Abstract
Deregulation of the Hedgehog (Hh) signaling pathway is associated with the development of human cancer including medullobastoma and basal cell carcinoma. Loss of Patched or activation of Smoothened in mouse models increases the occurrence of tumors. Likewise, in a Drosophila eye model, deregulated Hedgehog signaling causes overgrowth of eye and head tissues. Surprisingly, we show that cells with deregulated Hh signaling do not or only little contribute to the tissue overgrowth. Instead, they become more sensitive to apoptosis and may eventually be eliminated. Nevertheless, these mutant cells increase proliferation in the adjacent wild-type tissue, i.e., in a non-cell autonomous manner. This non-cell autonomous effect is position-dependent and restricted to mutant cells in the anterior portion of the eye. We also observe precocious non-cell autonomous differentiation in genetic mosaics with deregulated Hh signaling. Together, these non-cell autonomous growth and differentiation phenotypes in the Drosophila eye model reveal another strategy by which oncogenes may generate a supportive micro-environment for tumor growth.
Keywords: Hedgehog signaling, Costal-2, Patched, Non-cell autonomous overgrowth
1. Introduction
The Hedgehog (Hh) pathway is an important cell/cell signaling pathway in both vertebrates and invertebrates (reviewed in (Huangfu and Anderson, 2006; Ingham, 2008; Jiang and Hui, 2008). It was initially discovered in Drosophila melanogaster where it is required for embryonic segmentation (Nusslein-Volhard and Wieschaus, 1980). Since then, the Hh pathway has been shown to be involved in many biological processes including patterning, cell proliferation and cell fate specification as well as morphogenesis and homeostasis (Huangfu and Anderson, 2006; Jiang and Hui, 2008; Kalderon, 2005). In humans, deregulated, i.e., increased Hh signaling is associated with various cancers including basal cell carcinoma, medulloblastoma, rhabdomyosarcoma, glioma as well as breast, colorectal, pancreatic and prostate cancer (Jiang and Hui, 2008; Teglund and Toftgard, 2010). Therefore, a comprehensive understanding of the biology and pathology of the Hh pathway is essential for the development of treatments of these diseases.
The Hh pathway controls the activity of the transcription factor Cubitus interruptus (Ci; Gli in mammals) (Aza-Blanc et al., 1997). In the absence of Hh, the transmembrane protein Patched (Ptc; Ptch1 in mammals) keeps another transmembrane protein, Smoothened (Smo), in intracellular vesicles (Denef et al., 2000; Ingham et al., 2000; Nakano et al., 2004; Stegman et al., 2004). The absence of Smo enables several kinases including PKA, GSK-3 and CK1 to phosphorylate Ci (Chen et al., 1998; Price and Kalderon, 1999; Price and Kalderon, 2002; Sisson et al., 2006; Zhang et al., 2005) and mark it for ubiquitylation by the Slimb ubiquitin ligase (Jia et al., 2005; Jiang and Struhl, 1998; Noureddine et al., 2002; Ou et al., 2002; Smelkinson and Kalderon, 2006; Smelkinson et al., 2007). Ubiquitylation triggers partial degradation of newly synthesized full length Ci of 155kD (Ci155) to a 75kD protein (Ci75) that acts as a transcriptional repressor of Hh gene targets (Aza-Blanc et al., 1997; Methot and Basler, 1999; Wang and Price, 2008). This proteolysis occurs in a protein complex composed of Ci, the protein kinase Fused and Costal-2 (Cos2) which is a kinesin-like protein with similarity to Kif-7 in mammals (Cheung et al., 2009; Endoh-Yamagami et al., 2009; Farzan et al., 2008; Ogden et al., 2004; Robbins et al., 1997; Ruel et al., 2007; Sisson et al., 1997; Wang and Holmgren, 2000). Upon binding of Hh to its receptor Ptc, Smo translocates to the plasmamembrane and interacts with Cos2 to release full length Ci (Ci155). The release of Ci155 is triggered by phosphorylation of Cos2 and Smo by Fused (Jia et al., 2003; Liu et al., 2007; Lum et al., 2003; Nybakken and Perrimon, 2002; Nybakken et al., 2002; Ruel et al., 2007; Ruel et al., 2003; Zhu et al., 2003). Ci155 can now act as a transcriptional activator of Hh target genes.
Genetically, ptc, cos2 and PKA are negative regulators of the Hh pathway. Loss of these genes results in accumulation of Ci155 and promotes ligand-independent, deregulated Ci activity (Chen and Struhl, 1996; Jiang and Struhl, 1995; Li et al., 1995; Pan and Rubin, 1995; Sisson et al., 1997; Thomas and Ingham, 2003; Wang and Holmgren, 1999). Similarly, in humans, ligand-independent Gli-induced tumors are caused by loss of Ptch1 or by gain-of-function mutations of Smo. Gain-of-function mutations of Gli transcription factors can also contribute to tumors, most notably glioma (Jiang and Hui, 2008; Teglund and Toftgard, 2010).
Hh signaling is crucial for development of the Drosophila compound eye, which depends on a changing balance of proliferation and differentiation (Baker, 2007; Carthew, 2007; Roignant and Treisman, 2009). During the first two larval stages, the eye-antennal imaginal disc proliferates extensively (Carthew, 2007; Wolff and Ready, 1991a). In the 3rd larval stage (L3), cells at the posterior edge of the eye disc form a groove, called the morphogenetic furrow (MF) (Wolff and Ready, 1991a). For the following two days, the MF moves anteriorly across the eye disc. Cells at the MF arrest proliferation and the first five photoreceptor neurons per ommatidium begin to differentiate (Baker, 2007; Carthew, 2007; Roignant and Treisman, 2009). While the MF moves on, the remaining cells undergo one additional round of proliferation (second mitotic wave), before they permanently arrest proliferation and differentiate into additional photoreceptor neurons, cone, pigment and bristle cells (Baker, 2007; Carthew, 2007; Roignant and Treisman, 2009). After the MF stops in the early pupal stage, the cells anterior to the MF differentiate into head cuticle.
Hh signaling is required for movement of the MF across the eye disc (Heberlein et al., 1995; Heberlein et al., 1993). Photoreceptor neurons posterior to the MF express Hh, which induces decaplentaplegic (dpp) expression (Greenwood and Struhl, 1999; Heberlein et al., 1993). Both Hh and Dpp diffuse to anteriorly located cells which Dpp arrests in G1 (Firth and Baker, 2005; Horsfield et al., 1998). In turn, these cells start to differentiate and produce Hh, just pushing the MF further anteriorly. Posterior to the MF, Hh promotes the second mitotic wave through expression of the Notch ligand Delta and thus Notch signaling (Baonza and Freeman, 2005; Firth and Baker, 2005). Thus, the Hh pathway is needed for the transition from proliferating to differentiating state of the eye disc, making it a critical target for homeostasis.
