Abstract
Background
Paraquat, still used as an herbicide in some parts of the world, is now regarded as a dangerous environmental neurotoxin and is linked to the development Parkinson’s disease (PD). Paraquat interacts with cellular redox systems and causes mitochondrial dysfunction and the formation of reactive oxygen species, which in turn, plays a crucial role in the pathophysiology of PD. Various antioxidant therapies have been explored with the expectations that they deliver health benefits to the PD patients, however, no such therapies were effective. Here we have tested the neuroprotective efficacy of a novel water-soluble CoQ10 (Ubisol-Q10), in a rat model of paraquat-induced neurodegeneration in order to evaluate its potential application in the management of PD.
Results
We have developed a rat model of progressive nigrostriatal degeneration by giving rats five intraperitoneal injections of paraquat (10 mg/kg/injection), once every five days. Neuronal death occurred over a period of 8 weeks with close to 50% reduction in the number of tyrosine hydroxylase-positive cells. Ubisol-Q10, at 6 mg CoQ10/kg body weight/day, was delivered as a supplement in drinking water. The intervention begun after the completion of paraquat injections when the neurodegenerative process had already began and about 20% of TH-positive neurons were lost. Ubisol-Q10 treatment halted the progression of neurodegeneration and remaining neurons were protected. The outcomes were evaluated based on the number of surviving tyrosine hydroxylase-positive neurons in the substantia nigra region and improved motor skills in response to the Ubisol-Q10 intervention. To maintain this neuroprotection, however, continuous Ubisol- Q10 supplementation was required, if withdrawn, the neuronal death pathway resumed, suggesting that the presence of CoQ10 was essential for blocking the pathway.
Conclusion
The CoQ10, given orally as Ubisol-Q10 in drinking solution, was effective in blocking the progression of neurodegeneration when administered therapeutically (post-toxin injection), at a much lower concentration than other previously tested oil soluble formulations and well within the acceptable daily intake of 12 mg/kg/day. Such unprecedented neuroprotection has never been reported before. These results are very encouraging and suggest that Ubisol-Q10 should be further tested and developed as a therapy for halting the progression of PD.
Keywords: Parkinson’s disease, Oxidative stress, Neuronal cell death, Mitochondrial dysfunction, Water soluble CoQ10
Background
Parkinson’s disease (PD), the second most common neurodegenerative disorder, is characterised by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) region of the brain. PD affects approximately 1–2% of the population, above the age of 55 and with the steady growth of the ageing population, disease management is a growing concern for neurologists and other physicians. By the time the characteristic features of PD such as bradykinesia, rigidity, postural instability, and resting tremor become obvious, approximately 60-70% of DA neurons in the SNpc are lost [1]. Currently, there is no therapy available to halt the progression of this neurodegeneration. It has been possible, however, to alleviate the symptoms of the disease by providing dopamine replacement. Administration of levodopa is the most commonly utilized treatment for symptomatic relief [2], yet its prolonged application leads to drug induced dyskinesia, which severely affects the patient’s quality of life.
In the majority of cases the cause of PD remains unknown, but factors contributing to the pathogenesis of the disease are extensively studied. PD can be caused by environmental factors such as exposure to herbicides and pesticides or by genetic factors linked to gene mutations that increase the susceptibility to PD [3]. Although these genetic defects account for only 10% of PD cases, their identification brings about a better understanding of the disease pathophysiology and its progressive nature [4]. It is known that classical symptoms of PD can be caused by exposure to neurotoxin MPTP. In 1983 Langston’s group found PD like symptoms in young drug addicts who consumed heroin containing MPTP, a by-product in the synthesis of a synthetic heroin [5]. Later it was shown that MPTP injections cause selective loss of DA neurons in the SNpc region of certain strains of mice thereby creating animal models of PD [6-9]. Although MPTP is not an environmental toxin and humans are not commonly exposed to it, several epidemiological studies reveal a link between the use of herbicides and pesticides such as paraquat (PQ), maneb and rotenone and the incidence of PD [10]. It was subsequently discovered that the active metabolite of MPTP, MPP + and PQ have structural similarity. They enter the DA neurons via the dopamine transporter as well as trigger neurodegeneration [11]. Three independent studies in Texas, Taiwan and California show that exposure to PQ indeed causes an increased susceptibility to PD [12,13]. In rodents, PQ exposure leads to the loss of DA neurons in the SNpc region of the brain in a time and dose dependent manner [14]. Therefore, rat and mouse models of PQ-induced neurodegeneration have been developed to study the pathophysiology of the disease and to develop successful treatment strategies.
One consistent finding between the PD patients and animal models of PD (MPTP, PQ, rotenone) is the malfunctioning of complex I of the electron transport chain suggesting clearly, that mitochondrial dysfunction is at the centre of PD pathophysiology [4]. It seems that a blockade of complex I of the oxidative phosphorylation pathway by these toxins and the inability of DA neurons to cope with the excess of generated free radicals are the triggers of neuronal death. Therefore, it should be possible to interfere with the progression of neurodegenerative processes by applying antioxidants, such as CoQ10 and/or Vitamin E, which are capable of reducing the levels of free radicals. However, both these antioxidants are lipid soluble compounds, characterized by limited bioavailability and difficult to deliver systemically, especially to the brain. Numerous studies have shown that CoQ10 is effective in preventing cell death caused by toxins such as PQ, however, very high doses of CoQ10 (from oil soluble formulation available on the market) are required to provide neuroprotection in vivo[15]. Our collaborators at NRC (Ottawa, ON) have developed a nanomiscelle formulation of CoQ10 (Ubisol-Q10), which appears water soluble and contains CoQ10 and a derivatized form of α-tocopherol (vitamin E) [16,17]. The solubilization of CoQ10 is achieved due to amphipathic properties of PEG-derivatised α-tocopherol allowing the formation of stable and water soluble nanomicelles [16,17]. This formulation has been tested in several cell culture models and it has been shown to be efficient in protecting neurons from the toxic effects of PQ [18]. It has also been tested in vivo in rats exposed to PQ [14]. Prophylactic application of Ubisol-Q10 in this rat model of PD, provided as drinking solution prior to the PQ exposure and throughout the duration of experiments, clearly confirm its neuroprotective efficacy against PQ. The beneficial effects were achieved at a much lower dose of CoQ10 (6 mg/kg b.w.) compared to oil soluble formulation, which was used at 200 – 1600 mg/kg/day in mice [19].
