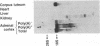Abstract
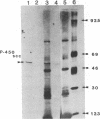
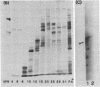
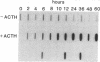
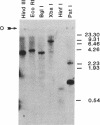
Two overlapping cDNA clones (pBSCC-1 and pBSCC-2) bearing inserts approximately equal to 425 and approximately equal to 950 base pairs long, respectively, which are specific for bovine cholesterol side-chain cleavage cytochrome P-450 (P-450scc), have been identified by using two differential hybridization screening procedures followed by hybrid-selected RNA translation. By using these cloned cDNAs as hybridization probes, an RNA species was identified that had the properties expected of mRNA specific for P-450scc with respect to tissue specificity, corticotropin (ACTH)-mediated regulation of synthesis, and size of the protein product synthesized in vitro. In RNA samples obtained from bovine adrenal cortex, from bovine corpus luteum, and from cultured bovine adrenocortical cells, it was found that P-450scc is encoded by mRNA species approximately equal to 2000 bases long, a majority of which are polyadenylylated. P-450scc mRNA was not detected in RNA samples prepared from bovine heart, liver, and kidney. Treatment of cultured bovine adrenocortical cells with ACTH resulted in the appearance of elevated levels of P-450scc mRNA within 8 hr. Thus, ACTH promotes the enhancement of P-450scc gene transcription or acts to stabilize the transcripts. When pBSCC-2 cDNA was used to probe high molecular weight bovine DNA following treatment with restriction endonucleases, a simple pattern of hybridization was observed indicating that P-450scc may be encoded by a single gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M., Adesnik M. A cytochrome P-450 multigene family. Characterization of a gene activated by phenobarbital administration. J Biol Chem. 1983 Sep 25;258(18):11285–11295. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V., Simpson E. R., Waterman M. R. Induction of synthesis of bovine adrenocortical cytochromes P-450scc, P-45011 beta, P-450C21, and adrenodoxin by prostaglandins E2 and F2 alpha and cholera toxin. Arch Biochem Biophys. 1984 Jun;231(2):271–279. doi: 10.1016/0003-9861(84)90388-6. [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Negishi M., Nebert D. W. Cytochrome P1-450 structural gene in mouse, rat, and rabbit: differences in DNA methylation and developmental expression of mRNA. DNA. 1982;1(3):231–238. doi: 10.1089/dna.1.1982.1.231. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Deshpande A. K., Chatterjee B., Roy A. K. Translation and stability of rat liver messenger RNA for alpha 2 mu-globulin in Xenopus oocyte. The role of terminal poly(A). J Biol Chem. 1979 Sep 25;254(18):8937–8942. [PubMed] [Google Scholar]
- DuBois R. N., Simpson E. R., Kramer R. E., Waterman M. R. Induction of synthesis of cholesterol side chain cleavage cytochrome P-450 by adrenocorticotropin in cultured bovine adrenocortical cells. J Biol Chem. 1981 Jul 10;256(13):7000–7005. [PubMed] [Google Scholar]
- DuBois R. N., Simpson E. R., Tuckey J., Lambeth J. D., Waterman M. R. Evidence for a higher molecular weight precursor of cholesterol side-chain-cleavage cytochrome P-450 and induction of mitochondrial and cytosolic proteins by corticotropin in adult bovine adrenal cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1028–1032. doi: 10.1073/pnas.78.2.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Pastewka J. V., Park S. S., Guengerich F. P., Gelboin H. V. Identification and quantitation of a 2.0-kilobase messenger ribonucleic acid coding for 3-methylcholanthrene-induced cytochrome P-450 using cloned cytochrome P-450 complementary deoxyribonucleic acid. Biochemistry. 1982 Dec 7;21(25):6574–6580. doi: 10.1021/bi00268a039. [DOI] [PubMed] [Google Scholar]