Hh signaling is also known to regulate proliferation (Chanut and Heberlein, 1995; Duman-Scheel et al., 2002; Heberlein et al., 1995). Consistently, we show here that deregulated, ligand-independent Hh signaling due to loss of the negative regulators cos2 and ptc causes overgrowth phenotypes of mosaic eyes and heads. Paradoxically, however, cos2 and ptc mutant cells have a growth-disadvantage and are eventually eliminated by apoptosis. In mosaic discs, proliferation is increased at the border to adjacent cos2+ tissue suggesting that the overgrowth is mediated through induction of non-cell autonomous proliferation. This effect is position-dependent and restricted to cos2 clones in or anterior to the MF. Finally, we demonstrate that cos2 clones not only cause non-cell autonomous precocious proliferation, but also non-cell autonomous differentiation. Together, these non-cell autonomous growth and differentiation phenotypes in the Drosophila eye model reveal another strategy by which oncogenes may generate a supportive micro-environment for tumor growth.
2. Results
2.1. cos2 mosaics display non-cell autonomous overgrowth
In a mutagenesis screen (see Experimental Procedures), we isolated three independent alleles of the Hh pathway gene costal-2 (cos2). In mosaics induced by the ey-FLP/FRT system, the three alleles behaved similarly and generated overgrowth of the eye (shown for one allele in Fig. 1A, B). Surprisingly, when comparing the relative representation of cos2 mutant tissue (marked in white due to loss of the white+ (w+) pigment transgene) and the wild-type or heterozygous tissue (referred to as cos2+ and marked in red due to the presence of the w+ pigment transgene), we noted that nearly the entire overgrown eye was red, i.e., cos2+ (Fig. 1 A, B), suggesting that this overgrowth was non-cell autonomous. Often, the red eyes had small portions of white cos2 mutant tissue, indicating that the cos2 clones were viable, but had a growth disadvantage over cos2+ tissue. In these mosaic eyes, the ommatidia were frequently roughened and expanded along the anterior margin even when this region was red (and therefore cos2+, Fig. 1A, B).
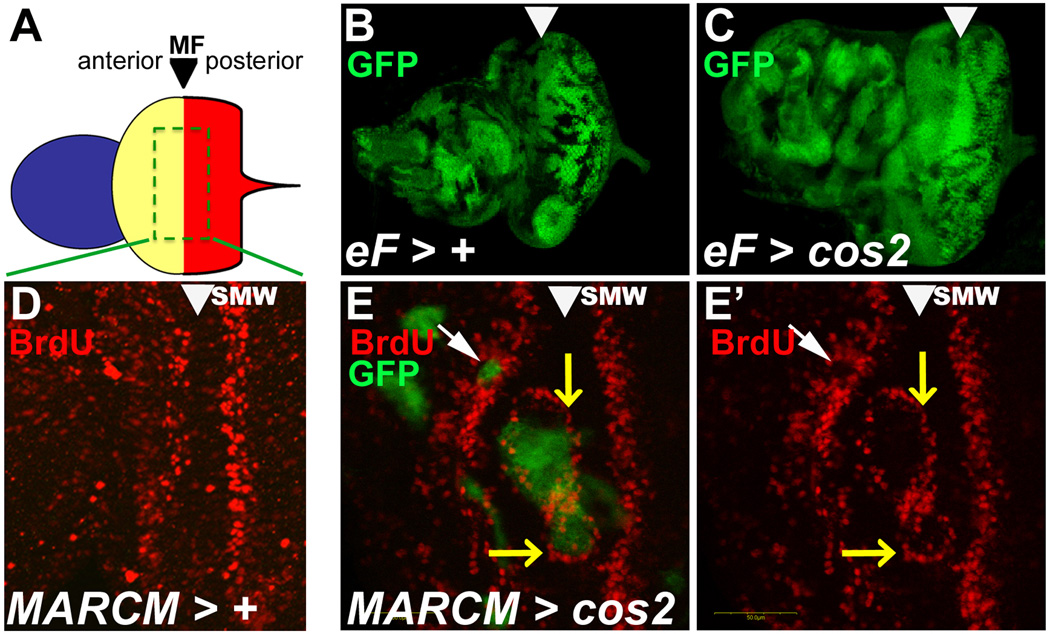
Figure 1. Non-cell autonomous overgrowth of eye and head cuticle in mosaics with deregulated Hh signaling.
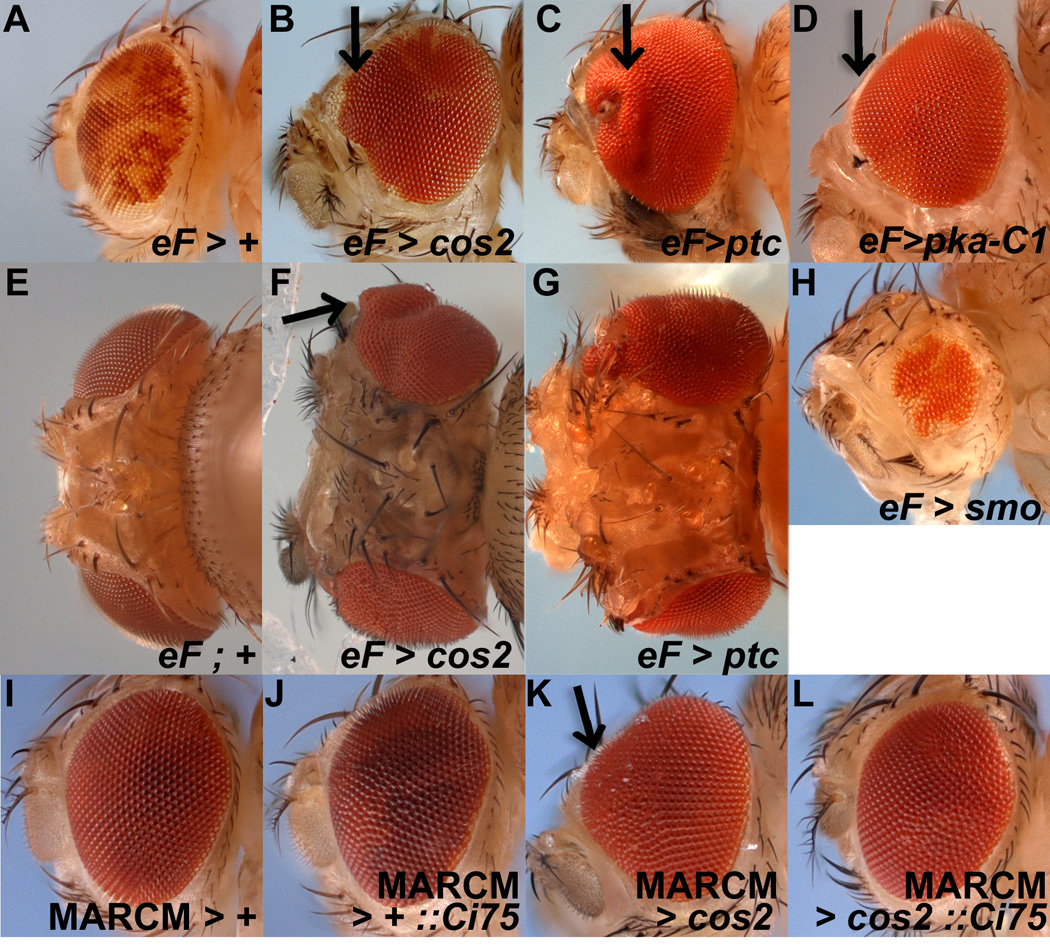
Anterior is to the left and images are from females. Mosaics were obtained by ey-FLP (eF) induced mitotic recombination, symbolized by >. MARCM-induced expression of transgenes such as Ci75 is symbolized by ::.
