Abstract
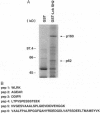
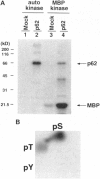
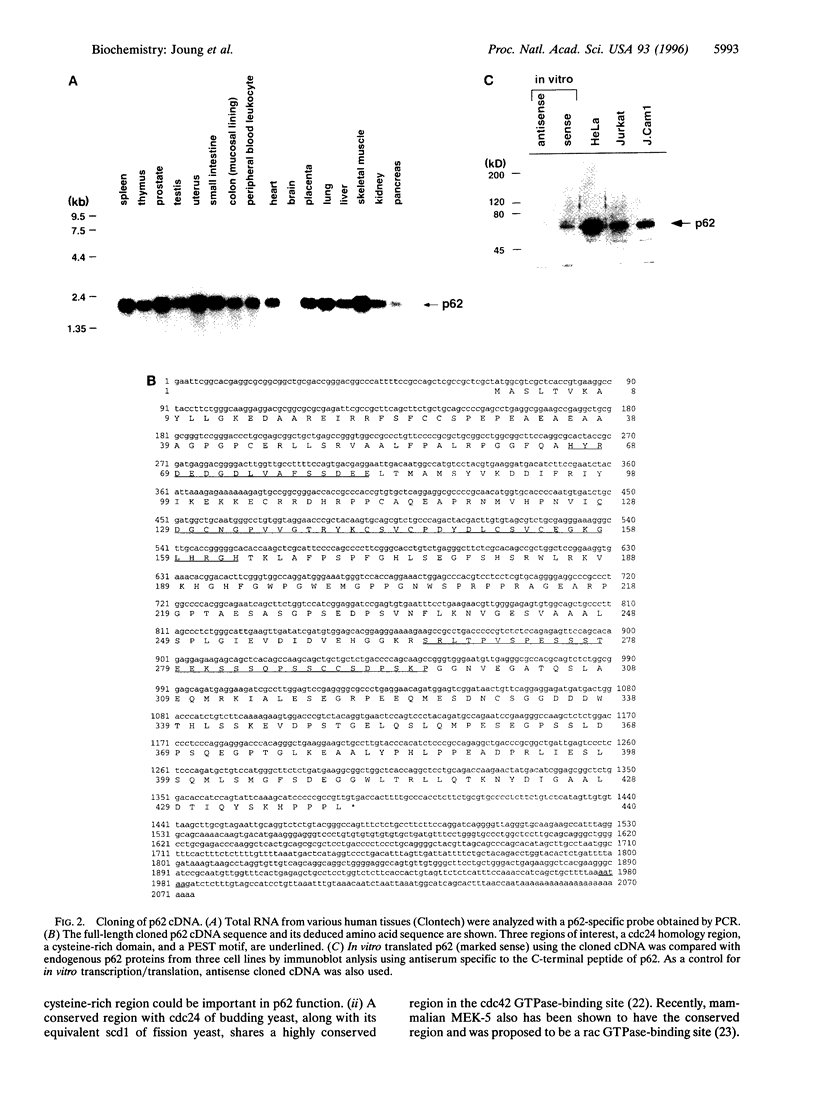
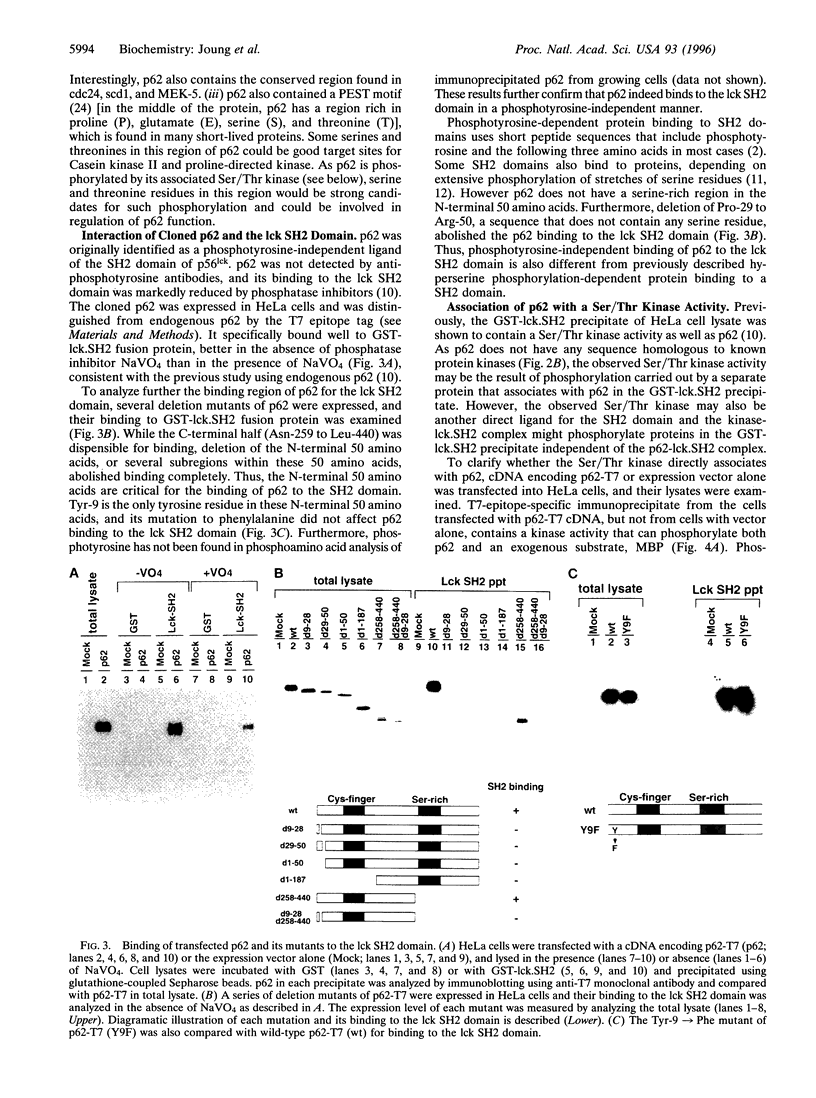
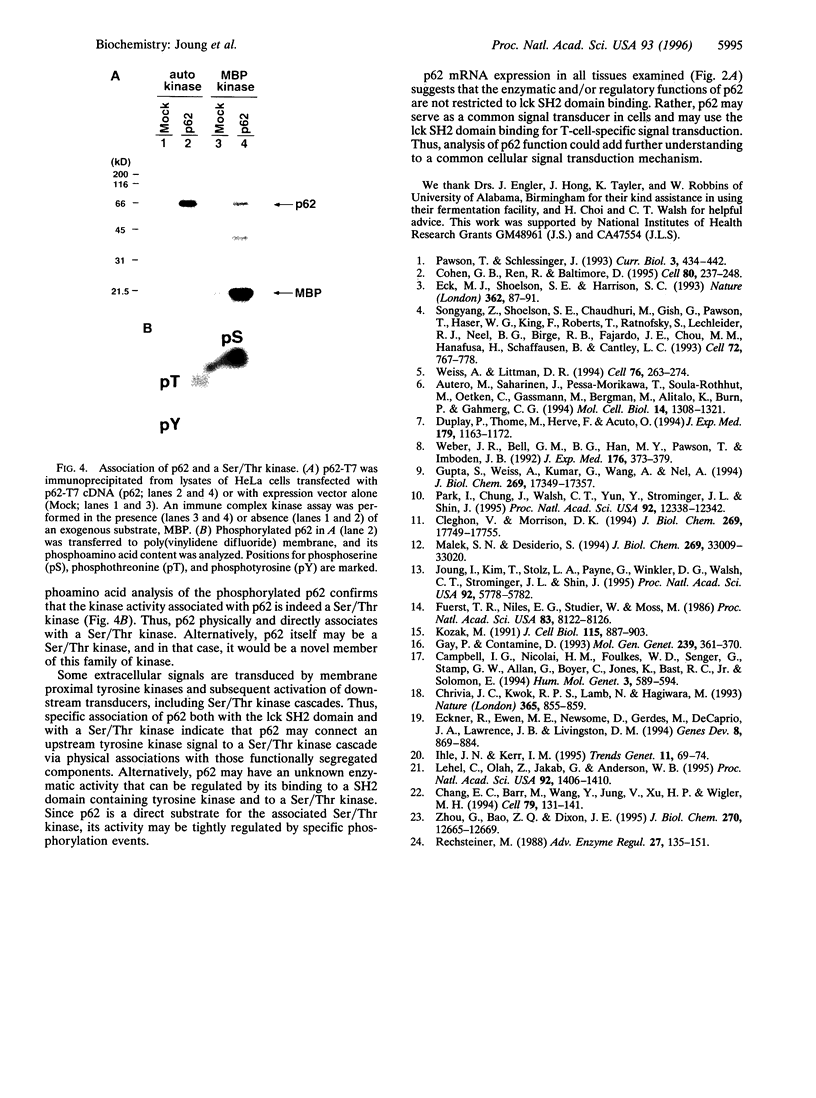
A novel human cDNA encoding a cytosolic 62-kDa protein (p62) that binds to the Src homology 2 (SH2) domain of p56lck in a phosphotyrosine-independent manner has been cloned. The cDNA is composed of 2074 nucleotides with an open reading frame encoding 440 amino acids. Northern analysis suggests that p62 is expressed ubiquitously in all tissues examined. p62 is not homologous to any known protein in the data base. However, it contains a cysteine-rich region resembling a zinc finger motif, a potential G-protein-binding region, a PEST motif, and several potential phosphorylation sites. Using T7-epitope tagged p62 expression in HeLa cells, the expressed protein was shown to bind to the lck SH2 domain. Deletion of the N-terminal 50 amino acids abolished binding, but mutagenesis of the single tyrosine residue in this region had no effect on binding. Thus, the cloned cDNA indeed encodes the p62 protein, which is a phosphotyrosine-independent ligand for the lck SH2 domain. Its binding mechanism is unique with respect to binding modes of other known ligands for SH2 domains.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autero M., Saharinen J., Pessa-Morikawa T., Soula-Rothhut M., Oetken C., Gassmann M., Bergman M., Alitalo K., Burn P., Gahmberg C. G. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994 Feb;14(2):1308–1321. