Abstract
Natural killer (NK) cells are part of the innate immune defense against infection and cancer, and are especially useful in combating certain viral pathogens. The utility of NK cells in human health has been underscored by a growing number of individuals who are deficient in NK cells and/or their functions. This can be in the context of a broader genetically-defined congenital immunodeficiency of which there are over forty presently known to impair NK cells. The abnormality of NK cells, however, in certain cases represents the majority immunological defect. In aggregate, these conditions are termed NK cell deficiency. Recent advances have added clarity to this diagnosis and identified defects in three different genes that can cause NK cell deficiency as well as some of the underlying biology. Appropriate consideration of these diagnoses and patients raises the potential for rational therapeutic options and further innovation.
Keywords: NK cells, Innate Immunity, NK cell deficiency, Primary Immunodeficiency, Cytotoxicity
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that are best known for their ability to mediate cytotoxicity and produce cytokines after the ligation of germline-encoded activation receptors.1 As a result, they have long been considered part of the innate immune system, but do have some newly appreciated adaptive roles as well.2 NK cells are best appreciated for innate defense against viral infections and in tumor cell surveillance, but are also increasingly recognized for participating in immunoregulation, coordination of immunity and modulating autoreactivity. NK cells are lymphocytes and major members of the innate lymphoid cell (ILC) family,3 which develop from CD34+ hematopoietic cells in the bone marrow and undergo terminal maturation in secondary lymphoid tissues.4 In humans, NK cells are classically identified by the absence of the T cell receptor complex and presence of neural cell adhesion molecule (denoted CD56 according to the cluster designation [CD] system). The majority of peripheral blood NK cells express low levels of CD56 as well as an IgG Fc receptor FcγRIIIA (CD16).4 These are considered mature and are referred to as CD56dim NK cells (which means that the fluorescent intensity for CD56 is slightly increased compared to cells negative for CD56 – Figure 1). A minority of peripheral blood NK cells express high levels of CD56 without expressing CD16 and are considered to comprise a developmentally immature but functionally enabled NK cell subset (otherwise known as CD56bright – Figure 1).5, 6 NK cells characteristically express a wide range of receptors, some of which are rather specific in their expression. These include receptors capable of inducing either activation or inhibitory signals and NK cell activities are accessed after a favorable balance of activation over inhibitory signals is achieved in their recognition of a target.7
Figure 1.
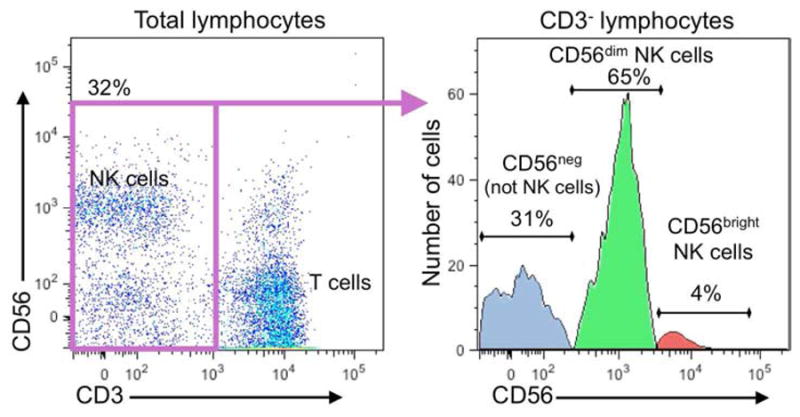
Flow cytometry depicting peripheral blood NK cell subsets according to CD56. PBMC from a normal donor were evaluated by FACS and gated lymphocytes analyzed for expression of CD3 and CD56 (left). NK cells are CD56+/CD3−. To illustrate the range of CD56 expression, all CD3− cells (non-T cells) were gated (purple box – left) and displayed as a histogram (right). Here there are 3 clear populations: CD56neg (blue peak), which are not NK cells, CD56dim NK cells (green peak), and CD56bright NK cells (red peak). In this example the CD56bright NK cells represent 4% of the CD3− cells or 5.8% of the total NK cells (CD56dim plus CD56bright). Experimental credit to Dr. Emily Mace, Baylor College of Medicine.
Regarding their role in infectious diseases, NK cells specialize in defense against certain T-cell elusive pathogens, most notably viruses.8 Many viruses have evolved strategies to evade the cytotoxic T lymphocyte (CTL) response by specifically downregulating class-I MHC in the infected cell.9 While this allows the virus to prevent its host cell from presenting viral protein-derived peptides to virus-specific CTLs, it also makes the infected cell more susceptible to NK cell defenses. Although NK cells are triggered by a large number of activation receptors, they are restrained by an extensive family of inhibitory receptors that can be ligated by class-I MHC.10 The most well known in humans is that of the Killer Cell Immunoglobulin-Like Receptor (KIR) family. As an example of the impact of NK cells, some viruses have further evolved specific NK cell evasion mechanisms to interfere with the KIR system, including virus-encoded class-I decoy molecules.11 The viruses that seem to be best targeted by NK cell mediated defenses are those of the Herpesvirus family, which are notorious for downmodulating class-I MHC.
Viruses can make infected host cells more susceptible to NK cell activities in ways other than simply decreasing NK cell inhibition. NK cells have activation receptors that can directly recognize particular viral antigens such as certain Natural Cytotoxicity Receptors (NCR), which can bind viral hemagglutinin.12 Some viruses are capable of inducing the expression of specific host-cell stress molecules that can serve ligands for NK cell activation receptors, thus representing an important paradigm by which NK cells combat disease. In this light, malignant cell transformation can also induce these cell stress-associated ligands, which when compounded by the fact that many cancer cells lose class-I MHC expression in evading tumor-specific CTL responses emphasizes the role of NK cells in tumor surveillance.13
After activation, NK cells are capable of three main functions to participate in immune defense. The first and best characterized is the ability to mediate contact dependent killing of target cells. This involves the mobilization of highly specialized organelles in NK cells known as lytic granules that contain the pore-forming molecule perforin and death-inducing enzymes such as granzymes.14 Once a killing program is triggered in an NK cell, the lytic granules are transported to the interface formed with the targeted cell and their contents secreted onto it. This function of cytotoxicity can be accessed by NK cell activation receptors as an innate immune defense or by recognition of IgG opsonized cells via CD16 to enable antibody-dependent cell mediated cytotoxicity (ADCC). Through ADCC NK cells have intimate interface with adaptive immunity and also enable the functions of certain therapeutic monoclonal antibodies.15 The second function of NK cells is the production of soluble factors to promote direct anti-disease effects as well as further induce or regulate immunity. These include a wide variety of cytokines, chemokines and other regulators. NK cells are probably best appreciated within this category for their ability to produce IFN-γ that has both antiviral and immune enhancing capabilities. The third, but less appreciated function of NK cells is that of promoting and regulating immunity through contact-dependent costimulatory and regulatory mechanisms. NK cells express, or can be induced to express a large number of relevant costimulatory and regulatory ligands and can localize to key regulatory sites including secondary lymphoid tissues where these contact-dependent roles in immune responses can be affected.1
While NK cells and their diverse functions serve important roles in numerous animal models of disease, they are also associated with human clinical conditions. Perhaps the clearest demonstration of the value of NK cells to humans, however, derives from their deficiency. NK cell deficiency (NKD) represents a small but increasingly appreciated subset of primary immunodeficiency diseases (PID) that present challenges both in diagnosis and clinical management.16 NK cell deficiencies also provide great insight into the value of and mechanisms underlying human NK cell functions.17–19 As an overarching theme, patients with NKD have susceptibility to Herpesviruses as well as select other viral pathogens. There are also distinct genetically-defined PIDs that include an impact upon NK cells and their functions.17–20 Many of these diseases also include susceptibility to viral infection and are informative from a mechanistic standpoint as they delineate specific molecular requirements of human NK cells.