Since PD is diagnosed when the symptoms appear and the neurodegeneration is already in progress, the prophylactic treatments, especially in sporadic cases of PD, are not relevent. Therefore, we designed a study to examine whether a therapeutic intervention with Ubisol-Q10 in rats already exposed to PQ could halt the on-going neurodegeneration and behavioural deterioration. Furthermore, we investigated whether a sustained supplementation of Ubisol-Q10 was needed to maintain the neuroprotection. Here we present our data showing that oral delivery of Ubisol-Q10, starting after the PQ injections, did halt neurodegeneration and prevented a loss of normal motor skills.
Results
Brain delivery of Coenzyme Q10
The brain CoQ10 levels were measured in rats which were given a 1 h access to Ubisol-Q10 supplemented water (at a concentration of 50 μg /ml) after a 24 h period of water deprivation. During this time rats drank on average 10 ml of solution containing 500 μg of CoQ10. Animals were sacrificed at different time points after the Ubisol-Q10 intake, CoQ10 was extracted and analysed by HPLC. The results are shown in Figure 1. We observed a modest, but time dependent elevation of CoQ10 in the brain, peaking at 3 h post-feeding, with values 30-50 % higher than the basal level, suggesting that its transfer to the brain parenchyma and subsequent metabolic turnover was taking place. A question whether these changes were sufficient to achieve a therapeutic neuroprotection against PQ was examined below (Figures 2, 3 and 4).
Figure 1.
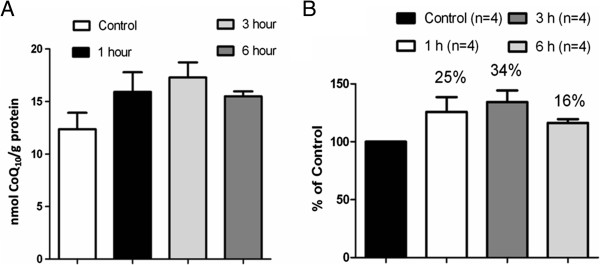
Bioavailability of Ubisol – Q10. (A) There is a gradual increase in the levels of CoQ10 in the rat brains that were sacrificed at the 1 and 3 hour time points following the 1 hour feeding with Ubisol – Q10 supplemented drinking water after the 24 hour water starvation in comparison with the control group that was fed with regular drinking water for 1 hour instead. The control group was sacrificed 1 hour following the water feeding. (B) There is a 25% increase in the level of CoQ10 at the 1 hour time point, 34% at the 3 hour time point and a decline to 16% at the 6 hour time point.
Figure 2.
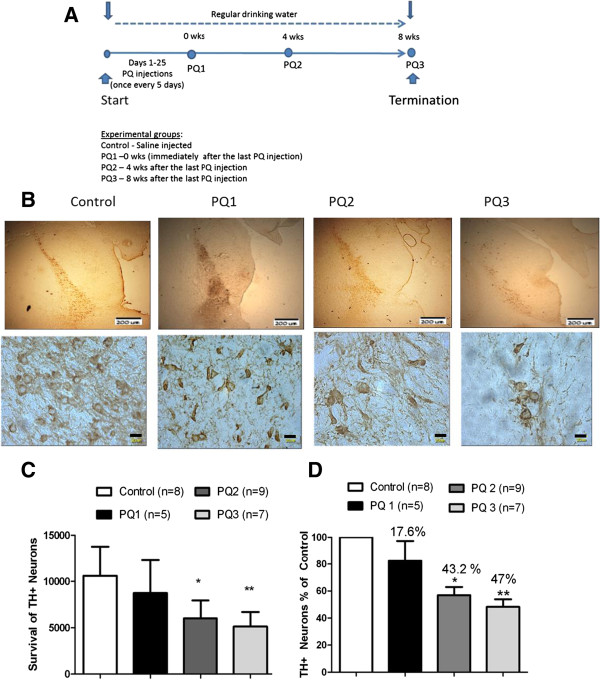
Progressive loss of DA neurons following PQ injections. (A) The experimental plan demonstrating the injection regime and treatment schedule. Immunohistochemistry performed using anti-tyrosine hydroxylase antibodies on the brain sections of animals. (B) Representative images of midbrain sections showing TH positive neurons at lower and higher magnifications from saline injected control group rats, PQ injected rats dissected 24 hours after the last injection (PQ1 group), PQ injected rats dissected four weeks after the last injection (PQ2 group), PQ injected rats dissected eight weeks after the last injection (PQ3 group). The area of SN which is to be counted is selected in every 6th section of the midbrain (sectioned at 30 microns thickness). (C) The total number of TH positive neurons in the SNpc region was counted at higher magnification using the stereology software purchased from the Stereology Resource Centre, Inc., Florida. There is a significant decrease in the number of TH - positive neurons (*p < 0.05) in the rats sacrificed four weeks and (**p < 0.05) eight weeks after the last injection in comparison with saline injected control group and no significance in rats sacrificed immediately after the last injection verses saline injected control groups. (D) The percentage decrease in TH positive neurons between the saline injected control and PQ groups. There is 43.3% and 47% decrease in the TH positive neurons in the rats sacrificed four and eight weeks after the last injection and only a 17.8% decrease in the number of TH positive neurons in the rats sacrificed 24 hours after the last injection. The bars in the upper (low magnification) panel are 200 μm and in the lower (high magnification) panel 20 μm.
