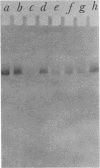
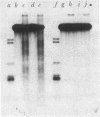
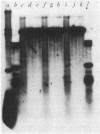
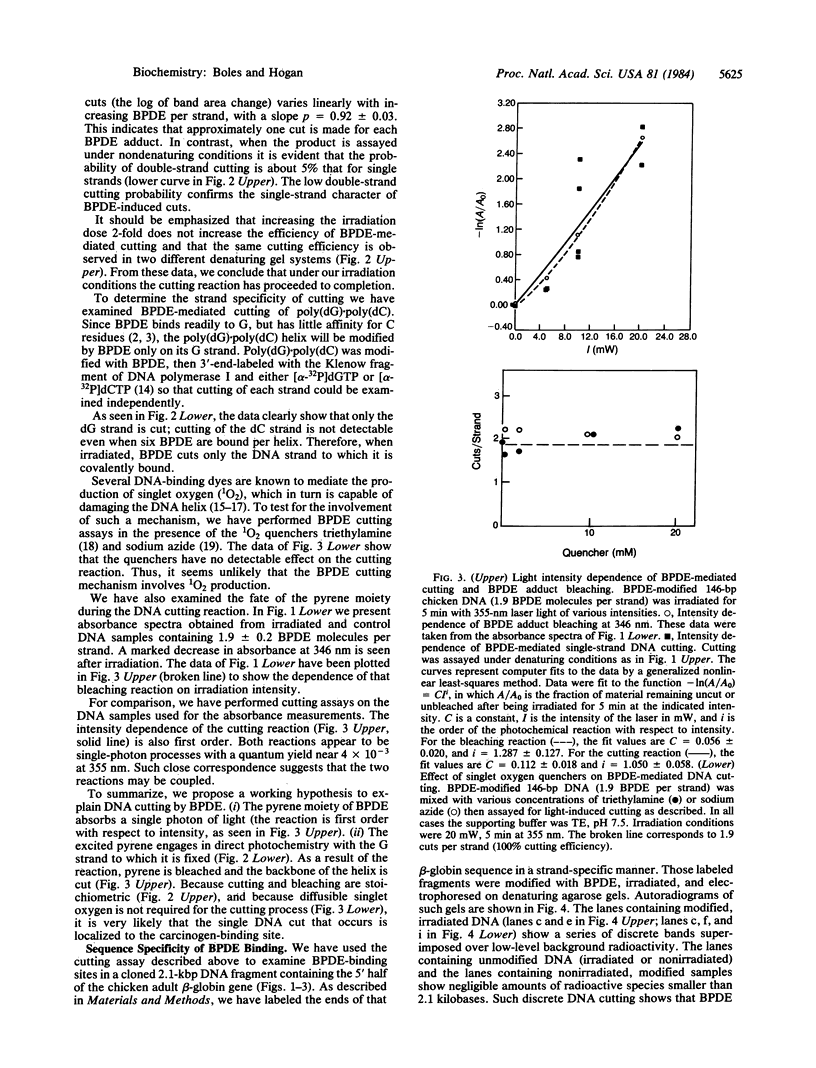
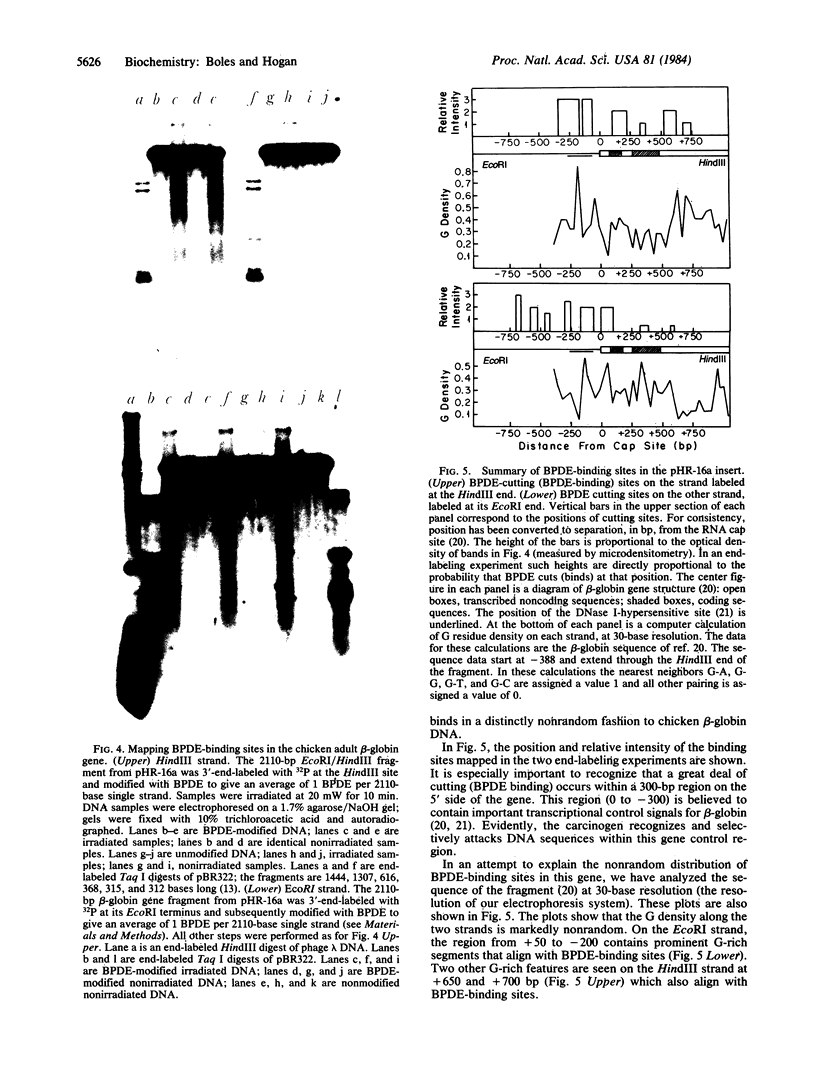
Abstract
Benzo[alpha]pyrene diol epoxide (BPDE) is a well-studied environmental carcinogen that binds covalently to DNA. Here we describe a photochemical technique that allows us to map BPDE-binding sites within cloned gene sequences. The technique is based upon our observation that, when irradiated with laser light at 355 nm, one single-strand DNA cut is produced at each BPDE binding site. In initial experiments we have studied the distribution of such cuts in cloned DNA from the chicken adult beta-globin gene. We find that BPDE binding in this gene sequence is distinctly nonrandom. While several prominent BPDE-binding sites are evident, a 300-base-pair sequence immediately 5' to the RNA cap site is most strongly attacked by the carcinogen. This region is believed to contain important transcriptional control sequences. We discuss the possibility that sequence-specific binding to such regulatory elements may be an important feature of the mechanism of the carcinogen.
Full text
PDF
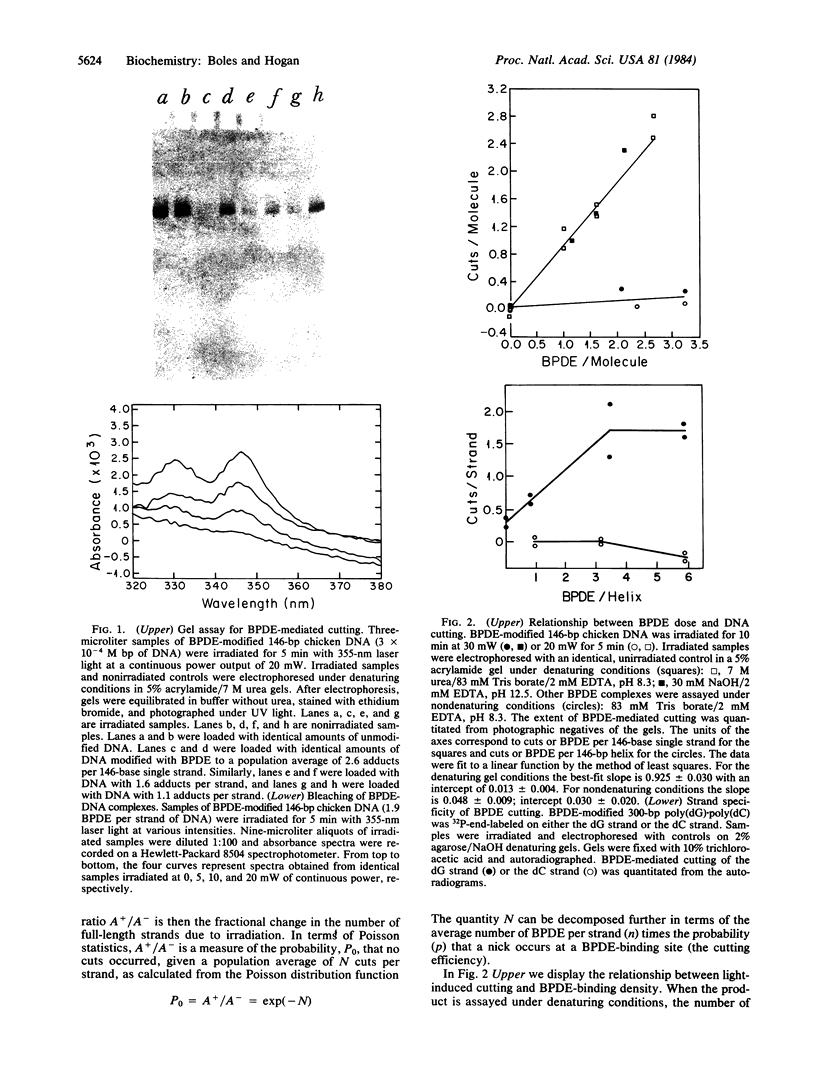

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buening M. K., Wislocki P. G., Levin W., Yagi H., Thakker D. R., Akagi H., Koreeda M., Jerina D. M., Conney A. H. Tumorigenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides in newborn mice: exceptional activity of (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5358–5361. doi: 10.1073/pnas.75.11.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geacintov N. E., Gagliano A., Ivanovic V., Weinstein I. B. Electric linear dichroism study on the orientation of benzo[alpha]pyrene-7,8-dihydrodiol 9,10-oxide covalently bound to DNA. Biochemistry. 1978 Nov 28;17(24):5256–5262. doi: 10.1021/bi00617a027. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Lo K. M., D'Andrea A. D. Preferred sites of strand scission in DNA modified by andi-diol epoxide of benzo[a]pyrene. Science. 1980 Aug 22;209(4459):929–931. doi: 10.1126/science.7403858. [DOI] [PubMed] [Google Scholar]
