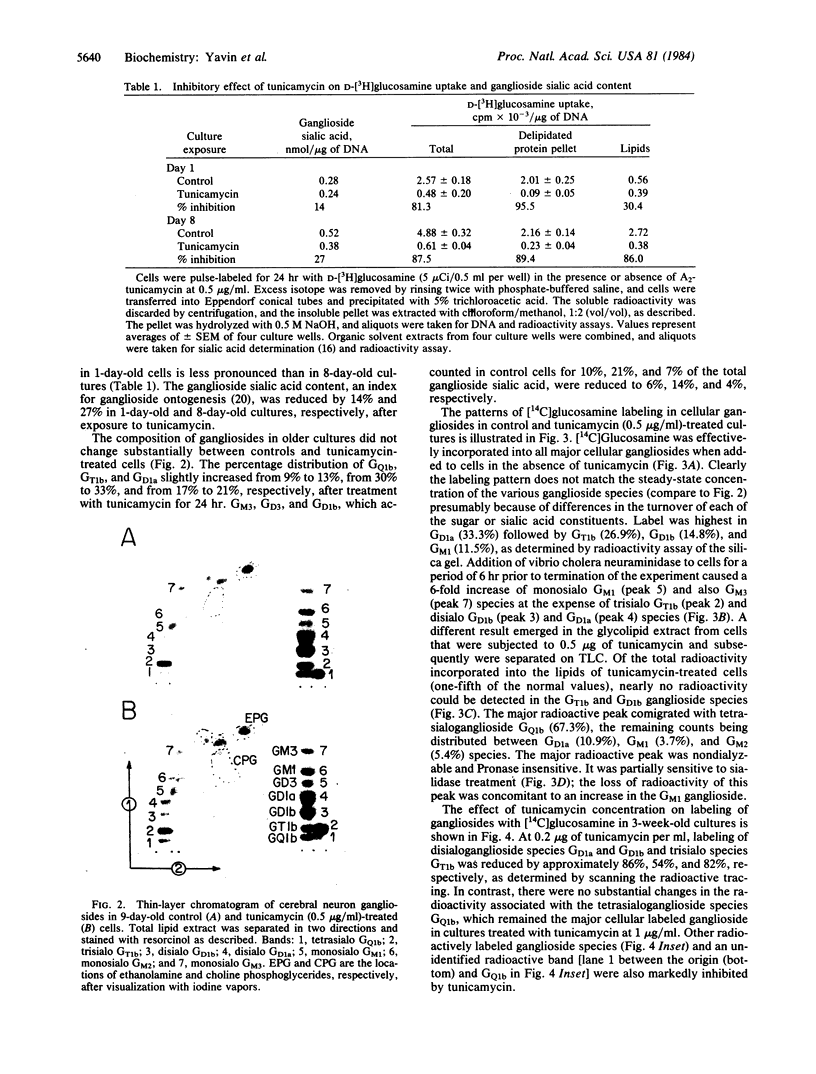
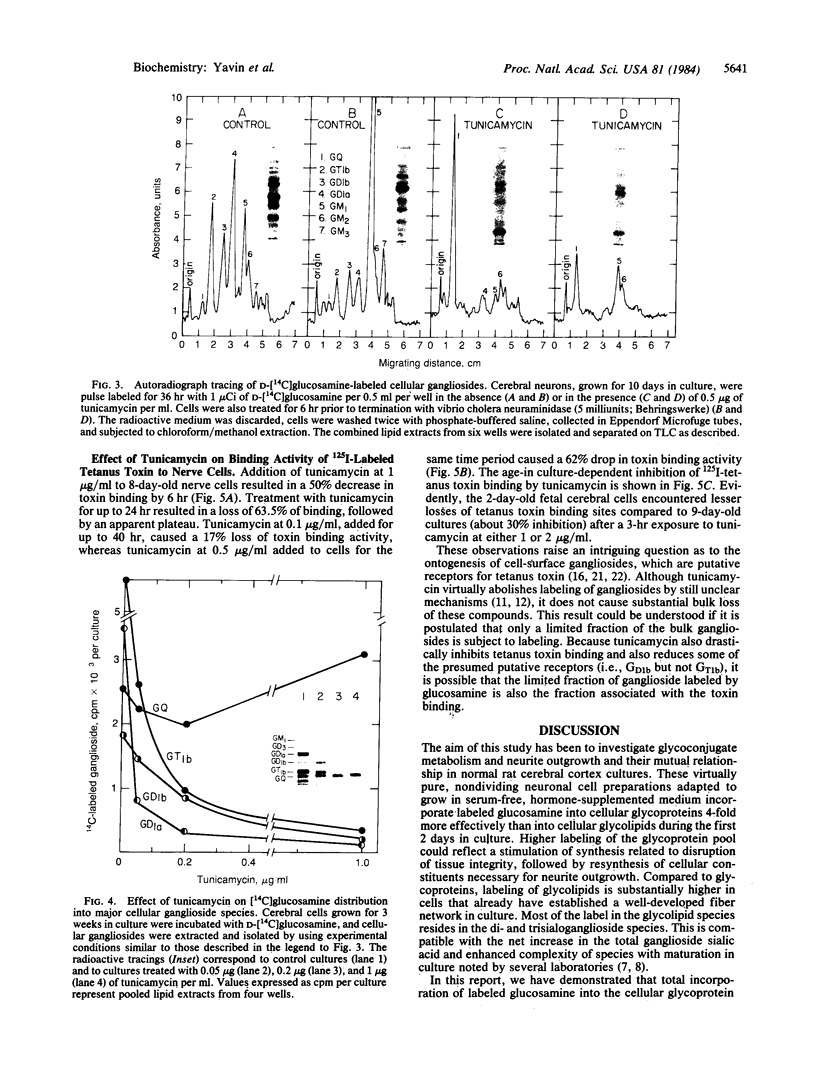
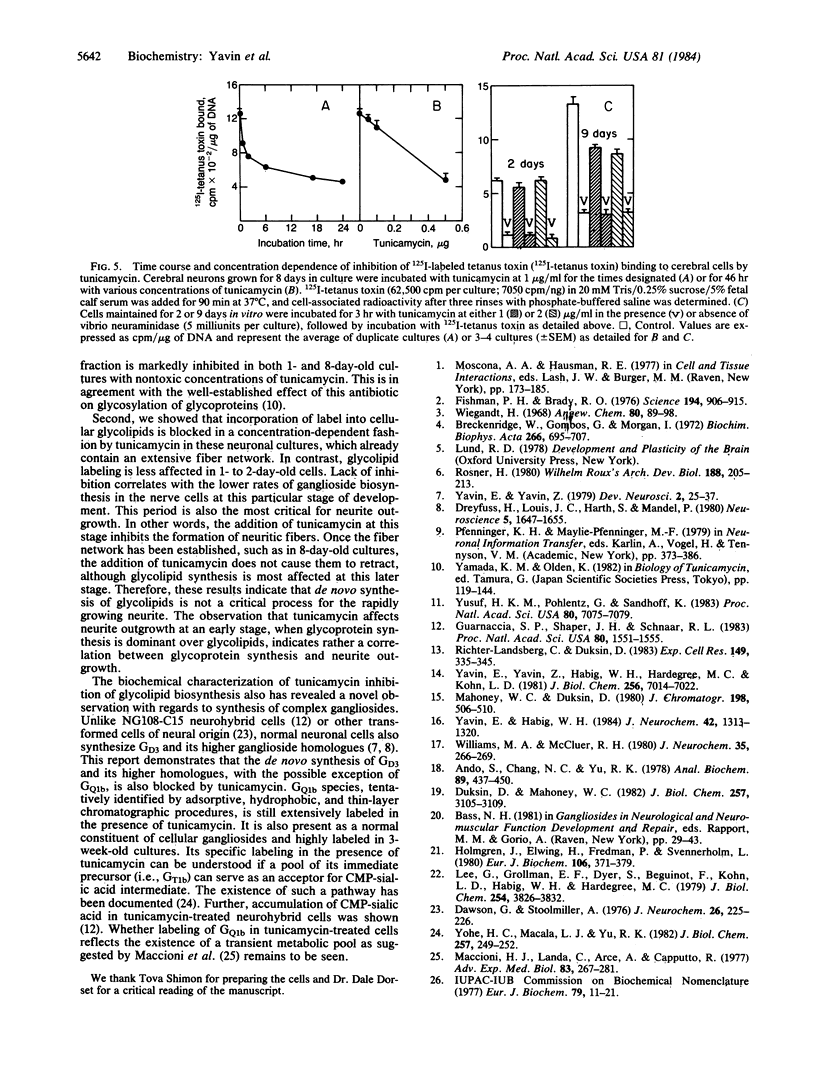
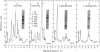Abstract
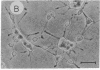
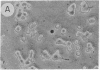
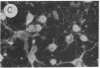
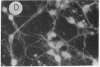
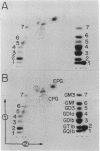
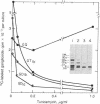
Fetal cerebral neurons at the initiation of active neurite outgrowth in culture incorporate 4-fold more [3H]glucosamine into glycoproteins than into the cellular lipid fraction. After 8 days or longer, when a well-developed fiber network is apparent, lipids are labeled more extensively than the glycoproteins. Labeling of the latter is inhibited 95% and 89% by 0.5 microgram of tunicamycin per ml added to 1-day-old and 8-day-old cultures, respectively. Labeling of glycolipids is inhibited 30% in 1-day-old and 86% in 8-day-old cultures. Tunicamycin blocks incorporation of glucosamine label into practically all ganglioside species except for a resorcinol-positive, sialidase-sensitive band tentatively identified as GQ1b tetrasialoganglioside (Svennerholm ganglioside nomenclature). It also substantially reduces binding of 125I-labeled tetanus toxin to intact