Figure 3.
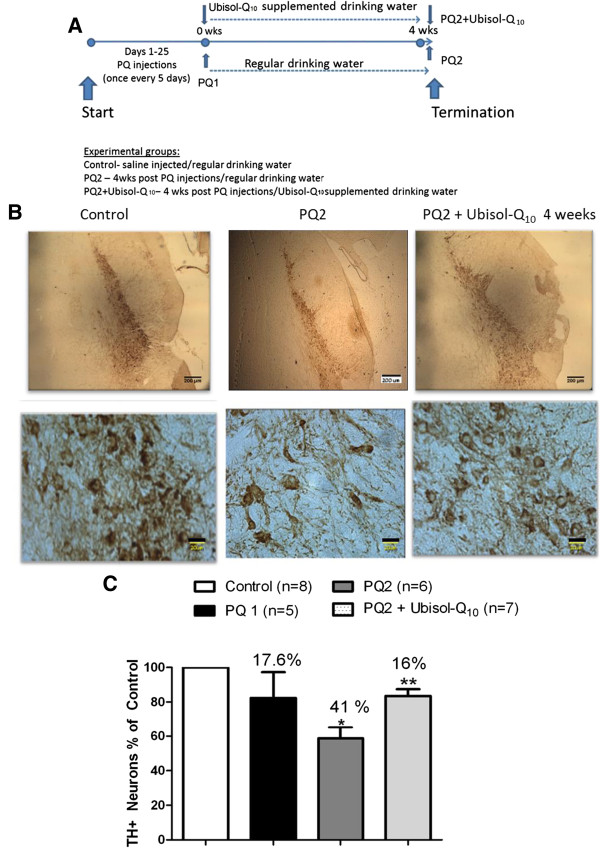
Halting the progression of neurodegeneration by Ubisol – Q10. (A) The experimental plan demonstrating the injection regime and treatment schedule. Immunohistochemistry performed using anti-tyrosine hydroxylase antibodies on the brain sections of animals. (B) Representative images of midbrain sections showing TH positive neurons at lower and higher magnifications from saline injected control group rats, PQ injected rats fed with regular drinking water dissected immediately after the last injection (PQ1), PQ injected rats fed with regular drinking dissected 4 weeks after last injection (PQ2), PQ injected rats fed with Ubisol – Q10 supplemented drinking water after last injection and dissected 4 weeks after last injection (PQ2 + Ubisol- Q10 4 weeks). The area of SN which is to be counted is selected in every 6th section of the midbrain (sectioned at 30 microns thickness). The total number of TH positive neurons in the SNpc region was counted at high magnification using the stereology software purchased from the Stereology Resource Centre, Inc., Florida. (C) The percentage decrease in TH positive neurons between the saline injected control group and the PQ injected treated and untreated groups. There is a significant 41% decrease in the TH positive neurons in the PQ2 group in comparison with the saline injected control group (*p < 0.05), whereas there is loss of 17% neurons in the Ubisol – Q10 treated group verses the control (**p < 0.05) indicating significant neuroprotection when compared to the PQ2 group. The bars in the upper (low magnification) panel are 200 μm and in the lower (high magnification) panel 20 μm.
Figure 4.
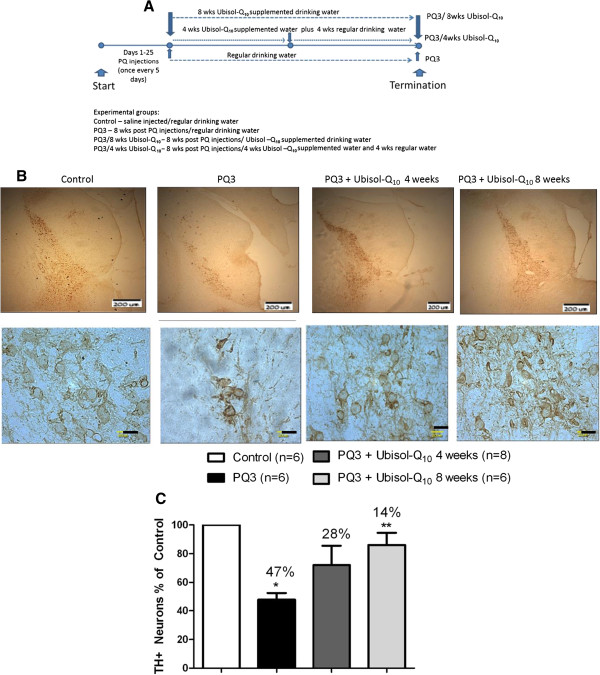
Sustained feeding of Ubisol-Q10 is needed for neuroprotection. (A) The experimental plan demonstrating the injection regime and treatment schedule. (B) Immunohistochemistry performed using anti-tyrosine hydroxylase antibodies on the brain sections of animals. Representative images of midbrain sections showing TH positive neurons at lower and higher magnifications from saline injected control group rats, PQ injected rats fed with regular drinking water dissected 8 weeks after last injection (PQ3), PQ injected rats fed with Ubisol – Q10 supplemented drinking water for 4 weeks followed by regular drinking water for 4 weeks after last injection with PQ and dissected 8 weeks after last injection (PQ3 + Ubisol – Q10 4 weeks group), PQ injected rats fed with Ubisol – Q10 supplemented drinking water for 8 weeks after last injection with PQ and dissected 8 weeks after last injection (PQ3 + Ubisol – Q10 8 weeks group). The area of SN which is to be counted is selected in every 6th section of the midbrain (sectioned at 30 microns thickness). The total number of TH positive neurons in the SNpc region was counted at high magnification using the stereology software purchased from the Stereology Resource Centre, Inc., Florida. (C) The percentage decrease in TH positive neurons between the saline injected control and PQ injected treated and untreated groups. There is a significant 47% decrease in the TH positive neurons in the PQ3 group in comparison with the saline injected control group (*p < 0.05) and loss of 28% neurons in the PQ3 + Ubisol-Q10 4 weeks group. A 14% decrease in TH positive neurons is seen in the treated PQ3 + Ubisol-Q10 8 weeks (**p < 0.05) indicating significant neuroprotection when compared to the PQ3 group. The bars in the upper (low magnification) panel is 200 μm and in the lower (high magnification panel) 20 μm.
