Abstract
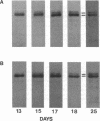
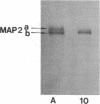
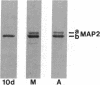
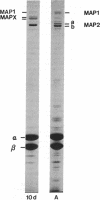
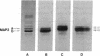
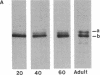
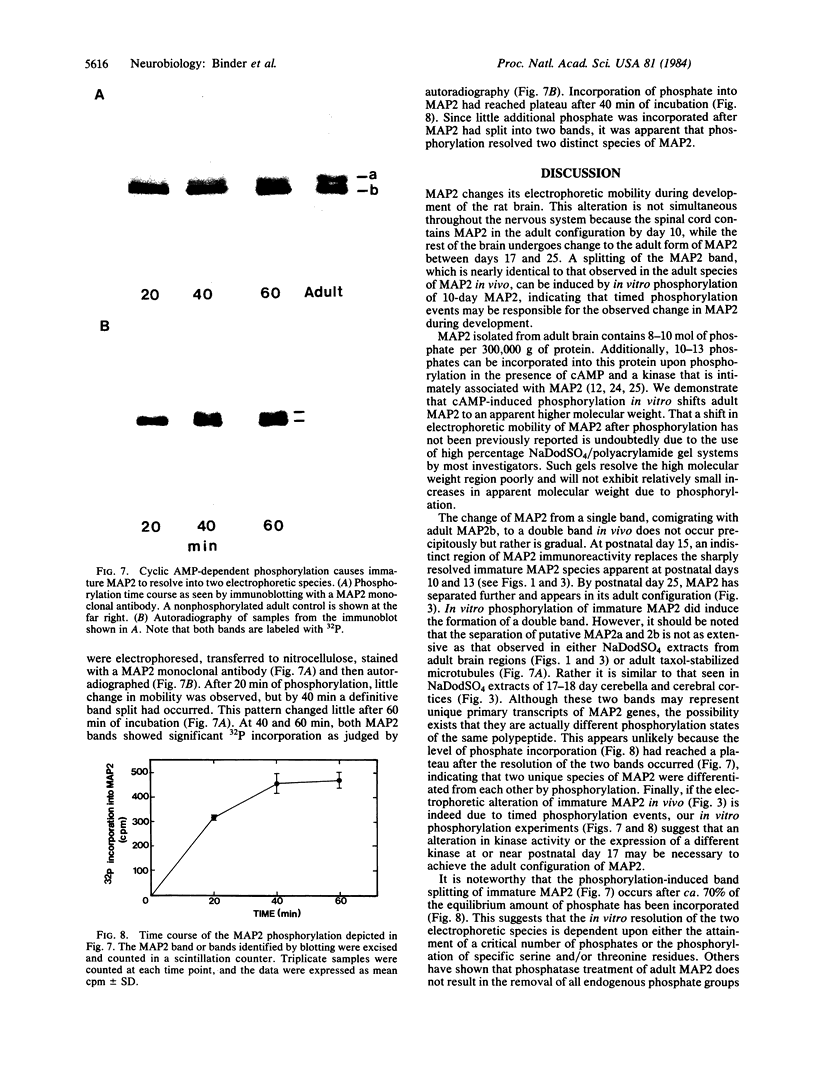
The electrophoretic pattern of the large microtubule-associated protein, MAP2, changes during rat brain development. Immunoblots of NaDodSO4 extracts obtained from the cerebral cortex, cerebellum, and thalamus at 10-15 days after birth reveal only a single electrophoretic species when probed with any of three MAP2 monoclonal antibodies. By contrast, adult MAP2 contains two immunoreactive species, MAP2a and MAP2b. The single band of MAP2 from immature brain electrophoretically comigrates with adult MAP2b. Between postnatal days 17 and 18, immature MAP2 simultaneously resolves into two species in both the cerebellum and cerebral cortex. Immunoblots of NaDodSO4 extracts from spinal cord demonstrate the adult complement of MAP2 by day 10, indicating that MAP2 does not change coordinately throughout the entire central nervous system. In vitro cAMP-dependent phosphorylation of immature MAP2 causes a band split reminiscent of that seen during brain development in vivo. The possibility that the developmentally regulated changes observed in MAP2 during brain maturation are due to timed phosphorylation events is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz S. A., Katagiri J., Binder H. K., Williams R. C., Jr Separation and characterization of microtubule proteins from calf brain. Biochemistry. 1977 Dec 13;16(25):5610–5617. doi: 10.1021/bi00644a035. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Rosenbaum J. L. The in vitro assembly of flagellar outer doublet tubulin. J Cell Biol. 1978 Nov;79(2 Pt 1):500–515. doi: 10.1083/jcb.79.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Vallee R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983 Jun;96(6):1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns R. G., Islam K. Characterisation of the chick brain high molecular weight multiply phosphorylated microtubule associated protein. Eur J Biochem. 1982 Feb;122(1):25–29. doi: 10.1111/j.1432-1033.1982.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Caceres A., Binder L. I., Payne M. R., Bender P., Rebhun L., Steward O. Differential subcellular localization of tubulin and the microtubule-associated protein MAP2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1984 Feb;4(2):394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoulet P., Edde B., Jeantet C., Gros F. Evolution of tubulin heterogeneity during mouse brain development. Biochimie. 1982 Mar;64(3):165–172. doi: 10.1016/s0300-9084(82)80466-5. [DOI] [PubMed] [Google Scholar]