This review will provide an overview of the substantive advances made in the understanding of NKD. It will also recap PIDs impacting NK cells to build upon previous reviews of this topic.
NKD definition
To be considered an NKD, the impact upon NK cells need represent the major immunological abnormality in the patient. While many diseases, drugs, infections and physiological states can affect NK cell numbers and/or function, the NKD diagnosis is reserved for abnormalities that are fixed over time and not secondary in nature. Specifically NKD should be inherent and hardwired. In several cases a genetic mechanism responsible for NKD has been identified. It is also anticipated that the majority of true NKD will be monogenic given the overall rarity and impact of lacking NK cells and/or their functions.
NKD can be divided into two major types depending upon whether or not NK cells are present in the peripheral blood.19 Classical NKD (CNKD) is defined as an absence of NK cells and their function among peripheral blood lymphocytes. Functional NKD (FNKD) is defined as the presence of NK cells within peripheral blood lymphocytes, having defective NK cell activity. In other words, in CNKD, NK cells are absent and in FNKD NK cells are present, but do not work. It should be re-emphasized that in both CNKD and FNKD the NK cell abnormality is the major immunological deficit resulting in inadequate host defense. CNKD and FNKD are further subdivided based upon the particular gene that is responsible for the phenotype if identified (Table 1). Although both diagnoses are presently considered to be quite rare, a definitive estimate of prevalence is currently unavailable.
Table 1.
Classification of natural killer cell deficiency
| a NKD type | Peripheral blood NK cells CD3−, CD56+ | CD56dim NK cell subset | CD56bright NK cell subset | NK cell function | Infectious susceptibility | Gene Defect | Inheritance |
|---|---|---|---|---|---|---|---|
| CNKD | Absent or very low | ? | ? | Absent | Herpesviruses | ? | ? |
| Subtype 1 (CNKD1) | Absent or very low | Absent or very low | Absent | Absent | VZV, HSV, CMV, HPV, Mtb, trychophyton. | GATA2 | AD |
| Subtype 2 (CNKD2) | Absent or very low | Absent or very low | Preserved | Absent | MCM4 | AR | |
| FNKD | Present | Should be present | Should be present | Absent or severely decreased | Herpesviruses Papillomaviruses | ? | ? |
| Subtype 1 (FNKD1) | Present | Present | Present | Decreased | HSV, EBV, HPV | FCGR3A | AR |
Abbreviations used: NKD – NK cell deficiency; CNKD – Classical NK cell deficiency; FNKD – Functional NK cell deficiency, HSV – Herpes Simplex Virus; EBV – Epstein Barr Virus; HPV – Human Papilloma Virus; AD – Autosomal Dominant; AR – Autosomal Recessive.
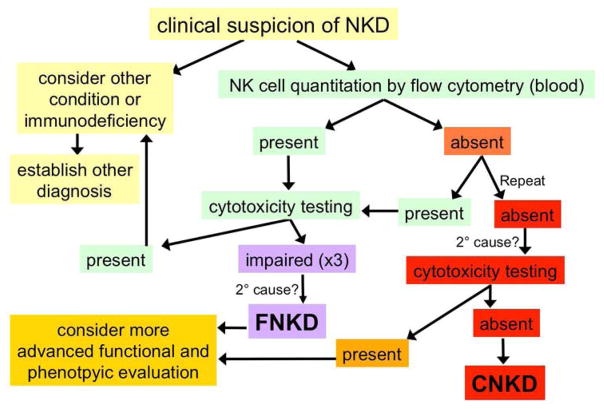
In order to be more specific, there are several important features in considering the CNKD or FNKD diagnoses (Table 2): 1) that the defect be stable over time; 2) that secondary causes of NK cell abnormalities be excluded as a cause (such as medication use-related, malignancy-related, or infection-related); 3) that other known primary immunodeficiency syndromes that can effect NK cell numbers and function be considered and at least rationally excluded; 4) for the purposes of an NKD diagnosis NK cells should be considered as those lymphocytes that are CD3−CD56+; 5) to be considered CNKD, NK cells be present at ≤1% of peripheral blood lymphocytes; 6) functional evaluation of NK cells be considered using reliable and validated assays on at least 3 occasions separated by 1 month each (51Cr-release cytotoxicity assay using K562 target cells is recommended, although normative ranges differ amongst clinical laboratories). An algorithm outlining an approach to a patient suspected to have NKD is presented in Figure 2.
Table 2.
Features of NKDa
| NK cell abnormalities represent the major immunologic abnormalityb |
| Defect is stable over timec |
| Secondary causes NK cell abnormalities excludedd |
| Exclude broader primary immunodeficiencies known to include an NK cell defecte |
| NK cells are evaluated as CD3−/CD56+f |
| In CNKD NK cells are ≤1% of peripheral blood lymphocytesg |
| Abnormal functional evaluations are repeatableh |
NKD overall characteristics to include both CNKD and FNKD except where specified
Some gene mutations will affect other immune cells, but to be considered an NKD the major impact on the patient should be derivative from the NK cell deficit.
It is of the essence that in the absence of a genetic abnormality known to cause NKD the NK cell defect be consistent.
Considering medications, malignancy, HIV-infection, severe physiologic or emotional stress.
See table 3
Most clinical laboratories use a reagent that identifies NK cells as CD56-PE+ or CD16-PE+. This is adequate for initial assessments.
The decreased population of NK cells should be stably decreased and not simply represent a single low value
Repetition of assays should be performed by a reliable validated assay (51Cr-release assay against K562 target cells is recommended for screening) on 3 separate occasions separated by a minimum of 1 month each applying laboratory-specific normative ranges.
Figure 2.
Diagnostic algorithm for NKD. An algorithmic approach to a patient suspected of having NKD is presented. Initial steps include considering alternative diagnoses as they are statistically more likely, as well as quantifying NK cells and their function in peripheral blood. Abnormal results should be repeated with a time interval of approximately one month. Absent NK cells are defined as ≤1% of peripheral blood lymphocytes. Cytotoxicity testing for screening is recommended by 51Cr-release assay using K562 target cells; normative ranges differ between laboratories and laboratory-specific ranges should be considered. Secondary (2°) causes should be considered as explanations for abnormalities. More advanced functional and phenotypic testing is presently in the domain of research-level interventions.
NKD should be clearly distinguished from any human abnormalities of natural killer T (NKT) cells. NKT cells are a subset of T cells that express certain NK cell surface determinants.21 They are not NK cells and are thus not part of a consideration of NKD. The presence or absence of NKT cells has been part of prior classifications of NKD (in what was previously referred to as absolute NKD),17, 18 but enough progress has been made regarding the biological and developmental understanding of NKT cells that they can be uncoupled from any present consideration of NKD. Thus the consideration of NKD should be specifically to NK cells and according to either the CNKD and FNKD subtype.19 In this light, as further understanding of historical published patients and cohorts improves the classification of certain cases is also subject to change.