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. G., Nicolai H. M., Foulkes W. D., Senger G., Stamp G. W., Allan G., Boyer C., Jones K., Bast R. C., Jr, Solomon E. A novel gene encoding a B-box protein within the BRCA1 region at 17q21.1. Hum Mol Genet. 1994 Apr;3(4):589–594. doi: 10.1093/hmg/3.4.589. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Barr M., Wang Y., Jung V., Xu H. P., Wigler M. H. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994 Oct 7;79(1):131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chrivia J. C., Kwok R. P., Lamb N., Hagiwara M., Montminy M. R., Goodman R. H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993 Oct 28;365(6449):855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cleghon V., Morrison D. K. Raf-1 interacts with Fyn and Src in a non-phosphotyrosine-dependent manner. J Biol Chem. 1994 Jul 1;269(26):17749–17755. [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Duplay P., Thome M., Hervé F., Acuto O. p56lck interacts via its src homology 2 domain with the ZAP-70 kinase. J Exp Med. 1994 Apr 1;179(4):1163–1172. doi: 10.1084/jem.179.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Eckner R., Ewen M. E., Newsome D., Gerdes M., DeCaprio J. A., Lawrence J. B., Livingston D. M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994 Apr 15;8(8):869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Contamine D. Study of the ref(2)P locus of Drosophila melanogaster. II. Genetic studies of the 37DF region. Mol Gen Genet. 1993 Jun;239(3):361–370. doi: 10.1007/BF00276934. [DOI] [PubMed] [Google Scholar]