- Hogan M. E., Dattagupta N., Whitlock J. P., Jr Carcinogen-induced alteration of DNA structure. J Biol Chem. 1981 May 10;256(9):4504–4513. [PubMed] [Google Scholar]
- Hogan M. E., Jardetzky O. Effect of ethidium bromide on deoxyribonucleic acid internal motions. Biochemistry. 1980 May 13;19(10):2079–2085. doi: 10.1021/bi00551a012. [DOI] [PubMed] [Google Scholar]
- Houba-Herin N., Calberg-Bacq C. M., Piette J., Van de Vorst A. Mechanisms for dye-mediated photodynamic action: singlet oxygen production, deoxyguanosine oxidation and phage inactivating efficiencies. Photochem Photobiol. 1982 Sep;36(3):297–306. doi: 10.1111/j.1751-1097.1982.tb04378.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- King H. W., Osborne M. R., Brookes P. The in vitro and in vivo reaction at the N7-position of guanine of the ultimate carcinogen derived from benzolalpyrene. Chem Biol Interact. 1979 Mar;24(3):345–353. doi: 10.1016/0009-2797(79)90082-6. [DOI] [PubMed] [Google Scholar]
- Mace H. A., Pelham H. R., Travers A. A. Association of an S1 nuclease-sensitive structure with short direct repeats 5' of Drosophila heat shock genes. Nature. 1983 Aug 11;304(5926):555–557. doi: 10.1038/304555a0. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Meehan T., Straub K. Double-stranded DNA steroselectively binds benzo(a)pyrene diol epoxides. Nature. 1979 Feb 1;277(5695):410–412. doi: 10.1038/277410a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Kasai H., Cho H., Harvey R. G., Jeffrey A. M., Jennette K. W., Weinstein I. Absolute configuration of a ribonucleic acid adduct formed in vivo by metabolism of benzo[a]pyrene. J Am Chem Soc. 1977 Jan 5;99(1):258–260. doi: 10.1021/ja00443a053. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Brookes P. Exceptional mutagenicity of a benzo(a)pyrene diol epoxide in cultured mammalian cells. Nature. 1976 May 6;261(5555):52–54. doi: 10.1038/261052a0. [DOI] [PubMed] [Google Scholar]