Paraquat model of progressive neurodegeneration
Long Evans Hooded rats were used to develop a model of progressive neurodegeneration. The rats were given 5 intraperitoneal injections of PQ (10 mg/ kg b.w./injection), one injection every five days over a period of 20 days. Animals were sacrificed at different time points for up to 8 weeks post-PQ exposure. Midbrain sections were prepared, immunostained with anti-tyrosine hydroxylase antibody and TH- positive neurons were counted using a stereologer, in an unbiased manner. As shown in Figure 2, a substantial percentage of DA neurons, i.e., close to 18%, were lost during the PQ injection period (PQ1 group). The neurons continued to die over the next several weeks reducing the number of TH-positive neurons by 43% at the end of week 4 (PQ2 group) and 47% by end of week 8 (PQ3 group) post-PQ exposure. The results confirmed that PQ triggered progressive neurodegeneration in this strain of rats, mimicking to some extent, changes occurring in Parkinsonian patients. This model is probably closest to what happens in humans as one month in a rat’s lifetime is equivalent to 2.5 years in human [20]. Therefore, this model was used to assess a therapeutic neuroprotection of CoQ10.
Therapeutic intervention with Ubisol-Q10
We have previously shown that prophylactic treatment with Ubisol – Q10 effectively protects rat brain from PQ toxicity [18]. In this study we applied the Ubisol-Q10 intervention after the completion of PQ injections. By this time, neurodegenerative processes in the brain had already triggered (Figure 2). The PQ-treated group of rats was placed on Ubisol-Q10 supplemented drinking water (containing 50 μg/ml of CoQ10) for 4 weeks (PQ2 + 4 wks Ubisol-Q10 group). This treatment began when nearly 18% of SN neurons were already lost (PQ1 group, Figure 2), but the question was whether the remaining vulnerable neurons could be saved. The generated data is summarized in Figure 3. The midbrain sections were immunostained with anti- TH antibodies, and the stained neurons were counted using a stereologer in an unbiased manner. As shown above (Figure 2), the PQ- treated rats drinking regular water (PQ2 group) lost over 40% of DA neurons over the period of 4 weeks, whereas rats drinking Ubisol – Q10 lost less than 20% (PQ2 + 4 wks Ubisol-Q10 group). Clearly, this Ubisol-Q10 treatment saved close to 17% of neurons which would have otherwise died as the consequence of PQ exposure. This unprecedented neuroprotection has never been reported in animal models of neurotoxicity and could offer hope to PD patients for better disease management.
We then examined how long Ubisol – Q10 supplementation would be required to maintain neuroprotection. In this set of experiments the PQ treated rats were either kept on Ubisol – Q10 for the full 8 weeks post-PQ or the treatment was withheld after 4 weeks and rats were given regular tap water for the additional 4 weeks (8 weeks total). There was a significant loss of DA neurons, approximately 47% in comparison with the saline injected control group indicating progressive neurodegeneration over a period of eight weeks (Figure 4). There was also significant neuroprotection in the rats that received the Ubisol – Q10 supplemented drinking water for eight weeks post injections. Since the Ubisol – Q10 intervention began after nearly 15% of DA neurons were already killed (Figure 2) no further loss of neurons was observed as a result of this intervention (Figure 4, only 14% of neuronal loss recorded). However, if the treatment was withheld after 4 weeks, the neurodegeneration resumed as evidenced by the reduced number of surviving neurons in this experimental group comparing to the group receiving Ubisol – Q10 for 8 weeks (28% versus 14%, respectively). Therefore, continuous Ubisol – Q10 supplementation was required to maintain the achieved level of neuroprotection.
Behaviour results
Deficiency in the motor function is a hallmark of PD. Next, we asked whether loss of DA neurons correlated with the deficiency of behavioural motor function following PQ treatment, and whether motor deterioration was blocked by Ubisol-Q10 treatment. We applied a horizontal beam walking test as described in the Method section. Results shown in Figure 5 indicated that the PQ3 group made more leg slips than either the control or the PQ3 + Ubisol-Q10 8 weeks in both the test phases or than the PQ3+ Ubisol-Q10 4 weeks group in the first test phase. The PQ3 + Ubisol-Q10 4 weeks increased its leg slips to the elevated levels of the PQ3 group in the second test phase. Multiple comparisons between groups confirmed that the number of leg slips of the PQ3 group was significantly greater than those of the other three groups (p < 0.05) in the 1st test phase but only remained significantly greater than that of the PQ3+/Ubisol-Q10 8 weeks group in 2nd test phase (p = .036). Thus even though the observed groups by phase interact was not significant, multiple comparison between groups reveal that only those rats that received Ubisol –Q10 in their drinking water over the complete post injection period maintained their superior performance similar to that of rats that were not exposed to potential neurodegenerative effects of PQ. From a behavioral aspect, treatment with the neuroprotectant agent only half way through the post-injection period was not sufficient to maintain its effect to the end of the experiment.
Figure 5.