Classical NK cell deficiency (CNKD)
CNKD is characterized by the both absence of NK cells and their function among peripheral blood lymphocytes. The single most well known case is that published in 1989 in the New England Journal of Medicine of an adolescent girl with multiple severe or disseminated herpesviral infections, including Varicella pneumonia, disseminated Cytomegalovirus (CMV) and Herpes Simplex Virus (HSV).22 She was stably deficient in NK cell cytotoxic activity as measured by K562 killing assays, and lacked “classical” CD56+/CD3− NK cells among PBMC by flow cytometry. This original case has served as the “typical” example of an NK cell deficiency and led to continued interest in pursuit of additional patients and answers.
Since this initial clear description of CNKD there have been at least 18 additional patients described phenotypically representing a total of 12 unrelated families 22–31. Of this group 42% (8/19) died prematurely. 53%% (10/19) have been described as experiencing severe consequences of herpesviral infections with cases present in 67% of the families represented. Of these severe Varicella Zoster virus (VZV) was most common occurring in 27% of patients, but CMV, Epstein-Barr virus (EBV) and HSV were all represented. Unusual consequences of human papilloma virus infection was identified in 16% and fungal infections in 10%. A number of patients (21%) experienced malignancies including an EBV-driven smooth muscle tumor, HPV-related cancers, and leukemia. Two patients have been successfully treated with hematopoietic stem cell transplantation (HSCT) 29, 32 while one died during the process. 17, 22 Other causes of death included EBV (2), CMV (1), VZV (1), cancer (2), and mycobacterial infection (1).
Further scientific advances have enabled the identification of two genetic mechanisms underlying CNKD. Thus, it is appropriate to refer to the CNKD subtypes according to genetic mechanism. The two presently identified genetic causes of CNKD can be labeled CNKD1 and CNKD2. Additional numerical designations (CNKD3, CNKD4, etc.) should be reserved for subsequent independent genetic mechanisms. CNKD without an identified genetic mechanism should just be referred to as CNKD (Table 1). Each of the two known genetic causes of CNKD is considered more specifically below.
CNKD1
Because the molecular mechanism of the 1989 CNKD case has been identified and this arguably represents the original description of a CNKD,22 it is given the CNKD1 designation. CNKD1 is caused by GATA2 haploinsufficiency.33 While GATA2 mutations can lead to a wide variety of clinical and immunological phenotypes, there is a subset of patients that present with hallmarks of NKD including the patient reported in 1989.33 GATA2 is a ubiquitously expressed hematopoietic transcription factor that promotes numerous genes of relevance and promotes survival and maintenance of hematopoietic cell subsets. A substantial number of GATA2 deficient patients present with infectious phenotypes characteristic of NKD including 78% with human papilloma virus (HPV) and 33% with severe or atypical manifestations of Herpesviruses.34 The latter includes disseminated VZV, CMV, and HSV. In several cases these infections have been ascribed as a cause of death, most notably HPV-derived anogenital cancers.
As mentioned above, the original CNKD1 patient died from complications of a hematopoietic stem cell transplantation that was performed to treat aplastic anemia. As is now appreciated, aplastic anemia can be a late complication of having GATA2 mutation. In this light, GATA2 mutation causes a variable clinical syndrome that is viewed by some as a progressive immunological exacerbation that evolves over decades and can include deficiency of monocytes and dendritic cells.35, 36 Six patients with GATA2 mutations have received HSCT with 5 successes, but it is unclear if these were NK cell predominant cases.37 What is also presently unclear in patients with GATA2 mutations is whether or not the NK cell deficiency occurs first, is a hardwired component of the mutation, or is just more pronounced in some patients. In this light, it is interesting that in a more comprehensive recent survey of human GATA2 mutation, HPV infection was the main infectious phenotype in the first decade of life.38 This suggests that the abnormal NK cell defenses may represent an early and even inherent aspect of this genetic disease.
Recently, a specific analysis of NK cells in GATA2 patients presenting with phenotypes suggestive of NKD has been performed.33 Half of these individuals were consistent with CNKD having ≤1% of NK cells among peripheral blood lymphocytes. Some of these patients, however, had NK cells in their peripheral blood. In all cases, however, NK cell cytotoxicity was defective – even when abundant NK cells were present. Thus, some GATA2 patients appear to be more characteristic of a FNKD. That said it is presently unclear if these individuals may eventually progress towards a total loss of NK cells. The experimental NK cell studies performed in CNKD1 have provided some additional insight and guidance. Even when NK cells were present, the developmentally immature minority CD56bright NK cell subset was uniformly absent. This could be recapitulated experimentally when differentiating NK cells in vitro from patient CD34+ hematopoietic stem cells.33 In healthy donor NK cells, the highest expression of GATA2 is found in the CD56bright subset,33 suggesting that the absence of this important intermediate in patients represents an inherent abnormality. In aggregate, these findings suggest a specific and important role for GATA2 in either a key phase of NK cell development or in NK cell survival. Further experimental work will hopefully gain insight from these patients and their particular mutations to better understand the role of GATA2 in NK cell biology.
Until further clarity can be gained surrounding the natural history and biology of GATA2 deficient patients with reference to NK cells, the subset with an NK cell-specific presentation should be considered as CNKD1 given the original patient described. Clinicians, however, should be aware of the potential for the NK cell-specific GATA2 patients to present as a FNKD.
CNKD2
A familial form of CNKD was defined in 2006 and has been listed as “Natural Killer Cell Deficiency, Familial Isolated” in the Online Mendelian Inheritance in Man (OMIM) database as entry #609981.26 The original report described a large consanguineous Irish cohort one of whom had recurrent EBV-driven lymphoproliferative disease despite having evidence of intact adaptive immunity against EBV. Two other family members had recurrent viral infections. All three had ≤1% NK cells in peripheral blood. All also had intrauterine growth retardation and some had adrenal insufficiency. The family was evaluated genetically by microsatellite homozygosity mapping and the locus was linked to chromosome 8 (8p11.23–q11.21). Each of the genes in this region was sequenced and the MCM4 gene was identified as appropriately segregating with the clinical phenotype in an autosomal recessive pattern.39 Two additional Irish families with similar phenotypes were also identified as having the same mutations in MCM4 and thus presumably deriving from a common founder effect.39, 40 One of the scientific groups sharing in this discovery approached the topic because of the endocrinological manifestations, but arrived at the same molecular, immunologic and mechanistic conclusion.40
The MCM4 gene encodes the mini chromosome maintenance (MCM) complex member 4. It is a member of the MCM 2–7 protein complex that enables helicase function during DNA replication. The MCM complex is recruited to DNA origin of recognition sites to direct DNA unwinding and ultimately polymerization.41 MCM4 is widely expressed and its function is considered essential for most cells. Complete MCM4 deficiency is embryonic lethal in mice.42 Thus, an obvious question is why do specific mutations in MCM4 result in CNKD2? The answer is not presently clear, but some evidence has been experimentally defined in patient cells and other in vitro systems. Firstly, MCM4 mutant patient fibroblasts and lymphocytes do appropriately assemble an MCM complex, but demonstrate excessive DNA breaks after aphidicolin39 or diepoxybutane40 stress, respectively. The fibroblast abnormality was shown to be complemented by re-introducing wild-type MCM4 expression in vitro.39 Thus, it must follow that certain human cell types rely more intensely upon MCM function or some particular aspect of the mutated region of MCM4. Presumably these cells would include either NK cells or cells that support NK cell development, as well as certain key cells of the endocrine system.
