecific recruitment.
The importance of humoral immune responses in breast cancer was further supported by a study from Schmidt et al. analyzing gene-expression patterns of 200 tumors from patients with node-negative breast cancer who underwent surgery but did not receive adjuvant systemic therapy [62]. They found that the B-cell metagene, a cluster of 60 genes containing transcripts for heavy and light chains of immunoglobulin, had a significant prognostic impact on metastasis-free survival in patients with highly proliferative, ER− cancer. This was confirmed in a study showing that a B-cell/plasma cell metagene was prognostic in highly proliferative ER+ tumors and ER− tumors, and had no prognostic value in low-proliferation ER+ tumors [63].
In a recently published study, Schmidt et al. reported IGKC to be a single marker that is as predictive and prognostic as the entire B cell metagene [64]. IGKC gene expression was associated with metastasis-free survival in a cohort of 965 patients with node-negative breast cancer and predicted response to anthracycline-based neoadjuvant chemotherapy in 845 patients. In addition, IGKC protein expression identified in paraffin-embedded tissues from 330 patients correlated with IGKC RNA levels and was significantly associated with metastasis-free survival. This work is significant because increased IGKC RNA or protein expression is related to increased numbers of immunoglobulin secreting plasma cells. This supports a mechanism whereby B-cell differentiation occurs within the tumor tissues [65]. In addition, the correlation between IGKC expression and clinical outcome implies that immunoglobulin produced by plasma cells in the breast tumor contributes to improved prognosis, providing evidence that these immunoglobulins are antigen-specific. Identification of the antigens could therefore provide a target for antibody-based immunotherapy. Finally, this work suggests that IGKC gene expression detected by PCR or protein expression detected by immunohistochemistry using a commercially available antibody could serve as a robust immunologic biomarker.
In addition to their role in promoting antibody responses discussed above, B cells, which can function as antigen-presenting cells, may also have a role in inducing CD8+ and CD4+ T-cell responses that can help control tumor growth and metastasis [66]. Therefore, despite the fact that the preponderance of studies evaluating immune infiltrates in solid tumors, including breast cancer, have focused on T cells, B cells clearly have a role as a component of the immune response in the tumor microenvironment.
Innate immune responses in breast cancer
The innate immune system provides an immediate response to pathogens, but does not confer long-lasting protective immunity. This response is nonspecific, and it has been less well studied than adaptive immunity with respect to its role in cancer. Cells of the innate immune system include macrophages, neutrophils, mast cells, NK cells, basophils, eosinophils and DCs. Due to the availability of cell-type specific antibodies, it is now understood that innate immune cells make up a component of the inflammatory infiltrate in the tumor microenvironment. This has led to increased interest in the role these cells play in tumor development and progression.
Macrophages
There is increasing recognition that other innate immune cells play important roles in linking innate and adaptive immune responses. Tumor-associated macrophages (TAMs) have been studied and found to have paradoxical roles in cancer. Polarization of TAMs has been well characterized (reviewed by Allavena et al. [67]). Briefly, classically activated macrophages (M1) induced by IFN-γ are antitumoral, characterized by high antigen-presenting capacity, IL-12 production, activation of Th1 responses and direct cytotoxic activity. Alternatively activated macrophages (M2) are induced by IL-4, IL-10, IL-13, immune complexes and glucocorticoids. They are poor antigen-presenting cells, suppress Th1 responses and promote angiogenesis and tissue remodeling. M2 macrophages therefore promote tumor growth. Macrophages have great plasticity; therefore, differentiating TAMs as either M1 or M2 is probably an oversimplification, as the function of TAMs is probably dictated by ongoing changes within the tumor microenvironment.
Several studies have looked at the prognostic significance of TAMs in breast cancer. One early study evaluated approximately 100 breast tumors by immunohistochemical staining with an anti-CD68 antibody to identify macrophages. There was a correlation between angiogenesis and TAMs, with highly vascular tumors having higher numbers of TAMs [68]. TAM density was also significantly associated with worse disease-free survival and OS. In a study from DeNardo et al. evaluating 179 treatment-naive breast cancer patients, the TAM density did not correlate with survival [32]. These investigators noted an inverse association between TAMs and CD8+ T cells; therefore, they hypothesized that an immune profile characterized by CD68low/CD4low/CD8high would improve prognostic stratification. They used the initial cohort (n = 179) to define the signature and then applied it to a validation cohort (n = 498). Kaplan–Meier analysis in the two cohorts showed that the CD68low/CD4low/CD8high signature identified patients with significantly reduced OS and RFS. To determine whether the presence of TAMs and CD8+ T cells could predict response to therapy, these investigators analyzed CD68 and CD8 mRNA in a cohort of 311 patients that had undergone fine-needle aspiration biopsy prior to neoadjuvant chemotherapy. Using median expression as a threshold, they found that the CD68high/CD8low group had a significantly lower pCR rate (7%), while the CD68low/CD8high group had a significantly higher pCR rate (27%) [32]. These are additional data suggesting that the immune microenvironment may provide biomarkers predicting response to therapy.