cells. There is 14% and 27% reduction in the total ganglioside sialic acid content in 1-day-old and 8-day-old cells treated for 24 hr with 0.5 microgram of tunicamycin per ml, but no substantial compositional changes are encountered. Tunicamycin blocks neurite outgrowth when added to cells soon after plating but causes no retraction or losses of fibers once the fiber network is established. Therefore, inhibition of neurite outgrowth by tunicamycin is not due to an effect on cellular gangliosides but can be correlated to an inhibition of protein glycosylation.
Full text
PDF
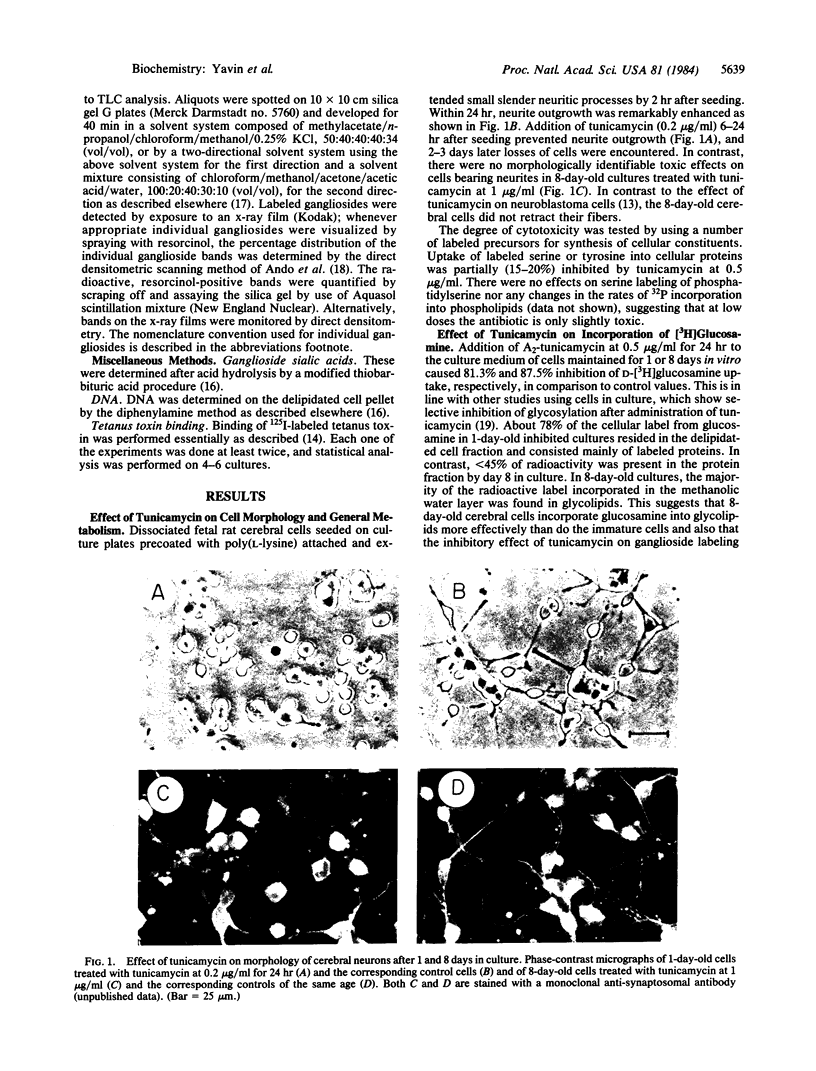
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Chang N. C., Yu R. K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal Biochem. 1978 Sep;89(2):437–450. doi: 10.1016/0003-2697(78)90373-1. [DOI] [PubMed] [Google Scholar]
- Breckenridge W. C., Gombos G., Morgan I. G. The lipid composition of adult rat brain synaptosomal plasma membranes. Biochim Biophys Acta. 1972 Jun 20;266(3):695–707. doi: 10.1016/0006-3002(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Dawson G., Stoolmiller A. C. Comparison of the ganglioside composition of established mouse neuroblastoma cell strains grown in vivo and in tissue culture. J Neurochem. 1976 Jan;26(1):225–226. doi: 10.1111/j.1471-4159.1976.tb04466.x. [DOI] [PubMed] [Google Scholar]