(A–H) Wild-type tissue is marked with red (w+) eye pigment while mutant tissue is white (w−). In wild type mosaics (A), the eye is a mixture of red and white eye tissue. In cos2 (B,C) and pka-C1 mosaics (D), not only are the eyes overgrown, but most of the pigment is red, i.e. cos2+ and pka-C1+, indicating that mostly wild type tissue contributes to the overgrown tissue. The overgrowth is most apparent at the anterior margin especially at the midline (arrows) of the eye (see also K). Some white tissue is apparent in the eye as well, indicating that the clones can survive, but have a growth disadvantage. Compared to controls (E), head cuticle also overgrows in cos2 (F) and ptc (G) mosaics. Reduced Hh signaling in smo mosaics (H) results in eyes of reduced size.
(I–L) Expression of Ci75 in cos2 mutant clones using the MARCM system suppresses the overgrowth in cos2 mosaics (K,L). Ectopic expression of Ci75 by itself in wild-type clones does not alter the size of the eye (J).
- y w ey-FLP; FRT42D P[w+] / FRT42D.
- y w ey-FLP; FRT42D P[w+] / FRT42D cos2P50.
- y w ey-FLP; FRT42D P[w+] / FRT42D ptcS2.
- y w ey-FLP; P[w+] FRT40 / P[y+] pka-C1K2 FRT40.
- y w ey-FLP; FRT42D P[w+] / CyO.
- y w ey-FLP; FRT42D P[w+] / FRT42D cos2H29.
- y w ey-FLP; FRT42D P[w+] / FRT42D ptcS2.
- y w ey-FLP; P[w+] FRT40 / P[y+] smo3 FRT40.
- y w hs-FLP; FRT42 P[tubP-GAL80]/FRT42; P[tubP-GAL4]/+.
- y w hs-FLP; FRT42 P[tubP-GAL80]/FRT42; P[tubP-GAL4]/UAS-Ci75.
- y w hs-FLP; FRT42 P[tubP-GAL80]/FRT42 cos2H29 ; P[tubP-GAL4]/+.
- y w hs-FLP; FRT42 P[tubP-GAL80]/FRT42 cos2H29 ; P[tubP-GAL4]/UAS-Ci75.
In addition to the effect on the eye itself, overgrowth was also seen in head cuticle and the antennae (Fig. 1E, F). Like the eye, the head and antennal structures developed (often with duplication of structures), indicating that differentiation occurred in these mosaics. Indeed, similar overgrowths with pattern duplications were seen in leg tissues (Suppl. Fig. S1) as well as wing tissues (Sisson et al., 1997) when those developing tissues were mosaic for cos2, indicating that the overgrowth was not an eye-specific effect.
2.2. The Hh pathway modulates proliferation non-cell autonomously
Overgrowth phenotypes of the eye have been seen with mutants that increase Hh signaling including ptc (Chanut and Heberlein, 1995; Ma and Moses, 1995; Wehrli and Tomlinson, 1995), pka-C1 (Johnson et al., 1995; Pan and Rubin, 1995; Shyamala and Bhat, 2002; Strutt and Mlodzik, 1997; Strutt et al., 1995) and ectopic expression of hh (Heberlein et al., 1995), while mutations that reduce Hh signaling such as smo have the opposite effect (Strutt and Mlodzik, 1997). These phenotypes have been attributed to altered regulation of the formation of the MF in the eye imaginal disc and the subsequent precocious formation of ommatidia. However, the potential non-cell autonomy of these overgrowth phenotypes as observed in cos2 mosaics has not been reported.
To determine whether the non-cell autonomy was a cos2-specific effect or general phenotype of deregulated Hh signaling, we tested mutants of other negative regulators of the Hh pathway for similar non-cell autonomous phenotypes. Like cos2, ey-FLP-induced mosaics of ptc and pka-C1 display non-cell autonomous overgrowth as seen by the predominantly red mosaic eye with the greatest effect at the anterior midline of the eye (Fig. 1C, D). Despite the overgrowth of ptc mosaic eyes, we never recovered any ptc mutant clones in adult flies indicating a strong non-autonomous effect. These mosaics also generated overgrowth of the head cuticle and antennae similar to cos2 (shown for ptc in Fig. 1G).
In contrast to these mutants, mosaic loss of smoothened (smo), a gene required for Hh signaling, in the developing eye formed eyes that were smaller with over-represented smo+ (red) tissue (Fig. 1H). smo mutant tissue is present, but small presumably due to increased cell death (Vrailas and Moses, 2006). However, eyes less than half the size of wild type suggests that the loss of smo mutant cells cannot be compensated for by new proliferation as it does in mosaics with cell-lethal mutations (see for example Fig. 3B). This observation implies that, in smo mosaics, a Hh-derived signal is missing that is required to increase proliferation of the adjacent tissue. In summary, these observations support the notion that Hh signaling controls cell proliferation non-cell autonomously during eye development. Because cos2 mosaics showed the strongest non-cell autonomous effects, we largely focus on cos2 for the characterization of the non-cell autonomous phenotypes.
Figure 3. Neither cos2 nor ptc mutant tissue is sufficient for overgrowth.
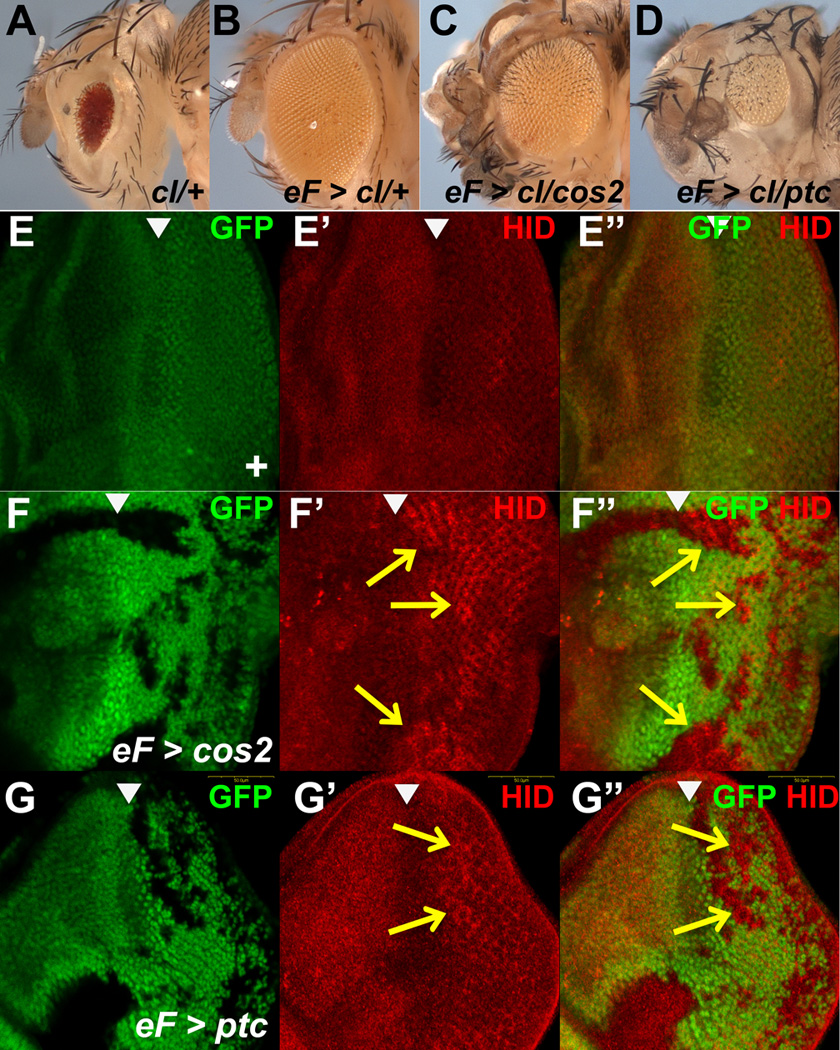
(A–D) Shown are fly eyes obtained by the cell-lethal method. (A) In the unflipped control, the GMR-hid transgene ablates most heterozygous cells and provides red eye pigment. (B) In trans to a wild type chromosome, the twin spot cells survive and re-populate the eye with a few red surviving cells from the dominant marker (GMR-hid). (C,D) Neither cos2 nor ptc mutant tissue generate a full sized adult eye. Especially ptc mutant eyes are significantly reduced in size (D).