As a second experimental clue, while patients were found to have substantively reduced numbers of NK cells, the depletion was reflected entirely within the CD56dim NK cell subset. This population contains the mature perforin-containing NK cells and interestingly the entirety of the CD56bright NK cell subset was preserved and potentially even increased.39 This finding generates two hypotheses to explain the effect of MCM4 mutation upon NK cells in CNKD2 patients. The first is derivative from the fact that the CD56bright NK cell subset contains the immature population that can serve as a developmental intermediate to CD56dim NK cells.5 Thus MCM4 mutation may be interrupting NK cell development at the CD56bright stage. The second hypothesis is that patient CD56dim NK cells are generated but have decreased survival in the face of the MCM4 mutation and thus are short-lived in CNKD2 patients. In this light decreased NK cell survival was documented in both NK cell subsets in patient cells.39 Thus at present either hypothesis is viable. Further work will likely determine which is correct as well as discern the surprising role of either the MCM complex and/or MCM4 in NK cells.
Diagnostically, CNKD2 can be suspected in the context of increased percentages of CD56bright NK cells (of total NK cells) in individuals with endocrine and/or growth abnormalities. These patients also have decreased NK cell cytotoxic function,26, 39 but it is presently unclear if this is a feature of: 1) extremely low total NK cell numbers; 2) decreased presence of mature CD56dim perforin-containing NK cells; or 3) an inherent inability of MCM4 mutated NK cells to kill.
Future of CNKD
It is expected that other genetic mechanisms underlying CNKD will be discovered in the near future. A CNKD2 “phenocopy” was described in a non-consanguinous French cohort and these individuals had growth retardation, and facial abnormalities.25 One died of disseminated CMV infection. The patient studied had a very small peripheral blood NK cell population that contained a preponderance of immature NK cells (defined in this report as CD56+/CD16−). Interestingly, separate studies of cultured patient T cells showed impaired IL-2 and IL-15-dependent survival.43 This is relevant as IL-15 in particular is a clear requirement for NK cell development and homeostasis.4 A separate family described over 30 years ago also has a similar NK cell phenotype.These individuals did not have growth retardation or endocrinological abnormalities. There were three affected family members all of whom experienced severe EBV infection.44 One case was immediately fatal, while a second (female) died later of progressive pulmonary decline. A third family member survived and has persistent NK cell abnormalities with near absent cytotoxicity and a preponderance of immature CD56bright NK cells (44 and JS Orange, unpublished). A separate recently reported spontaneous patient with pediatric melanoma and opportunistic fungal infection was also found to have an abnormal transition from CD56bright to CD56dim NK cells.45 It is presently unclear as to whether the molecular pathways affected in these cohorts will functionally overlap with that of CNKD2. That said, neither of the two family cohorts have been defined to have MCM4 mutations (despite this having been evaluated – unpublished results) and thus it is likely that the CNKD category will encompass additional genetic mechanisms that impact the CD56bright to CD56dim NK cell transition.
Additional detailed study of other individuals suggest that like in CNKD1, the CD56bright to CD56dim transition is not going to be the only step in NK cell differentiation or homeostasis targeted. An example is a recently described girl with an EBV-driven smooth muscle tumor. This patient had absent NK cell cytotoxic activity <1% NK cells in peripheral blood, but a normal ratio of CD56bright and CD56dim NK cells within the few that were identified.31 Her NK cells had some abnormal developmental signatures, however, as there were no CD57-expressing NK cells (a marker of terminal NK cell differentiation) and an increase in CD117+ NK cells (a hallmark of immaturity). This patient did not have MCM4 or GATA2 mutations (JSO unpublished results). Thus, it is probable that CNKD will comprise an array of genetic abnormalities that have the potential to selectively impact distinct steps in NK cell differentiation or NK cell subset survival. Further delineation of these will likely impact our understanding of not only this growing group of patients, but NK cell biology overall.
Functional NK cell deficiency (FNKD)
FNKD describes individuals who have normal numbers of NK cells present in peripheral blood, but ones that are functionally disabled in the face of otherwise effective immunity.46–51 An example of a well-known PID that results in absent NK cell function (cytotoxicity) would be perforin deficiency.52 Perforin deficiency, however, is not considered a FNKD because it also impairs the function of CTL. Thus the FNKD label is reserved for impact upon NK cells in relative isolation. It is anticipated that the FNKD category will be extensive, but has been more difficult to ascertain since the screening assay is one of cellular function. A recent study evaluating patients with severe and recurrent herpesvirus infections identified five with functional abnormalities,47 which was reflective of an historical study of similarly affected patients.48 The modern immunologic resolution applied in the current study suggested that specific phenotypic and functional aberrations may be present in each of the individuals with functional deficiency, but further analysis is needed.
While some of the patients with CNKD may present with NK cells in the peripheral blood, this may be akin to the “leaky SCID” phenomenon and requires further study of the natural history of those particular patients/mutations. It is also possible that some patients having CNKD that interferes with the CD56bright to CD56dim transition will present with numbers of peripheral blood total NK cells within the normal range. Since that would be a feature of increased immature NK cells with a paucity of mature cells, it is recommended that those patients (having abnormalities of NK cell development) be considered in the CNKD category.
The overarching theme in FNKD, however, is one of Herpesvirus susceptibility with the most common being HSV1.47, 48, 50 Abnormal susceptibility to or consequences of EBV, VZV, HPV and respiratory viruses, however, have all been described in FNKD.46,49,51,53 That said, here is likely some degree of selection bias in that in the majority only patients with abnormal susceptibility to Herpesviruses have been studied. Since there is presently one known genetic defect underlying FNKD, the same nomenclature as for CNKD should be utilized (Table 1). The first being designated FNKD1 and subsequent numbers reserved for additional genetic discoveries (i.e., FNKD2, FNKD3, etc).
FNKD1
The single known gene defect that causes FNKD is that of a particular mutation of the FCGR3A gene encoding CD16.49–51 As introduced above CD16 is the NK cell IgG Fc receptor and is best known for enabling ADCC. Thus far, FNKD1 has been described in three unrelated families, the first two almost 20 years ago. One had severe recurrent HSV stomatitis and recurrent herpetic whitlow.50 A second had progressive EBV infection and severe VZV infection requiring systemic therapy.49 Both had recurrent respiratory viral infections. A third patient was recently described and had EBV-driven monocentric Castleman’s disease and recalcitrant cutaneous warts.51 All patients had decreased spontaneous NK cell cytotoxicity against K562 target cells, but surprisingly none had abnormal ADCC.
The mutation underlying FNKD1 is recessive, rare and predicts homozygous substitution of Leucine with a Histidine at the 66th amino acid of CD16 (L66H).49–51 This alteration is in the distal Ig domain in the extracellular portion of CD16, which is not required for IgG binding (a function of the proximal Ig domain).54 The distal Ig domain has been recently shown to function in linking CD16 to the NK cell costimulatory receptor CD2.51 Thus the patient mutation does not affect ADCC, but does impair CD16 from being utilized as a costimulatory receptor when CD2 is ligated in the context of spontaneous NK cell cytotoxicity. The L66H mutation does not abrogate surface expression of CD16, but destroys an epitope present in the distal Ig domain recognized by anti-CD16 monoclonal antibody B73.1.49–51 The mutant CD16, however, is still recognized by the more commonly employed anti-CD16 3G8 monoclonal antibody. Thus, these two monoclonal antibodies can be used in screening for patients with this mutation using flow cytometry, as those affected will have NK cells that are recognized by 3G8, but not B73.1. FCGR3A gene sequencing must be applied to confirm, however, as patients with decreased NK cell expression of the B73.1 epitope without FCGR3A mutations have been identified.55
Future of FNKD
Although there are numerous anecdotal reports of individuals with susceptibility to infection and abnormal NK cell function, it is imperative that patients suspected of FNKD be rigorously considered and methodically evaluated (see Figure 2). The complexity in approaching FNKD lies in the fact that NK cell functions can be negatively affected by physiologic stress as well as certain therapeutic agents.56, 57 Thus, great caution must be applied in considering FNKD. That said, it is predicted that other specific receptor, or signaling molecule abnormalities will be discovered that impair specific NK cell subsets and/or NK cell functions in isolation of other major immunological effects. Enhanced NK cell subset and functional analyses in concert with careful evaluation of compelling patients will likely result in the significant growth of the FNKD diagnosis.