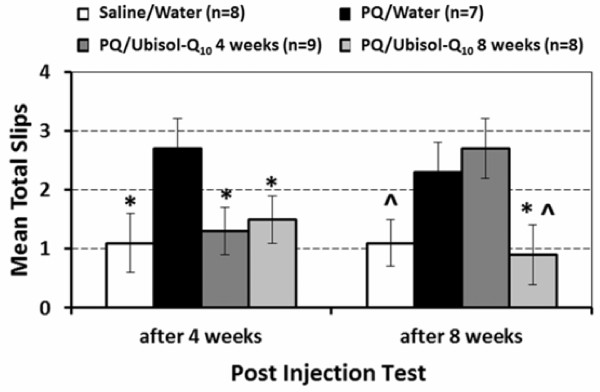
Mean total number of hind leg slips over three sessions in each post-injection test for each Injection/Treatment group. Vertical error lines represent ± SEM. Significant difference (p < 0.05) between the control or either of the two PQ/ Ubisol – Q10 groups and the PQ3 group in each injection test phase are designated by * and between the control and the PQ/Ubisol-Q10 8 weeks group and the PQ3 in the second injection phase by ^.
Toxicity assessment
The data presented above (Figure 4) clearly indicated that long-term treatments with Ubisol – Q10 would be required to maintain the neuroprotection. To ensure safety of such long treatments, we carried out a pilot toxicity study, in which rats were maintained on drinking water supplemented with Ubisol – Q10 at a dose 10 times higher (60 mg/kg/day) than that used for neuroprotection (6 mg/kg/day) for 2.5 months. During this time, the Ubisol – Q10 treated rats never displayed any signs of discomfort, no change in eating, drinking, grooming habits and no difference in body weight in comparison with rats drinking regular tap water over the same time period. At the conclusion of the experiments several tissues were collected and sent for histopathological examination by a board certified pathologist at the University of Guelph. No overt lesions of toxicological significance were observed in the Ubisol-Q10-treated animals (data not shown).
Discussion
For the first time ever, we have effectively established an animal model, using PQ, which accurately demonstrates the chronic and progressive neurodegeneration similar to that in PD patients. Compared to an acute model, our model effectively replicates PD by having slow, chronic degeneration of DA neurons in SNpc. However, progressive neurodegeneration has also been observed with continuous infusion of MPTP using an osmotic pump [21]. The progressive loss was evident in our results over the observed time span, with a decreased number of neurons remaining at each subsequent time interval. Our model allows us to intervene with treatment at any point after the initiation of the disease. Due to this we were also able to demonstrate for the first time, that when Ubisol-Q10 is administered therapeutically it effectively halts the progression of the neurodegeneration, even at low dosages. After demonstrating the success Ubisol-Q10 has in protecting the neurons therapeutically, it was shown that this treatment needs to be continuous. Halting the administration of the treatment would result in a continuation of the neurodegeneration initiated by the PQ toxin during the injection regime. With bioavailability data, we were also able to show that Ubisol-Q10 is effective at increasing the CoQ10 levels in the brain. All of the results were supported using behavioural, histochemical, and biochemical methodology.
An animal model of PD was developed when the neurotoxin MPTP was found to cause PD like symptoms and loss of DA neurons by blocking complex I of the electron transport chain [7]. MPTP establishes an acute model of PD, which is not realistic to the natural progression of the disease. Following this, it was found that environmental toxins, rotenone and PQ, can also block complex I of the electron transport chain. Further supported by epidemiological studies, a link between the use of these pesticides and the incidence of PD was established [22]. The development of the PQ animal model of PD is more relevant than the acute MPTP model as it more effectively mimics PD in patients.
In previous research, a variety of dosages and injection regimes of PQ have been used in rat models of PD [14,23]. The downfall of these PQ models was that they were not tested to ensure slow, progressive loss. However, progressive, continuous, and slow neuronal loss in the SNpc was tested and seen in our model during and after our 5 interpretational injection (1 every 5 days for 5 injections). Establishing this model is essential before testing any therapeutic treatment interventions as this is what characterizes PD in patients.
Additional research shows that the pathogenic mechanisms of PD are associated with mitochondrial dysfunction, oxidative stress and altered protein handling [4]. The involvement of mitochondria is considered a key to cell death observed in PD in both sporadic and familial cases.
Previous experiments in our lab have shown that Ubisol-Q10 is effective in protecting neurons against toxic insult in vivo and can protect DA neurons if administered prophylactically, that is, even before exposure to the environmental toxin, PQ [14]. However, PD is often not diagnosed until symptoms arise, which occurs when almost 50 – 60% neurons are lost.
Once the process is initiated by toxic insult, it is crucial to see if treatment administered therapeutically can halt further neurodegeneration. Ubisol-Q10 was tested therapeutically and it showed to have significant protection of the remaining DA neurons after both 4 weeks, and 8 weeks of treatment. This is one of the first experiments to show this. There are multiple explanations which could explain how Ubisol-Q10 protects the remaining neurons; initially it is plausible that the combined anti-oxidant nature of the two components of Ubisol-Q10 (CoQ10 and Vitamin E) could quench the levels of oxidative stress associated with the disease. It was shown that the carrier solution containing vitamin E alone did not have a significant effect on neuroprotection ([14] and data not shown). In other research, it’s been shown that lipid soluble CoQ10 (in high dosages) is an effective neuroprotective agent [19]. Past research on Ubisol-Q10 has shown it to be effective in stabilizing the mitochondria through inhibiting Bax [24]. Another hypothesis is that it could be protecting the mitochondria by increasing its overall energy output; as CoQ10 is naturally found in the electron transport chain.