(E–G) Anti-Hid antibody labelings of control (E), cos2 (F) and ptc (G) mosaic eye discs obtained by ey-FLP-induced mitotic recombination. In cos2 and ptc mutant clones posterior to the MF, Hid levels are elevated (arrows).
- (A) y w; FRT42 GMR-hid l(2)CL-R/+; ey-GAL4 UAS-FLP
- (B) y w; FRT42 GMR-hid l(2)CL-R/FRT42; ey-GAL4 UAS-FLP
- (C) y w; FRT42 GMR-hid l(2)CL-R/FRT42 cos2H29 y+; ey-GAL4 UAS-FLP
- (D) y w; FRT42 GMR-hid l(2)CL-R/FRT42 ptcC y+; ey-GAL4 UAS-FLP
- (E) y w ey-FLP; FRT42 ubi-GFP/CyO
- (F) y w ey-FLP; FRT42 cos2H29 /FRT42 ubi-GFP
- (G) y w ey-FLP; FRT42 ptcC /FRT42 ubi-GFP
2.3. cos2 acts via the canonical Hh pathway to promote non-cell autonomous proliferation
Next, we asked if cos2 regulates non-cell autonomous proliferation through the canonical Hh pathway, i.e., through Cubitus interruptus (Ci). Activation of the Hh signaling pathway normally blocks the proteolytic processing of this transcription factor, retaining the active, full-length Ci protein (Ci155), while preventing the formation the transcriptional repressor form (Ci75; see Introduction). Recent work in vertebrate systems has indicated that Hh, Ptc and their homologues can act independently of Gli transcription, i.e., in a non-canonical manner (Jenkins, 2009). We examined whether the overgrowth phenotype of cos2 mosaics is mediated through the canonical pathway, i.e., through Ci155. First, it has been reported that Ci155 accumulates in cos2 clones (Methot and Basler, 2000; Sisson et al., 1997; Wang et al., 2000; Wang and Holmgren, 1999; Wang and Holmgren, 2000) suggesting that proteolytic processing of Ci155 is blocked due to loss of Cos2 function.
Second, we determined if expression of the repressor form Ci75 can suppress the overgrowth phenotype of cos2 mosaics using the MARCM system (Lee and Luo, 2001) (Experimental Procedures). Expression of Ci75 in otherwise wild-type clones did not significantly affect eye size, although some disruption of the patterning of the ommatidia is apparent (Fig. 1J). In contrast, expression of Ci75 in cos2 mutant clones suppresses the overgrowth phenotype of cos2 mosaics (compare Fig. 1K and 1L). These results demonstrates that overexpression of Ci75 can overcome the accumulation of Ci155 and implies that the overgrowth of cos2 mosaics is due to the failure to convert Ci155 to the repressor form Ci75 in the mutant tissue. Thus, cos2 acts via the canonical Hh/Ci pathway to regulate growth.
2.4. cos2 mosaics induce proliferation at and anterior to the MF
We examined ey-FLP-induced cos2 mosaic eye-antennal imaginal discs from 3rd instar larvae. ey-FLP induces clones throughout the disc, i.e., in the most anterior region that forms the antenna, in the central region that forms the head capsule, and in the posterior region that represent the eye field (Fig. 2A, B). In cos2 mosaics, mutant clones are visible in all three regions of the disc, and all three regions are clearly overgrown relative to control discs of matched age (Fig. 2C). Noticeably, this overgrowth is predominantly GFP+ and therefore consists of mostly cos2+ cells, indicating that the mosaic loss of cos2 has a non-cell autonomous effect on inducing overgrowth.
Figure 2. Ectopic proliferation in cos2 mosaics eye disc at or anterior to the MF.
Shown are eye-antennal imaginal discs from 3rd instar larvae. The MF is marked by arrowheads. Clones were induced by ey-FLP (eF) induced mitotic recombination, symbolized by >.
(A) Schematic view of the eye-antennal disc. Anterior (blue) forms the antenna. Central (yellow) forms the head capsule, and posterior (red) forms the eye field which are separated by the morphogenetic furrow (MF).
(B,C) Size comparison of age-matched control (B) and cos2 mosaic discs (C). Although mutant clones (marked by the absence of GFP) are smaller in cos2 mosaics, the mosaic discs are larger and often deformed compared to control discs.
(D) The normal BrdU pattern in wild-type discs. SMW – second mitotic wave.
(E,E’) cos2 clones, positively marked by GFP due to MARCM, located in the MF stimulate BrdU incorporation at the boundary of the clone (white arrowhead). Clones anterior to the MF (yellow arrow) have reduced BrdU labeling.
- (B) y w ey-FLP; FRT42 ubi-GFP/FRT42
- (C) y w ey-FLP; FRT42 ubi-GFP/FRT42 cos2H29
- (D) y w hs-FLP UAS-mCD8-GFP; FRT42 cos2H29 /CyO; tubP[GAL4]/+
- (E,E’) y w hs-FLP UAS-mCD8-GFP; FRT42 tubP[GAL80]/FRT42 cos2H29 ; tubP[GAL4]/+
We examined cos2 clones in eye discs using BrdU incorporation as marker for proliferation to determine if cos2 could non-cell autonomously alter proliferation in cos2+ cells. Normally, proliferation occurs in two waves of cells – a broad asynchronous wave anterior to the MF and a synchronous wave posterior to the MF (second mitotic wave, SMW) (Fig. 2D). Cells in the MF are cell-cycle arrested in G1 (Firth and Baker, 2005; Horsfield et al., 1998). cos2 clones crossing the MF are associated with additional BrdU incorporation at the boundary of the clones (Fig. 2E, E’; yellow arrows). Within mutant clones located anterior to the MF, there is a marked decrease in BrdU labeling, indicating that many of these cells are not synthesizing DNA (Fig. 2E, E’; white arrows). However, there may be an increase in DNA synthesis in the cos2+ tissue adjacent to these clones as well, suggesting a non-cell autonomous effect on proliferation in cos2 mosaic discs. Clones crossing the SMW do not show changes in BrdU labeling.