Known PIDs associated with an NK cell abnormality
While CNKD and FNKD represent a specific subset of PIDs, there are at least 47 known genetically defined PIDs that include an impact upon NK cell number and/or function. They can be divided into diseases that: 1) impair NK cell development or survival; 2) impair the mechanics of cytotoxicity; 3) impair signaling for cytotoxicity; and 4) impair some other mechanism. They are listed in Table 3, which provides an overview of the NK cell defect and mechanism as well as a single key reference for each. By definition these are not a CNKD or FNKD because the NK cell component of the immunodeficiency represents a minority portion of the overall immunodeficiency. These diseases have been reviewed four times previously, and the reader is referred to those sources for a more comprehensive consideration of each condition and its underlying mechanistic impact upon NK cells.17–20 It is beyond the scope of this review to cover each of these diseases in Table 3 in detail. An overarching theme, however, continues to be a preponderance of viral susceptibilities to which the NK cell abnormalities may contribute.
Table 3.
Primary immunodeficiency diseases with an NK cell abnormality
| Disease | Gene(s)a | NK cell Defect | Mechanismb | Infectious Susceptibility | Referencec |
|---|---|---|---|---|---|
| Diseases impairing NK cell development or survival | |||||
| X-linked SCID | IL2RG | Absent NK cells | IL-15 receptor signaling | Multiple infections | 61 |
| Autosomal recessive SCIDd |
JAK3 IKZF1 ADA MTHFD1 |
Absent or low NK cells | IL-15 receptor signaling Developmental transcriptional programs Metabolic requirements Metabolic requirements |
Multiple infections |
62 63 61 64 |
| IPEX-like syndrome with growth hormone deficiency | STAT5B | Absent or low NK cells | IL-15 receptor signaling | Multiple infections | 65 |
| Bloom Syndrome | BLM | Cytotoxicity | Unclear | Fungi, bacteria | 66 |
| Fanconi’s Anemia | FANCA-G | Low NK cells | Bone marrow impairment | Multiple infections | 67 |
| Dyskeratosis Congenita | DKC1 | Low NK cells | Bone marrow impairment | Multiple infections | 68 |
| Diseases impairing the mechanics of cytotoxicity | |||||
| FHL2 | PRF1 | Cytotoxicity | Absent perforin | Herpesviruses | 52 |
| FHL3 | UNC13D | Cytotoxicity | Lytic granules cannot dock at synaptic membrane | Herpesviruses | 69 |
| FHL4 FHL5 |
STX11 STXBP2 |
Cytotoxicity | Lytic granules cannot fuse with synaptic membrane | Herpesviruses |
70 71 |
| Chediak-Higashi syndrome | LYST | Cytotoxicity | Abnormal lytic granule biogenesis | EBV, fungi | 72 |
| Griscelli syndrome type 2 | RAB27A | Cytotoxicity | Lytic granules cannot detach from microtubules | Herpesviruses, bacteria | 73 |
| Hermansky Pudliak syndrome |
AP3B1 BLOC1S6 |
Cytotoxicity | Abnormal lytic granule biogenesis | Herpesviruses, bacteria |
74 75 |
| Papilon-Lefevre syndrome | CTSC | Cytotoxicity | Ineffective maturation of lytic machinery | Herpesviruses, bacteria | 76 |
| Wiskott-Aldrich syndrome |
WASP WIPF1 |
Cytotoxicity | Defective actin organization at immunological synapse | Herpesviruses, Multiple infections |
77 78 |
| Autosomal Recessive Hyper-IgE syndrome | DOCK8 | Cytotoxicity | Defective actin accumulation at immunological synapse | HPV, multiple infections | 59 |
| May Hegglin Anomaly | MYH9 | Cytotoxicity | Defective lytic granule positioning | Intracellular bacteria | 79 |
| Leukocyte adhesion deficiency type I | ITGB2 | Cytotoxicity | Defective target cell binding and lytic granule organization | Multiple infections | 58 |
| Leukocyte adhesion deficiency type III | FERMT3 | Cytotoxicity | Defective target cell binding and NK cell activation | Multiple infections | 80 |
| Diseases inherently impairing signaling for cytotoxicity | |||||
| XLP type 1 | SH2D1A | Cytotoxicity | Receptor-induced NK cell activation (CD244) | EBV | 81 |
| XLP type 2 | XIAP | Low numbers +/− cytotoxicity | Unclear | EBV | 82 |
| Non-X linked lymphoproliferative syndrome | ITK | Low numbers +/− cytotoxicity | Unclear | EBV | 83 |
| PLC-γ-associated immunodeficiency | PLCG2 | Degranulation | Reduced activation-induced calcium flux | Respiratory infections | 60 |
| PKC-δ deficiency | PRKCD | Cytotoxicity | Unclear | EBV | 84 |
| CRAC channel deficiency |
ORAI1 STIM1 |
Degranulation | Activation-induced calcium flux for degranulation | Multiple infections |
85 86 |
| NEMO deficiency | IKBKG | Cytotoxicity | Activation signaling and NF-κB activation | Mycobacteria, bacteria, CMV | 87 |
| ALPS (caspase 8 deficiency) | CASP8 | Cytotoxicity | Activation signaling and NF-κB activation | Herpesviruses, bacteria | 88 |
| STAT1 deficiency | STAT1 | Cytotoxicity and cytokine production | Activation-induced transcription | HSV, CMV, Fungi, Mycobacteria | 89 |
| Diseases impairing other functions | |||||
| Bare Lymphocyte syndrome |
TAP1 TAP2 |
Cytotoxicity | Aberrant NK cell licensing | Multiple infections |
90 91 |
| Severe congenital neutropenia | ELANE | Cytotoxicity | Homeostasis and terminal differentiation via neutrophils | Bacteria | 92 |
| X-linked hyper-IgM-I | CD40LG | Cytotoxicity | Unclear | Enteroviruses, Bacteria, Pneumocystis | 93 |
| Netherton Syndrome | SPINK5 | Cytotoxicity | Unclear | Cutaneous infections | 94 |
| IL-12/IL-12 receptor deficiency | IL12B IL12RB1 | Cytokine production, cytotoxicity | Deficient IL-12 signaling | Mycobacteria, Salmonella | 95 |
| IL-21 receptor deficiency | IL21R | Cytotoxicity | Unclear | Multiple infections | 96 |
| X-linked immunodeficiency with Mg2+ defect | MAGT1 | Phenotype | Unclear | EBV, Multiple infections | 97 |
| Rett-syndrome like MECP2 duplication | MECP2 | Low numbers | Abnormal Tbet function | Fungi, Pneumonia | 98 |
| CD25 deficiency | IL2RA | Low numbers | Unclear | CMV | 99 |
| Ataxia Telangiectasia | ATM | Cytokine production | Unclear | Multiple infections | 100 |
The gene names listed are those of the approved nomenclature of the Human Genome Organization Gene Nomenclature Committee as confirmed on July 6th 2013 via http://www.genenames.org. The reader is referred to the reference cited in this table or to the website to find alternative names used or those more commonly applied in clinical immunology.