NK cells
NK cells are innate effector lymphocytes that can exert direct cellular cytotoxicity without previous sensitization [69]. Unlike T cells, which recognize antigens only in the context of their TCR/HLA molecule interactions, NK cells are not HLA-restricted. Rather, they are regulated by a different set of receptors, including killer-cell immunoglobulin-like receptors, Ly49 and NKG2. In fact, the interaction of NK cells with HLA molecules on the target cells can inhibit NK cell function. There is no direct evidence of an association between the presence of NK cells in the tumor microenvironment and clinical outcomes in breast cancer; however, there is indirect evidence that these cells may play a role in immune regulation. As was discussed above, tumor cells may lose expression of classical HLA class I molecules, allowing them to escape recognition by T cells. However, these cells may still be vulnerable to NK cell elimination. Expression of HLA-E and HLA-G, non-classical HLA class I molecules, plays a role in NK cell immune surveillance, as they can bind with inhibitory receptors on NK cells, thereby inhibiting proliferation and cytotoxic functions [70,71]. In a study evaluating 677 breast cancer patients, de Kruijf et al. stained tissue microarrays for classical HLA class I molecules, as well as HLA-E and HLA-G. In patients that had loss of classical HLA class I expression, expression of HLA-E and HLA-G resulted in worse RFS, suggesting that inhibition of NK cells may contribute to immune escape [72].
NK cells are also important for mediating response to trastuzumab therapy. As was discussed above, one mechanism of trastuzumab’s action is antibody-dependent cell-mediated cytotoxicity, a process that is dependent on immune effectors, primarily NK cells, binding via their Fc receptor to the IgG1 Fc portion of trastuzumab [73]. This in turn results in NK cell activation, release of cytotoxic granules and lysis of the trastuzumab-bound cancer cells [74]. Studies have shown that patients with Fc receptor polymorphisms that result in higher NK affinity for IgG1 have a better response to trastuzumab [75,76].
Neutrophils
Because there are many cells in the tumor microenvironment that secrete neutrophil-chemotactic substances, there is growing interest in studying tumor-associated neutrophils (TANs). Studies using CD66b antibodies to identify TANs have shown that the presence of TANs correlates with an adverse prognosis in multiple solid tumor types, including colorectal cancer, renal cell carcinoma and non-small-cell lung cancer [77–79]. There are no studies evaluating the presence of TANs and prognosis in breast cancer. However, there are studies showing that high levels of neutrophil elastase, a serine protease released from the primary granules of activated neutrophils, is associated with poor prognosis. Foekens et al. evaluated neutrophil elastase levels in breast tumors using ELISA and found that high levels of elastase correlated with poor clinical outcomes, including RFS and OS [80].
These data suggest that cells of the innate immune system, which make up part of the inflammatory infiltrate in the tumor microenvironment, play a role in the development and progression of tumors. Additional studies are warranted in order to determine not just their prognostic significance, but also to identify whether they may predict response to therapy or serve as therapeutic targets themselves.
Conclusion & future perspective
In this review, we have provided data showing that the ‘immune signature’ of the tumor microenvironment is complex and includes various immune cells that interact both with the tumor and with each other. Therefore, although the beneficial effects of the immune response to tumors has largely been attributed to T cells present in the inflammatory infiltrate, it is now clear that there are contributions from cells of both the adaptive and innate arms of the immune system. There is a growing body of literature that suggests that many of the cells present in the tumor microenvironment have dual roles. TAMs are one example of this, in that the M1 phenotype primarily exhibits antitumor effects, while the M2 phenotype promotes tumor growth. Despite our increased knowledge of tumor immunology, the current view of tumor immunity is in many ways overly simplified, since the immune cells tend to change their phenotype and functions at different stages of tumor development, as determined by cytokines and other factors present in the tumor microenvironment.
In the next 5 years, we anticipate that there will be additional investigations regarding immune cell types that have been less well studied, particularly cells of the innate immune system. We also anticipate additional investigations into the cytokines and chemokines elaborated by tumor cells and other elements of the microenvironment. These signals direct the phenotype and function of the immune cells recruited to the microenvironment; therefore, they probably dictate which cell types are most relevant for clinical outcomes. It is possible that strategies could be employed whereby the cytokine milieu present in the tumor microenvironment could be altered pharmacologically, thereby changing the composition of the inflammatory infiltrate.