In summary, cells within the cos2 clones have reduced levels of proliferation, while cos2+ cells adjacent to them increase their proliferation rate providing further support for the non-cell autonomous effects on proliferation seen in the cos2 mosaic flies.
2.5. Eyes predominantly mutant for cos2 and ptc have a growth disadvantage
To further characterize the non-cell autonomous phenotypes of cos2 and ptc mutants, we generated predominantly mutant eye discs by eliminating all wild-type and heterozygous cells using the ey-FLP cell-lethal method (Stowers and Schwarz, 1999). This method eliminates potential cell-cell signaling between mutant and non-mutant tissue which is necessary for non-cell autonomous interactions. Specifically, eyes are generated that are predominantly composed of mutant tissue because the homozygous twin spot dies due to a cell-lethal mutation and heterozygous tissue is removed using the dominant expression of the pro-apoptotic gene hid from an eye-specific driver (GMR-hid) (Stowers and Schwarz, 1999). The remaining tissue is homozygous for the chromosome arm which carries the mutation of interest. If this chromosome is wild-type, the tissue loss by the cell-lethal mutation and GMR-hid is completely compensated by new proliferation, generating a normal eye that is phenotypically white− (w−) due to loss of the cell-lethal clones (phenotypically w+) and heterozygous GMR-hid tissue (w+) (Fig. 3B). However, when that chromosome arm carries a mutation in either cos2 or ptc, the adult eye is reduced in size, which is particularly dramatic for ptc (Fig. 3C, D) and similar to viable ptc heteroallelic combinations with reduced levels of ptc (Thomas and Ingham, 2003). Thus, autonomously, cos2 and ptc are growth-impaired. In contrast, overgrowth of head cuticle and antennae is still observed (Fig. 3C, D). Because GMR-hid is not expressed in head and antenna, heterozygous cells survive in these tissues and can receive the signal for proliferation by mutant cells (cos2, ptc). Therefore, this analysis further supports the notion that the overgrowth of cos2 and ptc mosaics is due to non-cell autonomous interactions between mutant and non-mutant tissue which occurs in the antennae and head cuticle, but not in the eye in this mutant background.
We analyzed both cos2 and ptc mosaics for changes in gene expression associated with cell death. Protein levels of the pro-apoptotic protein Hid (Grether et al., 1995) are elevated posterior to the MF in both cos2 and ptc clones (Fig. 3F,G). Interestingly, Hid levels are not or only weakly increased in mutant clones anterior to the MF (Fig. 3F,G). In summary, the reduction of the size of predominantly mutant ptc and cos2 eyes (Fig. 3C,D) and the underrepresentation of mutant clones in mosaic eyes (Fig. 1A–D) is likely due to a combination of Hid-induced apoptosis and reduced proliferation (Fig. 2E).
2.6. cos2 mosaics induces precocious autonomous and non-cell autonomous differentiation
We also examined cos2 clones for changes in differentiation using antibodies to ELAV as markers for the developing photoreceptor neurons, which form in and posterior to the MF (Fig. 4A). In cos2 clones crossing the MF, an expansion of ELAV staining anterior to the furrow was observed (Fig. 4B’), suggesting that these cells were differentiating precociously. This expansion was attributed to altered and ectopic formation of the MF in the more anterior part of the disc as seen with other mutants in Hh signaling (Chanut and Heberlein, 1995; Ma and Moses, 1995; Ma et al., 1993; Pan and Rubin, 1995; Strutt and Mlodzik, 1997; Strutt et al., 1995; Wehrli and Tomlinson, 1995). However, in addition to the precocious autonomous differentiation in cos2 clones, ELAV staining was detected outside of the clonal boundary in regions adjacent to the MF (Fig. 4B’, see inset), indicating that the cos2 mutant cells were also able to promote differentiation non-cell autonomously. Evidence for non-cell autonomous precocious differentiation has previously been provided in pka mosaics (Strutt et al., 1995). cos2 clones located posterior to the MF differentiated normally and formed ELAV-positive clusters of developing photoreceptors independent of cos2 levels.
Figure 4. cos2 mutants generate precocious, autonomous and non-autonomous differentiation in the eye imaginal disc.
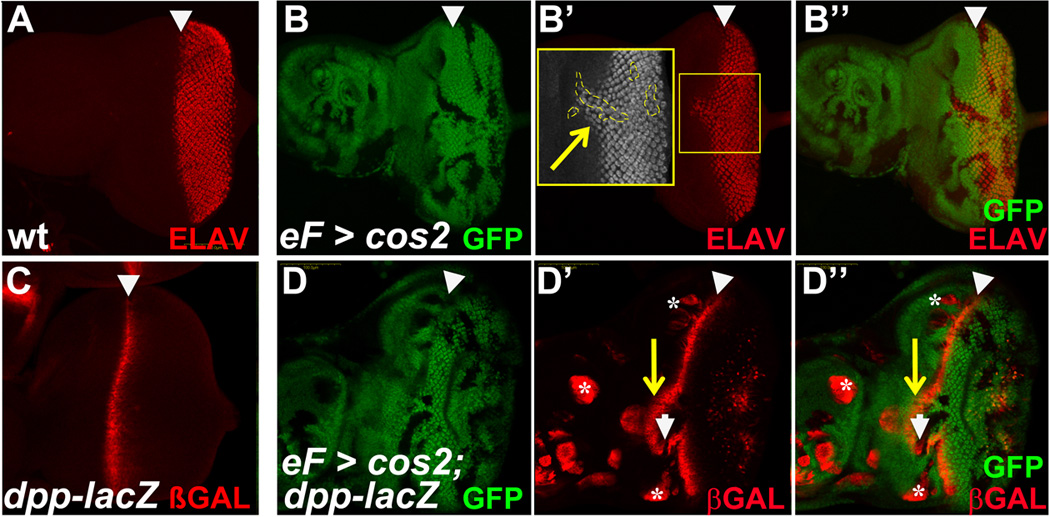
(A) ELAV marks the differentiating neuronal tissue posterior to the MF in control discs.
(B) In ey-FLP-induced cos2 mosaics, ELAV expression does extend anterior to the MF. This precocious expansion is associated with mutant clones, but is also seen in adjacent cos2+ tissue (shown in inset outlined in dashed line). ELAV-labeling is not grossly altered in cos2 clones posterior to the MF.
(C) dpp-lacZ marks the MF in wild-type eye discs.
(D) In ey-FLP-induced cos2 mosaics, multiple changes in dpp-lacZ expression are observed. The MF is shifted anterior around clones crossing the MF (arrow in D’). These clones reduce expression of dpp-lacZ (arrowhead). In contrast, clones further anterior and not associated with the MF autonomously induce dpp-lacZ expression (asterisk). β-GAL staining posterior to the MF is due to a weak GMR-lacZ reporter on the ey-FLP chromosome.