Mechanism that specifically underlies the NK cell abnormality. The listing of “unclear” signifies that not enough information relative to NK cells is available to directly define, or firmly infer the underlying mechanism.
For most diseases there multiple references that document the NK cell deficiency or NK cell abnormality. In some cases there are also additional references that define the defective mechanism experimentally. Due to space constraints a single reference was selected. Where possible it is one that is particularly mechanistically illustrative, or the most recent.
Abbreviations: SCID – Severe Combined Immunodeficiency; IPEX – Immunodeficiency, polyendocrinopathy, enteropathy, X-linked; FHL - Familial hematophagocytic lymphohistiocytosis; ALPS – autoimmune lymphoproliferative syndrome; NF-κB – nuclear factor kappa light chain enhancer of activated B cells; NEMO – NF-κB essential modulator; PLC – phospholipase C; PKC – protein kinase C; CRAC - Ca2+ release-activated Ca2+; XLP - X-linked lymphoproliferative syndrome; STAT1 – signal transducer and activators of transcription 1
Since the publication of previous reviews summarizing these diseases with regards to NK cells, there have been new insights into the known associations of NK cell defects with PIDs, previously identified PIDs that have now been defined to include an NK cell defect, as well as entirely new PIDs that include an NK cell defect. An example of a new insight into a known association is Leukocyte Adhesion Deficiency, type I (LAD1). Here it was known that NK cells with ITGB2 mutation did not adhere effectively to their target cells owing to absence of the LFA-1 integrin. Recent studies, however, have shown that an absent LFA-1 signal in LAD1 patient NK cells also prevents effective lytic granule organization in the subset of patient NK cells that can adhere to a target cell.58 An example of a previously known PID that has newly been associated with an NK cell abnormality is that of autosomal recessive hyper-IgE syndrome caused by dedicator of cytokinesis (DOCK) 8 gene mutation. These patient NK cells can bind to their target cell, but do not accumulate actin filaments at the lytic immunological synapse,59 which is required for effective cytotoxicity. This is especially interesting as patients with DOCK8 mutation have a high incidence of warts, the defense against which can be contributed to by NK cells. Finally, an example of a new PID that includes an NK cell defect is that of phospholipase C (PLC)-γ-associated immunodeficiency in which defective NK cell degranulation owing to aberrant activation-induced calcium flux has been documented.60 The list of these diseases will inevitably continue to grow and represents a unique mechanistic contribution to the field of NK cell biology. Importantly the link to NK cell defense may provide some further clues as to the full range of clinical phenotype in affected patients.
Treatment of NK cell deficiencies
A variety of treatments are reported as having been applied to patients with CNKD and FNKD. That said, there has never been an organized clinical trial of any therapy in these patients. Most therapeutic approaches have focused upon the susceptibility to Herpesviruses and the application of prophylactic antiviral drugs. Anecdotal cases have described perceived success, with the most common being the use of acyclovir, gancyclovir and related agents.22, 27, 47, 50 Breakthrough infections may require treatment with higher doses or parenteral forms. Therapies for papillomaviruses have also been described with more limited success, including topical agents, physical approaches and immunostimulants. Given the susceptibility to HPV, all patients diagnosed with NKD should be considered for HPV vaccination.
Systemic administration of cytokine therapies have also been described, either for antiviral effect or even for some potential effect upon the NK cells themselves. A recently reported example is that of IFN-α in CNKD1,33 which potentially induced some NK cell cytotoxic function. It has also been used for this purpose in FNKD.53 Theoretically, any therapeutic NK cell stimulatory cytokine has the potential to be of value, but this topic requires more specific evaluation.
For patients whose deficiency is perceived as more immediately life-threating, hematopoietic stem cell transplantation may be an option. This has been successfully applied in CNKD1,37 as well as in otherwise undefined CNKD.29 Overall therapeutic approaches to patients with CNKD and FNKD need further clarification and to be considered on a case-by-case basis.
Conclusion
A growing number of patients have been recognized that have immunodeficiency that impacts NK cells as the majority immune defect. A larger number of broader PIDs also include an NK cell abnormality, which has been mechanistically informative and potentially clinically useful. Detailed clinical and phenotypic evaluation of patients with NKD, however, has allowed paradigms to emerge, which include the susceptibility to herpesviruses and HPV as well as patients that lack NK cells (CNKD) or their functions (FNKD). Genetic advances have enabled the identification of three genes that can cause these conditions, GATA2, MCM4 and FCGR3A and further investigation is likely to uncover additional genetic mechanisms. The insight that these patients provide into NK cell biology is in its infancy as is the clinical and diagnostic approach to patients. Consideration of NKD, however, represents a first step in appropriately linking patients to a diagnosis. Collaborative efforts around diagnosed patients are likely to provide clearer paths to effective patient management and treatment.
Clinical Implications or Key Messages.
NK cells are vital components of human immune defense and can be deficient in number and/or function.
An NK cell deficiency can be considered when NK cell abnormalities represent the majority inherent immune defect.
What do we know?
NKD is rare but results in susceptibility to Herpesvirus and Papillomavirus infections
NKD types include those of number and function (CNKD) or just function (FNKD)
Two genes for CNKD (GATA2 and MCM4) and one for FNKD (FCGR3A) have been identified
At least some CNKD include impact upon NK cell development or NK cell developmental intermediates.
The mechanism underlying FNKD1 is abnormal interaction between mutant CD16 and an NK cell costimulatory receptor.
At least 47 known single gene PIDDs include an NK cell defect.
What is still unknown?
Prevalence estimates for NKD
Why the CNKD genes GATA2 and MCM4 can specifically effect NK cells.
What other genes underlie other CNKD and FNKD subtypes.
What are the truly effective treatment options for patients with NKD.
The mechanism by which all of the PIDD genes that impact NK cells result in abnormalities.
Acknowledgments
Funding sources:
NIH-NIAID R01067946
The Jeffrey Modell Foundation
The author would like to acknowledge the inspiration and encouragement derived from patients with NKD as well as their collaboration in investigating these conditions further. The author is also indebted for quality collaborations with referring physicians and NK cell biologists who have made this work possible. The author apologizes to the authors of relevant works that could not be cited herein due to bibliography limitations.