Previous research using oil soluble CoQ10 as a treatment for PD made it into clinical trials in 2011, though failed in phase 2. In their pre-clinical work the oil-soluble CoQ10 treatment was tested prophylactically on MPTP induced mouse model [19,25]. The oil soluble CoQ10 was shown to be effective, but only at high dosages. A possible explanation to the discontinuation of their clinical trial was because very large dosages (1,600 mg/kg/day) were required to show any neuroprotection. When this dosage is converted to a human dose (averaging 70 kg) they are required to take 112 g/day in order to obtain results, which is beyond the acceptable FDA approved dose for clinical trials (2.4 g). Therefore, in the clinical trial they were not receiving anywhere near the dose required to show positive results. However, our preclinical work, on our more accurate chronological model, treating both prophylactically and therapeutically has shown comparable neuroprotection but at a significantly lower dose (6 mg/kg/day). Therefore, if our dosage was converted for human treatment it would only be 0.42 g/day, which is not only lower then FDA approved amount for clinical trial (2.4 g) but also the approved maximum daily dosage for general supplement intake (1.2 g). Both the oil soluble CoQ10 and Ubisol-Q10 showed comparable bioavailability when administered, but in order to have comparable quantities in the brain the oil formulation needed to be given in a significantly higher dose [26].
The question remains why Ubisol-Q10 is more effective at lower doses than CoQ10. It is assumed that the water soluble composition makes absorption into the blood stream easier, therefore, making it possible to cross the blood brain barrier. It is evident in our bioavailability experiment that this formulation does shuttle CoQ10 into the brain, due to the increase of 35% after 3 hours. Though, the other significant finding was that once it is in the brain it does not accumulate. This means that there is no build-up of CoQ10 in the brain, which could be toxic to the neurons. The natural removal seen explains why when the treatment is withdrawn the effects are no longer sustained. Henceforth, in order to sustain neuroprotection the treatment must be continuous and in doing so neurotoxicity will not result. It is also important to note that the animals in this experiment were allowed to drink Ubisol-Q10 supplemented drinking water ad libitum and were not gavaged.
Our study has shown that the withdrawal of Ubisol-Q10 leads to continued neurodegeneration, which was triggered by the toxin during the injection period. Therefore, Ubisol-Q10 does not halt neurodegeneration by acting on the toxin, but rather by supporting the remaining neurons. This experiment was only conducted with sustained treatment over 8 weeks (with 1 month of treatment and a consecutive month of withdrawal). To ensure the results, more research needs to be conducted over longer time spans. Though the current data found supports sustained treatment regiments in order to withstand neurodegeneration. These findings were also supported by the behaviour data which shows that animals provided with Ubisol-Q10 treatment for a longer duration perform better throughout in the beam test compared to the animals where the treatment was withdrawn.
Conclusion
In conclusion we have shown that the PQ rat model of PD we used in the study shows slow progressive loss of DA neurons and hence mimics what is seen in patients suffering from PD. Our formulation can prevent the death of the remaining neurons in our PD model when administered after the process of neurodegeneration has been triggered and hence could be an effective therapeutic at any stage of the disease. Also, we found that Ubisol-Q10 has to be given continuously and cannot be withdrawn in order to continue neuroprotection. Bioavailability studies have shown that even though this formulation is provided at a low dose in order to provide significant neuroprotection, CoQ10 does cross the blood brain barrier and there is an increase in the levels of CoQ10 in the brain following administration of Ubisol-Q10. This formulation of CoQ10 is FDA-GRAS approved and preliminary toxicity results show that there is no overt toxicity even when the dose is increased to 10 times the required dose. Ubisol-Q10 is an effective neuroprotective agent that could be used effectively to halt the progression of Parkinson’s disease at low doses.
Methods
Animal care
All procedures involving animals were carried out in accordance with the Canadian Council for Animal Care guidelines and approved by the University of Windsor’s Animal Care Committee. Three months old male Long Evans Hooded rats were purchased from Charles River Laboratories. Rats in the same treatment group were housed together (3–4 per cage) for convenience and in order to prevent any hierarchy that could arise due to the extent of neurodegeneration. The rooms that housed the rats were maintained at 20°C in a reversed 12 h:12 h dark light cycle.
Paraquat neurotoxicity model and Ubisol-Q10 treatments
Rats received 5 intraperitoneal injections of PQ at a dose of 10 mg/kg body weight/injection dissolved in phosphate buffered saline (PBS), one injection every 5 days over a period of 20 days. Control rats received intraperitoneal injections of PBS alone. Brain tissue was examined immediately after the last PQ injection and, subsequently, 4 weeks and 8 weeks later. Supplementation of drinking water with Ubisol-Q10 at a concentration of 200 μg/ml (equivalent to 50 μg CoQ10/ml) begun on the day of the last PQ injection and it was continued for either 4 weeks or 8 weeks. Fresh drinking solutions were provided every second day. Sterile stock solution of Ubisol-Q10 at 200 mg/ml (equivalent to 50 mg CoQ10/ml) was provided by Zymes LLC (Hasbrouck, NJ). At the conclusion of experimental treatments, rats were perfused with Tyrodes buffer containing heparin, the tissues were fixed with 10% formalin, and the brains extracted and stored in the 10% formalin until processing for immunohistochemistry.
CoQ10 bioavailability study
Rats were deprived of water for a period of 24 hours prior to a full 1 h access to drinking water supplemented with Ubisol-Q10 at a concentration equivalent to 50 μg CoQ10/ml. The rats were sacrificed at 1, 3 and 6 h after the feeding. Brain tissue was collected and CoQ10 content was measured as previously described [27-29]. Briefly, samples were homogenized in cold PBS and subjected to repeated freezing/thawing steps to disrupt protein/lipid complexes. CoQ10 was extracted and analysed by HPLC following separation on a TSK-GEL ODS-100S column (4.6 mm × 150 mm, 7 μ particle size, TOSOH Biosep LLC, Montgomeryville), equipped with a 1 mm C18 guard column (Optimize Technologies Inc., Oregon City, OR). Absorbance at 275 nm was monitored and recorded using Beckman System Gold Software.