- (A) y w ey-FLP; FRT42 ubi-GFP/CyO
- (B) y w ey-FLP; FRT42 cos2H29 /FRT42 ubi-GFP
- (C) y w ey-FLP; ubi-GFP FRT40/CyO; dpp-lacZ
- (D) y w ey-FLP GMR-lacZ; FRT42 cos2H29 /FRT42 ubi-GFP; dpp-lacZ
Because the precocious differentiation was attributed to the altered and ectopic formation of the MF, we examined how the MF was modified in cos2 mosaics. We used the dpp-lacZ reporter, which is expressed along the developing MF in wild type discs (Fig. 4C). In cos2 mosaics, dpp-lacZ expression was significantly altered in a position-dependent manner in eye-antennal disc (Fig. 4D,D’). As predicted, the expression of dpp-lacZ was shifted towards the anterior by cos2 clones that cross the MF (yellow arrow in Fig. 4D’,D’’). In these clones, there is little to no dpp-lacZ expression detectable (white arrow in Fig. 4D’,D’’). However, in clones located anterior to the MF including in the antenna portion, loss of cos2 led to ectopic autonomous expression of dpp-lacZ in the mutant tissue (asterisks in Fig. 4D’,D’’). Ectopic expression of dpp-lacZ in these clones was not associated with ELAV expression, indicating that the levels of dpp expression in clones not in contact with the MF are usually not high enough for precocious differentiation. cos2 clones posterior to the MF do not induce dpp-lacZ expression.
3. Discussion
3.1. Mosaics with deregulated Hh signaling cause non-autonomous overgrowth
Signal-independent, deregulated Hh signaling causes cancer in humans and overgrowth in Drosophila (Chanut and Heberlein, 1995; Heberlein et al., 1995; Ma and Moses, 1995; Pan and Rubin, 1995; Strutt and Mlodzik, 1997; Strutt et al., 1995; Teglund and Toftgard, 2010; Wehrli and Tomlinson, 1995). However, the autonomous/non-cell autonomous nature of this overgrowth has not been determined. We show here that in mosaics with deregulated Hh signaling through loss of cos2, ptc or PKA-C1, the overgrown eye and head tissues are largely composed of non-mutant (heterozygous and wild-type) cells, i.e., non-cell autonomously, while the mutant cells themselves do not or only weakly contribute to the overgrowth. Instead, cos2 and ptc mutant clones do not proliferate and are sensitive to apoptosis as evidenced by increased Hid protein levels within clones and the small size of the eye in the cell-lethal background.
Non-cell autonomous overgrowth has also been observed for mosaics of hyperplastic discs, which encodes an ubiquitin ligase. In this case, the overgrowth has been attributed to a combined deregulation of Hh and Dpp signaling (Lee et al., 2002). Mutants outside of Hh signaling that cause non-cell autonomous overgrowth in genetic mosaics affect genes involved in ESCRT (endosomal sorting complex required for transport) function such as vacuolar sorting protein (vps) 20, vps22, vps23, vps25, vps28 and vps32 as well as the E1 ubiquitin-activating enzyme Uba1 (Herz and Bergmann, 2009; Herz et al., 2006; Herz et al., 2009; Lee et al., 2008; Moberg et al., 2005; Pfleger et al., 2007; Thompson et al., 2005; Vaccari and Bilder, 2005; Vaccari et al., 2009). Loss of ESCRT function results in accumulation of endocytosed cell surface proteins at the endosome resulting in deregulation of many signaling pathways (Herz et al., 2006; Moberg et al., 2005; Thompson et al., 2005; Vaccari and Bilder, 2005; Vaccari et al., 2009). Uba1 mutations affect every ubiquitin-dependent process in the cell including inactivation of cell signaling receptors. What these mutants all have in common is that they cause deregulation of cell signaling pathways.
Our data are in apparent contrast to a previous study which showed that cells with increased Ci activity regulate growth autonomously (Duman-Scheel et al., 2002). However, the experimental conditions used in that study are significantly different from ours. These authors strongly overexpressed Ci155 using the binary Gal4/UAS system. In our study, we use cos2 mutants which also causes accumulation of Ci155, but these levels are dependent on expression from the endogenous ci gene and do not reach the unphysiologically high levels resulting from binary expression of a Ci155 transgene.
3.2. Position-dependent effects in cos2 mosaics
Our results demonstrate that cos2, a regulator of the Hh pathway, acts in several distinct ways in the eye imaginal disc. Normally, as previously shown for the Hh pathway (Heberlein et al., 1995; Heberlein et al., 1993), cos2 regulates the progression of the MF as shown by precocious photoreceptor differentiation and dpp-lacZ expression associated with cos2 clones (Fig. 4). Furthermore, cos2 mutant clones promote proliferation non-cell autonomously as seen by the predominantly wild type tissue in the adult mosaic eyes (Fig. 1) and the changes in BrdU labeling (Fig. 2). cos2 is also required for the viability of cells in the developing eye (Fig. 3).
The regulation of these processes is position-dependent within the developing eye disc and involves both autonomous and non-cell autonomous effects. Our analysis shows that increased proliferation is detectable at the border with neighboring non-mutant cells when cos2 mutant clones are located either at or anterior to the MF. We did not detect any effect on proliferation in cos2 clones posterior to the MF. Therefore, because under normal developmental conditions, Hh signaling is required for MF progression anterior to the MF, the position-dependence of deregulated Hh activity at and anterior to the MF implies that only tissue in which Hh signaling is normally activated responds to deregulation of it, which was already noted by (Heberlein et al., 1995; Lebovitz and Ready, 1986; Ma et al., 1993). Other tissues are inert to deregulated Hh signaling. These findings explain why in the resulting adult mosaic flies, the overgrowth extends preferentially anteriorly of the eye and the head cuticle which is specified anterior to the MF (Fig. 1).
While proliferation and differentiation occurs anterior to the MF, cos2 and ptc mutant clones posterior to the MF accumulate levels of the pro-apoptotic Hid protein in an autonomous manner. cos2 mutant cells can survive to adulthood (Fig. 1A), but are strongly underrepresented compared to non-mutant cells. ptc mutant cells are completely absent in mosaic eyes (Fig. 1C). Therefore, these mutant cells are sensitive to apoptotic signaling and many of them are eliminated. This elimination could be due to cell competition (Baker, 2011; Johnston, 2009; Tamori and Deng, 2011). However, even by removing the cell competitive environment using the cell-lethal method, predominantly mutant cos2 and ptc eyes are strongly reduced in size (Fig. 3C, D) suggesting that the underrepresentation of cos2 and ptc mutant clones is not solely due to cell competition. It is more likely that the autonomous loss of cos2 and ptc is a combination of reduced proliferation and increased sensitivity to apoptotic signals. However, the same can be said about cells that have lost Hh activity: they are also upregulating hid and die by cell death (Werz et al., 2005; Vrailas and Moses, 2006). It is not uncommon for cells with the incorrect developmental information to increase expression of hid and die both in embryos and imaginal discs (Werz et al., 2005; Wolff and Ready, 1991b).