Abbreviations used
- ADCC
antibody-dependent cell mediated cytotoxicity
- CD
cluster designation
- CNKD
classical natural killer cell deficiency
- CTL
cytotoxic T lymphocyte
- CMV
Cytomegaloviru
- DOCK
dedicator of cytokinesis
- EBV
Epstein-Barr virus
- FNKD
Functional natural killer cell deficiency
- HPV
Human Papilloma virus
- HSCT
Hematopoietic stem cell transplantation
- HSV
Herpes simplex virus
- IFN
Interferon
- LAD
Leukocyte adhesion deficiency
- KIR
Killer Cell Immunoglobulin-Like Receptor
- MCM
mini chromosome maintenance
- NCR
Natural Cytotoxicity Receptors
- NK
natural killer
- NKT
natural killer T
- NKD
natural killer cell deficiency
- PBMCs
peripheral blood mononuclear cells
- PID
primary immunodeficiency
- PLC
phospholipase C
- VZV
Varicella Zoster virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013 doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–94. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 9.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23:96–103. doi: 10.1016/j.coi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–44. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisnic VJ, Krmpotic A, Jonjic S. Modulation of natural killer cell activity by viruses. Curr Opin Microbiol. 2010;13:530–9. doi: 10.1016/j.mib.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–60. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 13.Lam RA, Chwee JY, Le Bert N, Sauer M, Pogge von Strandmann E, Gasser S. Regulation of self-ligands for activating natural killer cell receptors. Ann Med. 2013 doi: 10.3109/07853890.2013.792495. [DOI] [PubMed] [Google Scholar]
- 14.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94:S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 17.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 18.Orange JS. NK cell deficiency syndromes. In: Rose B, editor. UpToDate. Waltham: 2006. [Google Scholar]
- 19.Orange JS. Unraveling human natural killer cell deficiency. J Clin Invest. 2012;122:798–801. doi: 10.1172/JCI62620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood SM, Ljunggren HG, Bryceson YT. Insights into NK cell biology from human genetics and disease associations. Cell Mol Life Sci. 2011;68:3479–93. doi: 10.1007/s00018-011-0799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–17. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 22.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 23.Akiba H, Motoki Y, Satoh M, Iwatsuki K, Kaneko F. Recalcitrant trichophytic granuloma associated with NK-cell deficiency in a SLE patient treated with corticosteroid. Eur J Dermatol. 2001;11:58–62. [PubMed] [Google Scholar]
- 24.Ballas ZK, Turner JM, Turner DA, Goetzman EA, Kemp JD. A patient with simultaneous absence of “classical” natural killer cells (CD3−, CD16+, and NKH1+) and expansion of CD3+, CD4−, CD8−, NKH1+ subset. J Allergy Clin Immunol. 1990;85:453–9. doi: 10.1016/0091-6749(90)90155-w. [DOI] [PubMed] [Google Scholar]
- 25.Bernard F, Picard C, Cormier-Daire V, Eidenschenk C, Pinto G, Bustamante JC, et al. A novel developmental and immunodeficiency syndrome associated with intrauterine growth retardation and a lack of natural killer cells. Pediatrics. 2004;113:136–41. doi: 10.1542/peds.113.1.136. [DOI] [PubMed] [Google Scholar]
- 26.Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78:721–7. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146:423–5. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Min S, Monaco-Shawver L, Orange J, Church J. Classical Natural Killer Cell Deficiency (CNKD): a new case. Clin Immunol. 2009;131:S61. [Google Scholar]
- 29.Notarangelo LD, Mazzolari E. Natural killer cell deficiencies and severe varicella infection. J Pediatr. 2006;148:563–4. doi: 10.1016/j.jpeds.2005.06.028. author reply 4. [DOI] [PubMed] [Google Scholar]
- 30.Wendland T, Herren S, Yawalkar N, Cerny A, Pichler WJ. Strong αβ and γδ TCR response in a patient with disseminated mycobacterium avium infection and lack of NK cells and monocytopenia. Immunol Lett. 2000;72:75–82. doi: 10.1016/s0165-2478(00)00169-3. [DOI] [PubMed] [Google Scholar]
- 31.Shaw RK, Issekutz AC, Fraser R, Schmit P, Morash B, Monaco-Shawver L, et al. Bilateral adrenal EBV-associated smooth muscle tumors in a child with a natural killer cell deficiency. Blood. 2012;119:4009–12. doi: 10.1182/blood-2011-10-385377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilmour KC, Fujii H, Cranston T, Davies EG, Kinnon C, Gaspar HB. Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood. 2001;98:877–9. doi: 10.1182/blood.v98.3.877. [DOI] [PubMed] [Google Scholar]
- 33.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121:2669–77. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–29. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–5. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–8. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715–20. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spinner MA, Sanchez LA, Hsu AP, Calvo KR, Cuellar-Rodriguez J, Hickstein DD, et al. GATA2 deficiency: extended clinical phenotype in 57 patients. J Clin Immunol. 2013;33:672. [Google Scholar]
- 39.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122:821–32. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122:814–20. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bochman ML, Schwacha A. The Mcm complex: unwinding the mechanism of a replicative helicase. Microbiol Mol Biol Rev. 2009;73:652–83. doi: 10.1128/MMBR.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet. 2007;39:93–8. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- 43.Eidenschenk C, Jouanguy E, Alcais A, Mention JJ, Pasquier B, Fleckenstein IM, et al. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177:8835–43. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 44.Fleisher G, Starr S, Koven N, Kamiya H, Douglas SD, Henle W. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100:727–30. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 45.Domaica CI, Fuertes MB, Uriarte I, Girart MV, Sardanons J, Comas DI, et al. Human natural killer cell maturation defect supports in vivo CD56(bright) to CD56(dim) lineage development. PLoS One. 2012;7:e51677. doi: 10.1371/journal.pone.0051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komiyama A, Kawai H, Yabuhara A, Mitsuhiko Y, Miyagawa Y, Ota M, et al. Natural killer cell immunodeficiency in siblings: defective killing in the absence of natural killer cytotoxic factor activity in natrual killer and lymphokine-activated killer cytotoxicities. Pediatr. 1990;85:323–30. [PubMed] [Google Scholar]
- 47.Ornstein BW, Hill EB, Geurs TL, French AR. Natural killer cell functional defects in pediatric patients with severe and recurrent herpesvirus infections. J Infect Dis. 2013;207:458–68. doi: 10.1093/infdis/jis701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez C, Kirkpatrick D, Read SE, Fitzgerald PA, Pitt J, Pahwa S, et al. Correlation between low natural killing of fibroblasts infected with herpes simplex virus type 1 and susceptibility to herpesvirus infections. J Infect Dis. 1983;147:1030–5. doi: 10.1093/infdis/147.6.1030. [DOI] [PubMed] [Google Scholar]
- 49.de Vries E, Koene HR, Vossen JM, Gratama J-W, von dem borne AEGK, Waaijer JLM, et al. Identification of an unusual Fcg receptor IIIa (CD16) on natural killer cells in a patient with recurrent infections. Blood. 1996;88:3022–7. [PubMed] [Google Scholar]
- 50.Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. Natural Killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103:408–13. doi: 10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. 2012;122:3769–80. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Risma KA, Frayer RW, Filipovich AH, Sumegi J. Aberrant maturation of mutant perforin underlies the clinical diversity of hemophagocytic lymphohistiocytosis. J Clin Invest. 2006;116:182–92. doi: 10.1172/JCI26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cac NN, Ballas ZK. Recalcitrant warts, associated with natural killer cell dysfunction, treated with systemic IFN-alpha. J Allergy Clin Immunol. 2006;118:526–8. doi: 10.1016/j.jaci.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Tamm A, Schmidt RE. The binding epitopes of human CD16 (Fc gamma RIII) monoclonal antibodies. Implications for ligand binding. J Immunol. 1996;157:1576–81. [PubMed] [Google Scholar]