- Fernandez H. L., Burton P. R., Samson F. E. Axoplasmic transport in the crayfish nerve cord. The role of fibrillar constituents of neurons. J Cell Biol. 1971 Oct;51(1):176–192. doi: 10.1083/jcb.51.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francon J., Lennon A. M., Fellous A., Mareck A., Pierre M., Nunez J. Heterogeneity of microtubule-associated proteins and brain development. Eur J Biochem. 1982 Dec 15;129(2):465–471. doi: 10.1111/j.1432-1033.1982.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Scherson T., Giveon D., Behar L., Littauer U. Z. Modulation of mRNA for microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4892–4896. doi: 10.1073/pnas.79.16.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Islam K., Burns R. Multiple phosphorylation sites of microtubule-associated protein (MAP2) observed at high ATP concentrations. FEBS Lett. 1981 Jan 26;123(2):181–185. doi: 10.1016/0014-5793(81)80282-7. [DOI] [PubMed] [Google Scholar]
- Izant J. G., McIntosh J. R. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Johnson K. A., Borisy G. G. Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol. 1977 Nov 25;117(1):33–52. doi: 10.1016/0022-2836(77)90021-3. [DOI] [PubMed] [Google Scholar]
- Payne M. R. Monoclonal antibodies to the contractile proteins. Cell Muscle Motil. 1983;4:137–177. [PubMed] [Google Scholar]
- Runge M. S., Schlaepfer W. W., Williams R. C., Jr Isolation and characterization of neurofilaments from mammalian brain. Biochemistry. 1981 Jan 6;20(1):170–175. doi: 10.1021/bi00504a028. [DOI] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Sloboda R. D., Rosenbaum J. L. Decoration and stabilization of intact, smooth-walled microtubules with microtubule-associated proteins. Biochemistry. 1979 Jan 9;18(1):48–55. doi: 10.1021/bi00568a008. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cameron B. F. Morphological evidence for the participation of microtubules in axonal transport. Ann N Y Acad Sci. 1975 Jun 30;253:472–506. doi: 10.1111/j.1749-6632.1975.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Extensive cAMP-dependent and cAMP-independent phosphorylation of microtubule-associated protein 2. J Biol Chem. 1983 Jun 25;258(12):7883–7886. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A., Denoulet P., Jeantet C. High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett. 1982 Aug 31;31(3):323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]