3.3. Significance for Hh-induced human cancers
Ligand-independent Hh signaling is associated with a subset of human tumors such as basal cell carcinoma, medulloblastoma, rhabdomyosarcoma, glioma as well as breast, colorectal, pancreatic and prostate cancer (reviewed in (Teglund and Toftgard, 2010). Many of the affected tissues in these tumors require sonic Hh (Shh) signaling for normal homeostasis. For example, in skin, Shh signaling is required for maintaining stem cell population, and for regulating hair f ollicle and sebaceous gland development (Athar et al., 2006). In the cerebellum, Shh controls proliferation of cerebellar cortical cells and therefore the overall size of the tissue (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). Deregulation of Shh signaling in these tissues by losing Ptch1 or increasing Smo and Gli functions can cause basal cell carcinoma and medulloblastoma (Athar et al., 2006; Teglund and Toftgard, 2010). However, it is unknown if these human malignancies are caused by autonomous or non-cell autonomous interactions. Therefore, our findings in Drosophila that ligand-independent Hh signaling causes non-cell autonomous proliferation may be significant for understanding of these tumors in humans. For example, ligand-independent signaling in these tumors may be needed to promote a supportive microenvironment for tumor growth. It would also be interesting to determine why tissues are inert to deregulated Hh signaling if they do not require Hh signaling for normal homeostasis such as the tissue posterior to the MF in the fly eye. Future studies may help identifying such principles and aid in developing therapies for treatment of these diseases.
4. Experimental Procedures
4.1. Fly stocks
The new cos2 (cos2H29, cos2L51, cos2P50) and ptcC alleles were isolated in a mutagenesis screen for modifiers of a cell death phenotype and will be published elsewhere (manuscript in preparation). Other mutants and transgenes used are: pka-C1B3, pka-C1K2, UAS-Ci75 (kind gift of Dan Kalderon); smo3 (kind gift of Mardelle Atkins); dpp-lacZ, ptcS2 and all ey-FLP/FRT, hs-FLP/FRT, MARCM and FRT cell-lethal stocks were obtained from the Bloomington Drosophila stock center in Bloomington, IN.
4.2. Mosaics
Mosaics were induced using several techniques. Generally, we used the FLP/FRT system with hs-FLP (Xu and Rubin, 1993) or ey-FLP (Newsome et al., 2000) as the enzymatic source and marking the non-mutant tissue using either ubi-GFP to express GFP in the larval tissue or P[w+] to generate red eye pigment in adults in a w− background. Mosaics were also generated using the MARCM (mosaic analysis using a repressible cell marker) technique which allows expression of transgenes such as UAS-Ci75 in mutant clones (Lee and Luo, 2001). Mosaics were induced by heat shocking 1st instar larvae 1 hour at 37°C. For MARCM analysis, a second heat shock in 2nd instar was added.
4.3. Immunohistochemistry
Imaginal discs were dissected from 3rd instar larvae and stained using standard protocols. Antibodies to the following primary antigens were used: BrdU (BD Biosciences); ELAV and Ci (Developmental Studies Hybridoma Bank); HID (kind gift from Hong Dong Ryoo); β-GAL (Promega). Cy3-conjugated anti-guinea pig and anti-mouse (Jackson ImmunoResearch) and AlexaFluor 546-conjugated anti-mouse and anti-rabbit (Invitrogen) were used as secondary antibodies.
4.4. Imaging
Adult eyes, heads and legs were imaged using a Zeiss AxioImager using CZ projection software. Legs were collected in ethanol:glycerol then mounted in isopropanol for imaging. GraphPad Prism 5 was used for graphing in combination with Image J software. Confocal images were taken using either an Olympus Fluoview 500 or Fluoview 1000 Laser Confocal Microscope and digital images processed using the associated software. Figures were assembled using Adobe Photoshop.
Supplementary Material
Supplemental Figure S1. Overgrowth with pattern duplication is apparent in the legs. Genotype: y w hs-FLP; FRT42 ubi-GFP / FRT42 cos2H29
Highlights.
Signal-independent, deregulated Hh signaling inhibits apoptosis non-autonomously
Notch signaling is used as a relay mechanism
Hh-induced Notch signaling induces expression of an IAP non-autonomously
Non-autonomous control of apoptosis by increased Hh activity is position-dependent
Acknowledgments
We are grateful to our colleagues who have shared their knowledge and resources, especially Konrad Basler, Hugo Bellen, Steve Cohen, Phil Ingham, Georg Halder, Dan Kalderon, Graeme Mardon, Pascal Meier, Hyung Don Ryoo, the Bloomington Stock Center in Indiana, and the Developmental Studies Hybridoma Bank in Iowa. We like to thank Jillian Lindblad and Jake Hernandez for excellent technical assistance. J. Henri Bayle improved the quality of the manuscript. This research was supported in part by the Cancer Center Support Grant CA #16672 to the DNA Analysis Facility. AB is grateful for support by the NIH (GM068016).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Athar M, Tang X, Lee JL, Kopelovich L, Kim AL. Hedgehog signalling in skin development and cancer. Exp Dermatol. 2006;15:667–677. doi: 10.1111/j.1600-0625.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Baker NE. Patterning signals and proliferation in Drosophila imaginal discs. Curr Opin Genet Dev. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Baker NE. Cell competition. Curr Biol. 2011;21:R11–R15. doi: 10.1016/j.cub.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Baonza A, Freeman M. Control of cell proliferation in the Drosophila eye by Notch signaling. Dev Cell. 2005;8:529–539. doi: 10.1016/j.devcel.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Carthew RW. Pattern formation in the Drosophila eye. Curr Opin Genet Dev. 2007;17:309–313. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut F, Heberlein U. Role of the morphogenetic furrow in establishing polarity in the Drosophila eye. Development. 1995;121:4085–4094. doi: 10.1242/dev.121.12.4085. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gallaher N, Goodman RH, Smolik SM. Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc Natl Acad Sci U S A. 1998;95:2349–2354. doi: 10.1073/pnas.95.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, Peterson AS. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr. Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Ascano M, Jr, Ogden SK, Sanial M, Brigui A, Plessis A, Robbins DJ. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr. Biol. 2008;18:1215–1220. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev. Cell. 2005;8:541–551. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Wolff T, Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Herz HM, Bergmann A. Genetic analysis of ESCRT function in Drosophila: a tumour model for human Tsg101. Biochem Soc Trans. 2009;37:204–207. doi: 10.1042/BST0370204. [DOI] [PubMed] [Google Scholar]
- Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A. Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS One. 2009;4:e4165. doi: 10.1371/journal.pone.0004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Development. 1998;125:5069–5078. doi: 10.1242/dev.125.24.5069. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Ingham PW. Hedgehog signalling. Curr Biol. 2008;18:R238–R241. doi: 10.1016/j.cub.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr Biol. 2000;10:1315–1318. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Jia J, Tong C, Jiang J. Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes Dev. 2003;17:2709–2720. doi: 10.1101/gad.1136603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Zhang L, Zhang Q, Tong C, Wang B, Hou F, Amanai K, Jiang J. Phosphorylation by double-time/CKIepsilon and CKIalpha targets cubitus interruptus for Slimb/beta-TRCP-mediated proteolytic processing. Dev Cell. 2005;9:819–830. doi: 10.1016/j.devcel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80:563–572. doi: 10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Grenier JK, Scott MP. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–4170. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- Johnston LA. Competitive interactions between cells: death, growth, and geography. Science. 2009;324:1679–1682. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D. The mechanism of hedgehog signal transduction. Biochem Soc Trans. 2005;33:1509–1512. doi: 10.1042/BST0331509. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Ready DF. Ommatidial development in Drosophila eye disc fragments. Dev Biol. 1986;117:663–671. doi: 10.1016/0012-1606(86)90335-0. [DOI] [PubMed] [Google Scholar]