Finally, as our understanding of the immune aspects of the breast tumor microenvironment increases, we anticipate that future clinical trials evaluating novel therapeutics will stratify patients based on the composition of their inflammatory infiltrate. Consistent with this, we anticipate that as novel breast cancer therapeutics are investigated in clinical trials, assessing the effects of those agents on immune aspects of the tumor microenvironment will become part of routine biopsies performed to assess predictive biomarkers. We envision trials evaluating immunotherapeutic agents in combination with standard therapeutic modalities. Studying immunotherapeutic agents in the neoadjuvant setting would provide investigators with the opportunity to assess immune responses in both the blood and tumor, thus helping to guide rational combinations of standard-of-care treatment modalities and novel immunotherapeutics.
Practice Points.
-
Prognostic impact of intratumoral immune response
Virtually all solid tumors contain immune cells at various densities, ranging from gross inflammation to subtle infiltration, that require specific antibodies for identification.
Multiple studies have shown that the immune infiltrate, whether detected pathologically or using gene-expression profiles, is prognostic in breast cancer.
-
Intratumoral immune response as a predictive marker for response to chemotherapy
The presence of tumor-infiltrating lymphocytes has been shown to predict response to neoadjuvant chemotherapy, with patients having significant tumor-infiltrating lymphocytes being more likely to achieve a pathologic complete response.
The presence of an immune infiltrate also predicts response to trastuzumab therapy, which is consistent with one of trastuzumab’s mechanisms of action being antibody-dependent cellular cytotoxicity, an immune cell-mediated mechanism.
-
Lymphocyte composition in the breast tumor microenvironment
The cell composition of immune infiltrates in the tumor microenvironment is heterogeneous, dictated by cytokines and chemokines elaborated by tumor cells and other elements of the microenvironment. It is likely that the phenotype and function of these cells changes as the tumor develops.
The prognostic significance of the immune infiltrate is likely due to the interactions between the various cell types and the tumor cells, as well as the relative proportion of immune cell types present within the tumor microenvironment.
-
Innate immune responses in breast cancer
While it has long been acknowledged that adaptive immune responses to tumors exist and impact prognosis, there is a growing appreciation that cells of the innate immune system contribute to the inflammatory infiltrate, modulate the adaptive immune responses and affect clinical outcomes.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
EA Mittendorf is supported in part by the grant NCI R00CA133244. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18(1):11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. Manuscript updating the original 2000 description of the ‘Hallmarks of cancer’. This update identified ‘evading immune destruction’ as a hallmark and identified inflammation as an enabling characteristic. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen VN, Vaske CJ, Ursini-Siegel J, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA. 2012;109(8):2802–2807. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪▪.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. Important paper demonstrating that the presence of tumor-infiltrating lymphocytes were predictive of response to neoadjuvant chemotherapy, with patients with tumors that had a higher density of tumor-infiltrating lymphocytes experiencing higher rates of pathologic complete response. [DOI] [PubMed] [Google Scholar]
- 6.Ignatiadis M, Singhal SK, Desmedt C, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol. 2012;30(16):1996–2004. doi: 10.1200/JCO.2011.39.5624. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed ZM, Going JJ, Edwards J, Mcmillan DC. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev. 2012;38(8):943–955. doi: 10.1016/j.ctrv.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Rakha EA, Aleskandarany M, El-Sayed ME, et al. The prognostic significance of inflammation and medullary histological type in invasive carcinoma of the breast. Eur J Cancer. 2009;45(10):1780–1787. doi: 10.1016/j.ejca.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexe G, Dalgin GS, Scanfeld D, et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67(22):10669–10676. doi: 10.1158/0008-5472.CAN-07-0539. [DOI] [PubMed] [Google Scholar]
- 12.van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 13.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14(16):5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. Describes the prognostic significance of immune-related gene-expression profiles. [DOI] [PubMed] [Google Scholar]
- 15.Staaf J, Ringner M, Vallon-Christersson J, et al. Identification of subtypes in human epidermal growth factor receptor 2-positive breast cancer reveals a gene signature prognostic of outcome. J Clin Oncol. 2010;28(11):1813–1820. doi: 10.1200/JCO.2009.22.8775. [DOI] [PubMed] [Google Scholar]
- 16.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teschendorff AE, Gomez S, Arenas A, et al. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West NR, Milne K, Truong PT, MacPherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13(6):R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2012;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 20.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through transactivation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. First manuscript describing chemotherapeutic agents causing immunogenic cell death. [DOI] [PubMed] [Google Scholar]
- 23.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94(2):259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loi S, Michiellis S, Lambrechts D, et al. Tumor PIK3CA mutations, lymphocyte infiltration, and recurrence-free survival (RFS) in early breast cancer (BC): results from the FinHER trial. J Clin Oncol. 2012;30(15S):8S. [Google Scholar]