- 55.Lenart M, Trzyna E, Rutkowska M, Bukowska-Strakova K, Szaflarska A, Pituch-Noworolska A, et al. The loss of the CD16 B73. 1/Leu11c epitope occurring in some primary immunodeficiency diseases is not associated with the FcgammaRIIIa-48L/R/H polymorphism. Int J Mol Med. 2010;26:435–42. [PubMed] [Google Scholar]
- 56.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 57.Cederbrant K, Marcusson-Stahl M, Condevaux F, Descotes J. NK-cell activity in immunotoxicity drug evaluation. Toxicology. 2003;185:241–50. doi: 10.1016/s0300-483x(02)00613-3. [DOI] [PubMed] [Google Scholar]
- 58.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, et al. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood. 2013;121:2627–37. doi: 10.1182/blood-2012-06-437012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131:840–8. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–8. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–87. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 62.Roberts JL, Lengi A, Brown SM, Chen M, Zhou YJ, O’Shea JJ, et al. Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood. 2004;103:2009–18. doi: 10.1182/blood-2003-06-2104. [DOI] [PubMed] [Google Scholar]
- 63.Goldman FD, Gurel Z, Al-Zubeidi D, Fried AJ, Icardi M, Song C, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the Ikaros gene. Pediatr Blood Cancer. 2012;58:591–7. doi: 10.1002/pbc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keller MD, Ganesh J, Heltzer M, Paessler M, Bergqvist AG, Baluarte HJ, et al. Severe combined immunodeficiency resulting from mutations in MTHFD1. Pediatrics. 2013;131:e629–34. doi: 10.1542/peds.2012-0899. [DOI] [PubMed] [Google Scholar]
- 65.Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–92. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- 66.Etzioni A, Lahat N, Benderly A, Katz R, Pollack S. Humoral and cellular immune dysfunction in a patient with Bloom’s syndrome and recurrent infections. J Clin Lab Immunol. 1989;28:151–4. [PubMed] [Google Scholar]
- 67.Korthof ET, Svahn J, de Latour RP, Terranova P, Moins-Teisserenc H, Socie G, et al. Immunological profile of Fanconi anemia: A multicentric retrospective analysis of 61 patients. Am J Hematol. 2013 doi: 10.1002/ajh.23435. [DOI] [PubMed] [Google Scholar]
- 68.Cossu F, Vulliamy TJ, Marrone A, Badiali M, Cao A, Dokal I. A novel DKC1 mutation, severe combined immunodeficiency (T+B-NK- SCID) and bone marrow transplantation in an infant with Hoyeraal-Hreidarsson syndrome. Br J Haematol. 2002;119:765–8. doi: 10.1046/j.1365-2141.2002.03822.x. [DOI] [PubMed] [Google Scholar]
- 69.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–73. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 70.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cote M, Menager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18–2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–73. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 73.Wood SM, Meeths M, Chiang SC, Bechensteen AG, Boelens JJ, Heilmann C, et al. Different NK cell-activating receptors preferentially recruit Rab27a or Munc13–4 to perforin-containing granules for cytotoxicity. Blood. 2009;114:4117–27. doi: 10.1182/blood-2009-06-225359. [DOI] [PubMed] [Google Scholar]
- 74.Badolato R, Parolini S. Novel insights from adaptor protein 3 complex deficiency. J Allergy Clin Immunol. 2007;120:735–41. doi: 10.1016/j.jaci.2007.08.039. quiz 42–3. [DOI] [PubMed] [Google Scholar]
- 75.Badolato R, Prandini A, Caracciolo S, Colombo F, Tabellini G, Giacomelli M, et al. Exome sequencing reveals a pallidin mutation in a Hermansky-Pudlak-like primary immunodeficiency syndrome. Blood. 2012;119:3185–7. doi: 10.1182/blood-2012-01-404350. [DOI] [PubMed] [Google Scholar]
- 76.Meade JL, de Wynter EA, Brett P, Sharif SM, Woods CG, Markham AF, et al. A family with Papillon-Lefevre syndrome reveals a requirement for cathepsin C in granzyme B activation and NK cell cytolytic activity. Blood. 2006;107:3665–8. doi: 10.1182/blood-2005-03-1140. [DOI] [PubMed] [Google Scholar]
- 77.Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99:11351–6. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanzi G, Moratto D, Vairo D, Masneri S, Delmonte O, Paganini T, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med. 2012;209:29–34. doi: 10.1084/jem.20110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanborn KB, Mace EM, Rak GD, Difeo A, Martignetti JA, Pecci A, et al. Phosphorylation of the myosin IIA tailpiece regulates single myosin IIA molecule association with lytic granules to promote NK-cell cytotoxicity. Blood. 2011;118:5862–71. doi: 10.1182/blood-2011-03-344846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gruda R, Brown AC, Grabovsky V, Mizrahi S, Gur C, Feigelson SW, et al. Loss of kindlin-3 alters the threshold for NK cell activation in human leukocyte adhesion deficiency-III. Blood. 2012;120:3915–24. doi: 10.1182/blood-2012-02-410795. [DOI] [PubMed] [Google Scholar]
- 81.Tangye SG, Phillips JH, Lanier LL, Nichols KE. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165:2932–6. doi: 10.4049/jimmunol.165.6.2932. [DOI] [PubMed] [Google Scholar]
- 82.Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–82. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119:1350–8. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cgamma and Cbeta: a novel mechanism for PI3K-independent enhancement of FcepsilonRI-induced mast cell mediator release. Cell Signal. 2008;20:625–36. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maul-Pavicic A, Chiang SC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci U S A. 2011;108:3324–9. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fuchs S, Rensing-Ehl A, Speckmann C, Bengsch B, Schmitt-Graeff A, Bondzio I, et al. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol. 2012;188:1523–33. doi: 10.4049/jimmunol.1102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109:1501–9. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–8. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 89.Vairo D, Tassone L, Tabellini G, Tamassia N, Gasperini S, Bazzoni F, et al. Severe impairment of IFN-gamma and IFN-alpha responses in cells of a patient with a novel STAT1 splicing mutation. Blood. 2011;118:1806–17. doi: 10.1182/blood-2011-01-330571. [DOI] [PubMed] [Google Scholar]
- 90.Furukawa H, Yabe T, Watanabe K, Miyamoto R, Miki A, Akaza T, et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I) Hum Immunol. 1999;60:32–40. doi: 10.1016/s0198-8859(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 91.Markel G, Mussaffi H, Ling KL, Salio M, Gadola S, Steuer G, et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood. 2004;103:1770–8. doi: 10.1182/blood-2003-06-2114. [DOI] [PubMed] [Google Scholar]
- 92.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordonez-Rueda D, Barlogis V, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209:565–80. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostenstad B, Giliani S, Mellbye OJ, Nilsen BR, Abrahamsen T. A boy with X-linked hyper-IgM syndrome and natural killer cell deficiency. Clin Exp Immunol. 1997;107:230–4. doi: 10.1111/j.1365-2249.1997.284-ce1174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renner ED, Hartl D, Rylaarsdam S, Young ML, Monaco-Shawver L, Kleiner G, et al. Comel-Netherton syndrome defined as primary immunodeficiency. J Allergy Clin Immunol. 2009;124:536–43. doi: 10.1016/j.jaci.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guia S, Cognet C, de Beaucoudrey L, Tessmer MS, Jouanguy E, Berger C, et al. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111:5008–16. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
- 96.Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210:433–43. doi: 10.1084/jem.20111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–6. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang T, Ramocki MB, Neul JL, Lu W, Roberts L, Knight J, et al. Overexpression of methyl-CpG binding protein 2 impairs T(H)1 responses. Sci Transl Med. 2012;4:163ra58. doi: 10.1126/scitranslmed.3004430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goudy K, Aydin D, Barzaghi F, Gambineri E, Vignoli M, Ciullini Mannurita S, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146:248–61. doi: 10.1016/j.clim.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reichenbach J, Schubert R, Feinberg J, Beck O, Rosewich M, Rose MA, et al. Impaired interferon-gamma production in response to live bacteria and Toll-like receptor agonists in patients with ataxia telangiectasia. Clin Exp Immunol. 2006;146:381–9. doi: 10.1111/j.1365-2249.2006.03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]