Toxicity study
A group of rats (4 rats) were kept for 2.5 months on drinking water supplemented Ubisol- Q10 at a concentration 2 μg/ml (equivalent to 50 μg/ml of CoQ10) or 10 times the dose used in the neuroprotection study. Animals were weighed once a week to ensure their health. The rats were then perfused with heparin containing Tyrodes buffer and formalin fixed tissue – heart, lung, liver and kidney were sent to the Animal Health Laboratory, University of Guelph. Hematoxylin & Eosin -stained histological sections of the tissues were evaluated by a board-certified veterinary pathologist.
Rat horizontal beam walking test
All rats were assessed for performance on a horizontal beam-walking test for motor skills/motor deficits as measured by leg slips. The aluminium beam was 1.68 metres in length, 2 centimetres in width and 0.75 metres from the ground. A mirror was placed behind the beam, measuring 1.78 metres in length and 0.3 metres in height. Four weeks after the last injection, rats underwent one trial per day for four consecutive days (one training trial and three test trials). Eight weeks after the last injection another three test trials were performed (one trial per day). In the training trial, rats ran down the beam to the holding cage on a flat platform three times, each time with different distances between the holding cage and starting position. The first position was a quarter of the beam length, the second was half, and the last was the entire distance of the beam. This last distance is where mice were placed for the subsequent test trails.
Rats received a small slice of apple in the holding cage located on a table at the end of the beam. The rat had up to 2 minutes to cross the beam. The test trials were recorded using a standard video camera, located 2 metres perpendicular to the beam. The number of hind leg slips made from either leg during each test trial was later noted from viewing the recorded video clips. The number of limb slips for each rat was summed over the three test sessions in each phase because rats made too few slips in each session to analyse this behaviour over trials within each session. The statistical analysis of each rat’s total number of leg slips over each test series was carried by a two-way ANOVA (Groups x Test phase with repeated measures on the second factor). Effects from these analyses were considered significant at p < 0.05. Post-hoc comparisons between groups at each test phase were carried out by Least Squares Difference post-hoc multiple comparisons test multiple comparisons and significant differences between groups were considered at p < 0.05 (one-tail) based on the prediction that rats not given post-injection Ubisol-Q10 in their drinking water would show deficits associated with PQ-induced neurodegeneration.
Immunohistochemistry
The brains kept in 10% formalin were transferred to 30% sucrose (w/v) three days before sectioning. The midbrain region was sectioned at a thickness of 30 μm and 64 sections were collected in total. The sections were subjected to immunohistochemistry with anti-tyrosine hydroxylase antibody (1:1000 dilution) purchased from Pel-Freeze Biologicals, USA. Prior to overnight incubation with the primary antibody at 4°C, the sections were incubated in 1% H2O2 for 5 minutes to block endogenous peroxidase, DAKO universal blocking solution (purchased from Diagnostics Canada Inc., Mississauga) for 30 minutes and in normal goat serum (prepared as per instructions on anti-rabbit Vecstatin ABC Kit, Vector Laboratories) for 30 minutes in order to block the binding of non-specific goat IgG. The sections were washed in Tris buffered saline (TBS) twice for 5 minutes between the blocking steps in order to remove any excess blocking reagents. After overnight incubation the slides were washed in TBS twice and incubated in biotinylated anti-rabbit IgG raised in goat (anti-rabbit Vecstatin ABC Kit) for 1.5 hours. The slides were again washed twice for 5 minutes with TBS, following which they were incubated in avidin biotin complex (ABC reagent) for 45 minutes. Then the two five minute washes with TBS were repeated, and the peroxidase substrate 3, 3′ diaminobenzidine (DAB) prepared as per the instructions to specifically stain the DA neurons. The sections were then dehydrated with 95% ethanol and xylene and coverslipped to be able to visualise under the microscope.
The number of TH-positive neurons in the SN were counted using the Stereology Software provided by the Stereology Resource Center, Chester, MD as previously described [30,31]. The SN region was outlined at low magnification and the neurons were counted at high magnification.
Statistical analysis
Differences among means were analysed using one way analysis of variance (ANOVA) and pairwise comparisons between means were analyzed by post hoc Bonferroni’s multiple comparison test. The software used was GraphPad Prism ver. 4.0.
Abbreviations
PD: Parkinson’s disease; SN: Substantia nigra; SNpc: Substantia nigra pars compacta; DA: Dopaminergic; PQ: Paraquat; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPP+: 1-methyl-4-phenylpyridinium; NRC: National Research Council; PEG: Polyethylene glycol; PBS: Phosphate Buffered Saline; TBS: Tris Buffered Saline; GLP: Good lab practices; ABC: Avidin Biotin Complex; ANOVA: Analysis of variance; FDA: Food and Drug Administration; GRAS: Generally recognised as safe.
Competing interests
Dr Shelley Weinstock is paid employee of the Zymes LLC, the company which has the licence for Ubisol - Q10, hence the financial interest.
Authors’ contributions
KM, MS, JKS, JC and SP contributed to the planning and execution of the experiments and writing the manuscript. KM, SL, KH, HM and PL were involved in performing injection and feeding of different regiments, dissections, immunohistochemical analysis and biochemical analysis. JC, CK and DL were involved in the design and execution of behavioural tests, analysis of rotorod results and animal care. MS, HBB and SW prepared water-soluble CoQ10 and placebo formulations. All authors read and approved the final manuscript.
Contributor Information
Krithika Muthukumaran, Email: muthuku@uwindsor.ca.
Samantha Leahy, Email: leahys91@gmail.com.
Kate Harrison, Email: k8harrison25@gmail.com.
Marianna Sikorska, Email: marianna.sikorska@bell.net.
Jagdeep K Sandhu, Email: Jagdeep.Sandhu@nrc-cnrc.gc.ca.
Jerome Cohen, Email: jcohen@uwindsor.ca.
Corrine Keshan, Email: keshan@uwindsor.ca.
Daniel Lopatin, Email: lopatin@uwindsor.ca.