- 25▪.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2796–2801. doi: 10.1073/pnas.1104303108. Recent manuscript detailing the composition of the inflammatory infiltrate in breast tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteside TL, Ferrone S. For breast cancer prognosis, immunoglobulin κ chain surfaces to the top. Clin Cancer Res. 2012;18(9):2417–2419. doi: 10.1158/1078-0432.CCR-12-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong RA, Leffers N, Boezen HM, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund LT, Bremnes RM. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116(18):4318–4325. doi: 10.1002/cncr.25333. [DOI] [PubMed] [Google Scholar]
- 30.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. Describes the prognostic significance of CD8+ T cells in a large population of breast cancer patients. [DOI] [PubMed] [Google Scholar]
- 32.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittendorf EA, Sharma P. Mechanisms of T-cell inhibition: implications for cancer immunotherapy. Expert Rev Vaccin. 2010;9(1):89–105. doi: 10.1586/erv.09.144. [DOI] [PubMed] [Google Scholar]
- 34.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–A45. doi: 10.1016/s0264-410x(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 35.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat Med. 2001;7(10):1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura K, Bahar R, Natsume W, Sakiyama S, Tagawa M. Secretion of interleukin-10 from murine colon carcinoma cells suppresses systemic antitumor immunity and impairs protective immunity induced against the tumors. Cancer Gene Ther. 2002;9(1):109–115. doi: 10.1038/sj.cgt.7700418. [DOI] [PubMed] [Google Scholar]
- 41.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 43.Pages F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 44.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 45.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Clin Med. 1998;188(12):2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Clin Med. 1996;184(1):19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Qi Y, Hu J, Tang L, Zhao S, Shan B. Expression of Th17 cells in breast cancer tissue and its association with clinical parameters. Cell Biochem Biophys. 2012;62(1):153–159. doi: 10.1007/s12013-011-9276-3. [DOI] [PubMed] [Google Scholar]
- 49.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Clin Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪▪.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi: 10.1200/JCO.2006.05.9584. Report of a large series demonstrating that the presence of Tregs, identified as FOXP3+ cells, has prognostic significance in breast cancer. [DOI] [PubMed] [Google Scholar]
- 51.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 53.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4+CD25+ T cells: multiple pathways on the road. J Cell Physiol. 2007;211(3):590–597. doi: 10.1002/jcp.21001. [DOI] [PubMed] [Google Scholar]
- 55.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35(6):1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 56.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–1466. [PubMed] [Google Scholar]
- 58.Marsigliante S, Biscozzo L, Marra A, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139(1):33–41. doi: 10.1016/s0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 59.Ito T, Saga S, Nagayoshi S, et al. Class distribution of immunoglobulin-containing plasma cells in the stroma of medullary carcinoma of breast. Breast Cancer Res Treat. 1986;7(2):97–103. doi: 10.1007/BF01806794. [DOI] [PubMed] [Google Scholar]
- 60.Klein U, Kuppers R, Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V κ genes with no or little somatic mutation throughout life. Eur J Immunol. 1993;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- 61.Coronella JA, Spier C, Welch M, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169(4):1829–1836. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt M, Bohm D, von Torne C, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 63.Bianchini G, Qi Y, Alvarez RH, et al. Molecular anatomy of breast cancer stroma and its prognostic value in estrogen receptor-positive and -negative cancers. J Clin Oncol. 2010;28(28):4316–4323. doi: 10.1200/JCO.2009.27.2419. [DOI] [PubMed] [Google Scholar]
- 64▪▪.Schmidt M, Hellwig B, Hammad S, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin κ C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18(9):2695–2703. doi: 10.1158/1078-0432.CCR-11-2210. Recent manuscript showing that the presence of a single marker was as significant as an entire metagene profile in predicting clinical outcome. [DOI] [PubMed] [Google Scholar]
- 65.Whiteside TL, Ferrone S. IGKC and prognosis in breast cancer – response to Schmidt. Clin Cancer Res. 2013;19(1):305. doi: 10.1158/1078-0432.CCR-12-3200. [DOI] [PubMed] [Google Scholar]
- 66.Dilillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin–Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. Review of tumor-associated macrophages detailing the factors effecting their polarization to antitumor versus tumor-promoting phenotypes. [DOI] [PubMed] [Google Scholar]
- 68.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 69.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors I Distribution of reactivity and specificity. Int J Cancer. 1975;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 70.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 71.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189(7):1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Kruijf EM, Sajet A, van Nes JG, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185(12):7452–7459. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 73.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 74.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamura K, Shimizu C, Hojo T, et al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22(6):1302–1307. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 76.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 77.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 78.Rao HL, Chen JW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE. 2012;7(1):e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129(7):1651–1660. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 80.Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG, Jochum M. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63(2):337–341. [PubMed] [Google Scholar]
Website
- 101. [Accessed 29 December 2012];ClinicalTrials.gov: Pre-Operative, Single-Dose Ipilimumab and/or Cryoablation in Early Stage/Resectable Breast Cancer. http://clinicaltrials.gov/show/NCT01502592.