- Lee JD, Amanai K, Shearn A, Treisman JE. The ubiquitin ligase Hyperplastic discs negatively regulates hedgehog and decapentaplegic expression by independent mechanisms. Development. 2002;129:5697–5706. doi: 10.1242/dev.00159. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lee TV, Ding T, Chen Z, Rajendran V, Scherr H, Lackey M, Bolduc C, Bergmann A. The E1 ubiquitin-activating enzyme Uba1 in Drosophila controls apoptosis autonomously and tissue growth non-autonomously. Development. 2008;135:43–52. doi: 10.1242/dev.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ohlmeyer JT, Lane ME, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao X, Jiang J, Jia J. Fused-Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes Dev. 2007;21:1949–1963. doi: 10.1101/gad.1557407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- Ma C, Moses K. Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–831. doi: 10.1016/s0092-8674(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Suppressor of fused opposes hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development. 2000;127:4001–4010. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Nystedt S, Shivdasani AA, Strutt H, Thomas C, Ingham PW. Functional domains and sub-cellular distribution of the Hedgehog transducing protein Smoothened in Drosophila. Mech Dev. 2004;121:507–518. doi: 10.1016/j.mod.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Noureddine MA, Donaldson TD, Thacker SA, Duronio RJ. Drosophila Roc1a encodes a RING-H2 protein with a unique function in processing the Hh signal transducer Ci by the SCF E3 ubiquitin ligase. Dev Cell. 2002;2:757–770. doi: 10.1016/s1534-5807(02)00164-8. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Nybakken K, Perrimon N. Hedgehog signal transduction: recent findings. Curr Opin Genet Dev. 2002;12:503–511. doi: 10.1016/s0959-437x(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Nybakken KE, Turck CW, Robbins DJ, Bishop JM. Hedgehog-stimulated phosphorylation of the kinesin-related protein Costal2 is mediated by the serine/threonine kinase fused. J Biol Chem. 2002;277:24638–24647. doi: 10.1074/jbc.M110730200. [DOI] [PubMed] [Google Scholar]
- Ogden SK, Ascano M, Jr, Stegman MA, Robbins DJ. Regulation of Hedgehog signaling: a complex story. Biochem Pharmacol. 2004;67:805–814. doi: 10.1016/j.bcp.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou CY, Lin YF, Chen YJ, Chien CT. Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 2002;16:2403–2414. doi: 10.1101/gad.1011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Rubin GM. cAMP-dependent protein kinase and hedgehog act antagonistically in regulating decapentaplegic transcription in Drosophila imaginal discs. Cell. 1995;80:543–552. doi: 10.1016/0092-8674(95)90508-1. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Harvey KF, Yan H, Hariharan IK. Mutation of the gene encoding the ubiquitin activating enzyme ubal causes tissue overgrowth in Drosophila. Fly (Austin) 2007;1:95–105. doi: 10.4161/fly.4285. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–4339. doi: 10.1242/dev.126.19.4331. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Treisman JE. Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 2009;53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel L, Gallet A, Raisin S, Truchi A, Staccini-Lavenant L, Cervantes A, Therond PP. Phosphorylation of the atypical kinesin Costal2 by the kinase Fused induces the partial disassembly of the Smoothened-Fused-Costal2-Cubitus interruptus complex in Hedgehog signalling. Development. 2007;134:3677–3689. doi: 10.1242/dev.011577. [DOI] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, Therond PP. Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nat Cell Biol. 2003;5:907–913. doi: 10.1038/ncb1052. [DOI] [PubMed] [Google Scholar]
- Shyamala BV, Bhat KM. A positive role for patched-smoothened signaling in promoting cell proliferation during normal head development in Drosophila. Development. 2002;129:1839–1847. doi: 10.1242/dev.129.8.1839. [DOI] [PubMed] [Google Scholar]
- Sisson BE, Ziegenhorn SL, Holmgren RA. Regulation of Ci and Su(fu) nuclear import in Drosophila. Dev Biol. 2006;294:258–270. doi: 10.1016/j.ydbio.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Smelkinson MG, Kalderon D. Processing of the Drosophila hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr Biol. 2006;16:110–116. doi: 10.1016/j.cub.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Smelkinson MG, Zhou Q, Kalderon D. Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Dev Cell. 2007;13:481–495. doi: 10.1016/j.devcel.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegman MA, Goetz JA, Ascano M, Jr, Ogden SK, Nybakken KE, Robbins DJ. The Kinesin-related protein Costal2 associates with membranes in a Hedgehog-sensitive, Smoothened-independent manner. J Biol Chem. 2004;279:7064–7071. doi: 10.1074/jbc.M311794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Mlodzik M. Hedgehog is an indirect regulator of morphogenetic furrow progression in the Drosophila eye disc. Development. 1997;124:3233–3240. doi: 10.1242/dev.124.17.3233. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Wiersdorff V, Mlodzik M. Regulation of furrow progression in the Drosophila eye by cAMP-dependent protein kinase A. Nature. 1995;373:705–709. doi: 10.1038/373705a0. [DOI] [PubMed] [Google Scholar]
- Tamori Y, Deng WM. Cell competition and its implications for development and cancer. J Genet Genomics. 2011;38:483–495. doi: 10.1016/j.jgg.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Thomas C, Ingham PW. Hedgehog signaling in the Drosophila eye and head: an analysis of the effects of different patched trans-heterozygotes. Genetics. 2003;165:1915–1928. doi: 10.1093/genetics/165.4.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, Bilder D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci. 2009;122:2413–2423. doi: 10.1242/jcs.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrailas AD, Moses K. Smoothened, thickveins and the genetic control of cell cycle and cell fate in the developing Drosophila eye. Mech Dev. 2006;123:151–165. doi: 10.1016/j.mod.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Amanai K, Wang B, Jiang J. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 2000;14:2893–2905. doi: 10.1101/gad.843900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. The subcellular localization and activity of Drosophila cubitus interruptus are regulated at multiple levels. Development. 1999;126:5097–5106. doi: 10.1242/dev.126.22.5097. [DOI] [PubMed] [Google Scholar]
- Wang QT, Holmgren RA. Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development. 2000;127:3131–3139. doi: 10.1242/dev.127.14.3131. [DOI] [PubMed] [Google Scholar]
- Wang Y, Price MA. A unique protection signal in Cubitus interruptus prevents its complete proteasomal degradation. Mol Cell Biol. 2008;28:5555–5568. doi: 10.1128/MCB.00524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Tomlinson A. Epithelial planar polarity in the developing Drosophila eye. Development. 1995;121:2451–2459. doi: 10.1242/dev.121.8.2451. [DOI] [PubMed] [Google Scholar]
- Werz C, Lee TV, Lee PL, Lackey M, Bolduc C, Stein DS, Bergmann A. Mis-specified cells die by an active gene-directed process, and inhibition of this death results in cell fate transformation in Drosophila. Development. 2005;132:5343–5352. doi: 10.1242/dev.02150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991a;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991b;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Zheng L, Suyama K, Scott MP. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 2003;17:1240–1252. doi: 10.1101/gad.1080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Overgrowth with pattern duplication is apparent in the legs. Genotype: y w hs-FLP; FRT42 ubi-GFP / FRT42 cos2H29