Harvey Miller, Email: Harvey.miller@nrc.ca.
Henryk Borowy-Borowski, Email: Henryk.Borowy-Borowski@nrc.ca.
Patricia Lanthier, Email: pat.lanthier@sympatico.ca.
Shelly Weinstock, Email: shelleyweinstock@me.com.
Siyaram Pandey, Email: spandey@uwindsor.ca.
Acknowledgement
This work was supported by an operating grant from CIHR to SP and a Therapeutic Development Initiative Grant from Michael J. Fox Foundation to Zymes LLC. We thank Mr. Joseph Szecsei, Windsor, Ontario for his generous donation to acquire the Stereologer System. We thank Parvati Dadwal, Katie Facecchia and Alyson Laframboise for their help with technical troubleshooting. We also thank Karishma Desai for reviewing the manuscript.
References
- de Lau LM, Schipper CM, Hofman A, Koudstaal PJ, Breteler MM. Prognosis of Parkinson Disease Risk of Dementia and Mortality: The Rotterdam Study. Arch Neurol. 2005;15(8):1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Lees A, Obeso J. Levodopa therapy for Parkinson’s disease: challenges and future prospects.le first published online. Mov Disord. 2008;15(S3):S495–S496. doi: 10.1002/mds.22048. [DOI] [PubMed] [Google Scholar]
- Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;15(22):2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;15:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;15:979–983. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Riachi NJ, Arora PK, Sayre LM, Harik SI. Potent Neurotoxic Fluorinated 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Analogs as Potential Probes in Models of Parkinson Disease. J Neurochem. 1988;15(4):1319–1321. doi: 10.1111/j.1471-4159.1988.tb10610.x. [DOI] [PubMed] [Google Scholar]
- Sundstorm ME, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;15:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Sonsalla PK. The MPTP-treated mouse as a model of Parkinsonism: how good is it? Neurochem Int. 1992;15(Suppl):299S–303S. doi: 10.1016/0197-0186(92)90256-q. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;15:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, la Vecchia C, Nicotera P. Paraquat and Parkinson’s disease. Cell Death Differ. 2010;15:1115–1125. doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- Rappold P, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, Sen N, Javitch JA, Tieu K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci USA. 2011;15(51):20766–20771. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson’s disease: a case–control study in Taiwan. Neurology. 1997;15(6):1583–1588. doi: 10.1212/WNL.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz M. Well-water consumption and Parkinson’s Disease in rural California. Environ Health Perspect. 2009;15(12):1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayajulu-Niţu M, Sandhu J, Cohen J, Sikorska M, Sridhar T, Matei A, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and Parkinsonism in adult rats: neuroprotection and amelioration of symptoms by water-soluble formulation of Coenzyme Q10. BMC Neurosci. 2009;15:88. doi: 10.1186/1471-2202-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;15:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowy-Borowski H, Sodja C, Docherty J, Walker PR, Sikorska M. Unique technology for solubilization and delivery of highly lipophilic bioactive molecules. J Drug Target. 2004;15:415–424. doi: 10.1080/10611860412331285233. [DOI] [PubMed] [Google Scholar]
- Sikorska M, Borowy-Borowski H, Zurakowski B, Walker PR. Derivatised alpha-tocopherol as a CoQ10 carrier in a novel water-soluble formulation. Biofactors. 2003;15(1–4):173–183. doi: 10.1002/biof.5520180220. [DOI] [PubMed] [Google Scholar]
- Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol Dis. 2005;15:618–625. doi: 10.1016/j.nbd.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;15(6):1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- Andrello N, Santos E, Araujo M, Lopes L. Rat’s age versus human’s age: what’s the relationship? Arq Bras Cir Dig. 2012;15(1):49–51. doi: 10.1590/S0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schlüter OM, Lenzi, Gesi M, Ruffoli R, Ferrucci F, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Südhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and α-synuclein. Proc Natl Acad Sci USA. 2004;15(9):3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;15(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson’s disease in young adult rats. Neurobiol Dis. 2005;15(2):360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, Antonsson B, Pandey S. Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis. 2006;15(8):1359–1369. doi: 10.1007/s10495-006-8417-4. [DOI] [PubMed] [Google Scholar]
- Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem. 2009;15(5):1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu R, McDermott MP, Dicenzo R, de Blieck EA, Hyson HC, Beal MF, Bednarczyk EM, Bogdanov M, Metakis LJ, Browne SE, Lorenzo BJ, Ravina B, Kieburtz K. A randomized study of the bioavailability of different formulations of coenzyme Q(10) (ubiquinone) J Clin Pharmacol. 2007;15(12):1580–1586. doi: 10.1177/0091270007307571. [DOI] [PubMed] [Google Scholar]
- Graves S, Sikorska M, Borowy-Borowski H, Ho RJ, Bui T, Woodhouse C. Analysis of coenzyme Q10 content in human plasma and other biological samples. Methods Mol Biol. 1998;15:353–365. doi: 10.1385/0-89603-472-0:353. [DOI] [PubMed] [Google Scholar]
- Tang PH, Miles MV, Miles L, Quinlan J, Wong B, Wenisch A, Bove K. Measurement of reduced and oxidized coenzyme Q9 and coenzyme Q10 levels in mouse tissues by HPLC with coulometric detection. Clin Chim Acta. 2004;15(1–2):173–184. doi: 10.1016/j.cccn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Souchet N, Laplante S. Seasonal and geographical variations of sterol composition in snow crab hepatopancreas and pelagic fish viscera from Eastern Quebec. Comp Biochem Physiol B Biochem Mol Biol. 2007;15(3):378–386. doi: 10.1016/j.cbpb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram K. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in aprimate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;15(52):18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Hengemihle JM, Jucker M, Calhoun ME, Ingram DK, Mouton PR. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods. 1998;15(1–2):101–108. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]