- Funkenstein B., Waterman M. R., Masters B. S., Simpson E. R. Evidence for the presence of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin in fresh granulosa cells. Effects of follicle-stimulating hormone and cyclic AMP on cholesterol side chain cleavage cytochrome P-450 synthesis and activity. J Biol Chem. 1983 Aug 25;258(16):10187–10191. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hardwick J. P., Gonzalez F. J., Kasper C. B. Transcriptional regulation of rat liver epoxide hydratase, NADPH-Cytochrome P-450 oxidoreductase, and cytochrome P-450b genes by phenobarbital. J Biol Chem. 1983 Jul 10;258(13):8081–8085. [PubMed] [Google Scholar]
- Heins B., Beato M. Hormonal control of uteroglobin secretion and preuteroglobin mRNA content in rabbit endometrium. Mol Cell Endocrinol. 1981 Feb;21(2):139–150. doi: 10.1016/0303-7207(81)90051-4. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Altieri M., Chen Y. T., Nakamura M., Tukey R. H., Nebert D. W., Negishi M. Characterization of cytochrome P2-450 (20-S) mRNA. Association with the P1-450 genomic gene and differential response to the inducers 3-methylcholanthrene and isosafrole. Eur J Biochem. 1983 Jul 15;134(1):13–18. doi: 10.1111/j.1432-1033.1983.tb07524.x. [DOI] [PubMed] [Google Scholar]
- Kramer R. E., Du Bois R. N., Simpson E. R., Anderson C. M., Kashiwagi K., Lambeth J. D., Jefcoate C. R., Waterman M. R. Cell-free synthesis of precursor forms of mitochondrial steroid hydroxylase enzymes of the bovine adrenal cortex. Arch Biochem Biophys. 1982 May;215(2):478–485. doi: 10.1016/0003-9861(82)90106-0. [DOI] [PubMed] [Google Scholar]
- Kramer R. E., Rainey W. E., Funkenstein B., Dee A., Simpson E. R., Waterman M. R. Induction of synthesis of mitochondrial steroidogenic enzymes of bovine adrenocortical cells by analogs of cyclic AMP. J Biol Chem. 1984 Jan 25;259(2):707–713. [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Miller W. L. Molecular cloning of DNA complementary to bovine adrenal P450scc mRNA. Biochem Biophys Res Commun. 1984 Apr 16;120(1):264–270. doi: 10.1016/0006-291x(84)91443-8. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Fujii-Kuriyama Y., Muramatsu M. Multiplicity of deoxyribonucleic acid sequences with homology to a cloned complementary deoxyribonucleic acid coding for rat phenobarbital-inducible cytochrome P-450. Biochemistry. 1983 Mar 1;22(5):1223–1229. doi: 10.1021/bi00274a036. [DOI] [PubMed] [Google Scholar]
- Negishi M., Nebert D. W. Structural gene products of the Ah complex. Increases in large mRNA from mouse liver associated with cytochrome P1-450 induction by 3-methylcholanthrene. J Biol Chem. 1981 Mar 25;256(6):3085–3091. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE D., HECHTER O. Studies on ACTH action in perfused bovine adrenals: the site of action of ACTH in corticosteroidogenesis. Arch Biochem Biophys. 1954 Aug;51(2):457–469. doi: 10.1016/0003-9861(54)90501-9. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Kasper C. B. Genetic polymorphisms for a phenobarbital-inducible cytochrome P-450 map to the Coh locus in mice. J Biol Chem. 1983 Aug 25;258(16):9585–9588. [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur J Biochem. 1967 Oct;2(3):275–285. doi: 10.1111/j.1432-1033.1967.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Hannah R. R., Negishi M., Nebert D. W., Eisen H. J. The Ah locus: correlation of intranuclear appearance of inducer-receptor complex with induction of cytochrome P1-450 mRNA. Cell. 1982 Nov;31(1):275–284. doi: 10.1016/0092-8674(82)90427-5. [DOI] [PubMed] [Google Scholar]
- Yago N., Kobayashi S., Sekiyama S., Kurokawa H., Iwai Y. Further studies on the submitochondrial localization of cholesterol side chain-cleaving enzyme system in hog adrenal cortex by sonic treatment. J Biochem. 1970 Dec;68(6):775–783. doi: 10.1093/oxfordjournals.jbchem.a129414. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]