- Dreyfus H., Louis J. C., Harth S., Mandel P. Gangliosides in cultured neurons. Neuroscience. 1980;5(9):1647–1655. doi: 10.1016/0306-4522(80)90028-7. [DOI] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Guarnaccia S. P., Shaper J. H., Schnaar R. L. Tunicamycin inhibits ganglioside biosynthesis in neuronal cells. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1551–1555. doi: 10.1073/pnas.80.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Lee G., Grollman E. F., Dyer S., Beguinot F., Kohn L. D., Habig W. H., Hardegree M. C. Tetanus toxin and thyrotropin interactions with rat brain membrane preparations. J Biol Chem. 1979 May 25;254(10):3826–3832. [PubMed] [Google Scholar]
- Maccioni H. J., Landa C., Arce A., Caputto R. The biosynthesis of brain gangliosides--evidence for a "transient pool" and an "end product pool" of gangliosides. Adv Exp Med Biol. 1977;83:267–281. [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Separation of tunicamycin homologues by reversed-phase high-performance liquid chromatography. J Chromatogr. 1980 Oct 24;198(4):506–510. doi: 10.1016/s0021-9673(00)80521-x. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C., Duksin D. Role of glycoproteins in neuronal differentiation. Inhibition of neurite outgrowth and the major cell surface glycoprotein of murine neuroblastoma cells by a purified tunicamycin homologue. Exp Cell Res. 1983 Dec;149(2):335–345. doi: 10.1016/0014-4827(83)90347-6. [DOI] [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Yavin E., Habig W. H. Binding of tetanus toxin to somatic neural hybrid cells with varying ganglioside composition. J Neurochem. 1984 May;42(5):1313–1320. doi: 10.1111/j.1471-4159.1984.tb02789.x. [DOI] [PubMed] [Google Scholar]
- Yavin E., Yavin Z., Habig W. H., Hardegree M. C., Kohn L. D. Tetanus toxin association with developing neuronal cell cultures. Kinetic parameters and evidence for ganglioside-mediated internalization. J Biol Chem. 1981 Jul 10;256(13):7014–7022. [PubMed] [Google Scholar]
- Yohe H. C., Macala L. J., Yu R. K. In vitro biosynthesis of the tetrasialoganglioside GQ1b. J Biol Chem. 1982 Jan 10;257(1):249–252. [PubMed] [Google Scholar]
- Yusuf H. K., Pohlentz G., Sandhoff K. Tunicamycin inhibits ganglioside biosynthesis in rat liver Golgi apparatus by blocking sugar nucleotide transport across the membrane vesicles. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7075–7079. doi: 10.1073/pnas.80.23.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]