- Phillips D. H. Fifty years of benzo(a)pyrene. Nature. 1983 Jun 9;303(5917):468–472. doi: 10.1038/303468a0. [DOI] [PubMed] [Google Scholar]
- Piette J., Calberg-Bacq C. M., Van de Vorst A. Alteration of guanine residues during proflavine mediated photosensitization of DNA. Photochem Photobiol. 1981 Mar;33(3):325–333. doi: 10.1111/j.1751-1097.1981.tb05425.x. [DOI] [PubMed] [Google Scholar]
- Prusik T., Geacintov N. E., Tobiasz C., Ivanovic V., Weinstein I. B. Fluorescence study of the physico-chemical properties of a benzo(a)pyrene 7,8-dihydrodiol 9,10-oxide derivative bound covalently to DNA. Photochem Photobiol. 1979 Feb;29(2):223–232. doi: 10.1111/j.1751-1097.1979.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Pulkrabek P., Leffler S., Grunberger D., Weinstein I. B. Modification of deoxyribonucleic acid by a diol epoxide of benzo[a]pyrene. Relation to deoxyribonucleic acid structure and conformation and effects on transfectional activity. Biochemistry. 1979 Nov 13;18(23):5128–5134. doi: 10.1021/bi00590a016. [DOI] [PubMed] [Google Scholar]
- Wang J., Hogan M., Austin R. H. DNA motions in the nucleosome core particle. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5896–5900. doi: 10.1073/pnas.79.19.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Nickol J., Felsenfeld G. Repeated sequence organization and RNA transcription map of the chicken adult beta-globin gene region. J Biol Chem. 1981 Feb 25;256(4):1502–1506. [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yang N. C., Ng L. K., Neoh S. B., Leonov D. A spectrofluorimetric investigation of calf thymus DNA modified by BP diolepoxide and 1-pyrenyloxirane. Biochem Biophys Res Commun. 1978 Jun 14;82(3):929–934. doi: 10.1016/0006-291x(78)90872-0. [DOI] [PubMed] [Google Scholar]
- de Mol N. J., Beijersbergen van Henegouwen G. M., van Beele B. Singlet oxygen formation by sensitization of furocoumarins complexed with, or bound covalently to DNA. Photochem Photobiol. 1981 Dec;34(6):661–666. [PubMed] [Google Scholar]