- Gupta S., Weiss A., Kumar G., Wang S., Nel A. The T-cell antigen receptor utilizes Lck, Raf-1, and MEK-1 for activating mitogen-activated protein kinase. Evidence for the existence of a second protein kinase C-dependent pathway in an Lck-negative Jurkat cell mutant. J Biol Chem. 1994 Jun 24;269(25):17349–17357. [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Joung I., Kim T., Stolz L. A., Payne G., Winkler D. G., Walsh C. T., Strominger J. L., Shin J. Modification of Ser59 in the unique N-terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5778–5782. doi: 10.1073/pnas.92.13.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehel C., Olah Z., Jakab G., Anderson W. B. Protein kinase C epsilon is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1406–1410. doi: 10.1073/pnas.92.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S. N., Desiderio S. A cyclin-dependent kinase homologue, p130PITSLRE is a phosphotyrosine-independent SH2 ligand. J Biol Chem. 1994 Dec 30;269(52):33009–33020. [PubMed] [Google Scholar]
- Park I., Chung J., Walsh C. T., Yun Y., Strominger J. L., Shin J. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12338–12342. doi: 10.1073/pnas.92.26.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv Enzyme Regul. 1988;27:135–151. doi: 10.1016/0065-2571(88)90014-3. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Weber J. R., Bell G. M., Han M. Y., Pawson T., Imboden J. B. Association of the tyrosine kinase LCK with phospholipase C-gamma 1 after stimulation of the T cell antigen receptor. J Exp Med. 1992 Aug 1;176(2):373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Zhou G., Bao Z. Q., Dixon J. E. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995 May 26;270(21):12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]