Abstract
Objective:
Cognitive control deficits are commonly seen in Depression of Bipolar Disorder (BDd) and Unipolar Major Depressive Disorder (UDd). Because failure to differentiate BDd from UDd has significant clinical consequences we aimed to identify differential patterns of neural activity in BDd versus UDd underlying response inhibition and motor control in adolescents.
Methods:
Functional MRI was used to compare 12 BDd adolescents (mean age= 15.5±1.2) with age- and sex-matched ten UDd and ten healthy control (HC) adolescents during the performance of well-validated Go/NoGo task. NoGo response inhibition versus Go motor control blocks was used in whole-brain analysis and results were corrected for multiple comparisons.
Results:
There were no significant behavioral or neuroimaging findings between adolescents with BDd and UDd. However, both groups relative to HC showed significantly higher left superior temporal and left caudate activity during the NoGo condition. Moreover, left anterior cingulate (ACC) activity relative to HC was significantly higher only in BDd – not UDd – adolescents during the NoGo condition, and left caudate activity was higher only in UDd – not BDd – adolescents during the Go condition. In addition, several neural regions including dorsolateral prefrontal (DLPFC) were positively correlated with increased reaction time in UDd – not BDd – adolescents.
Conclusions:
Despite some similarities, neural correlates of depression are different in BDd and UDd relative to HC, and greater recruitment of ACC resources during response inhibition can help distinguish BDd.
Keywords: adolescence, depression, bipolar disorder, anterior cingulate, neuroimaging
Résumé
Objectif:
Les déficits de contrôle cognitif sont souvent observés dans la dépression du trouble bipolaire (dTB) et dans le trouble dépressif majeur unipolaire (dTU). Parce que ne pas différencier entre la dTB et la dTU a des conséquences cliniques significatives, nous avons cherché à identifier les modèles différentiels d’activité neurale dans la dTB comparativement à la dTU en ce qui concerne l’inhibition sous-jacente de la réponse et le contrôle moteur chez les adolescents.
Méthodes:
L’imagerie par résonance magnétique fonctionnelle (IRMf) a servi à comparer 12 adolescents souffrant de dTB (âge moyen = 15,5±1,2) avec 10 adolescents souffrant de dTU appariés selon l’âge et le sexe, et dix adolescents témoins en santé (TS) durant la performance d’une tâche Go/NoGo bien validée. L’inhibition de la réponse à NoGo par opposition aux blocs de contrôle moteur de GO a été utilisée dans une analyse du cerveau en entier et les résultats ont été corrigés pour de multiples comparaisons.
Résultats:
Il n’y avait pas de résultats de comportement ou de neuroimagerie significatifs entre les adolescents souffrant de dTB et de dTU. Cependant, les deux groupes comparés aux TS montraient une activité temporale gauche supérieure et caudale gauche significativement plus élevée durant la condition NoGo. En outre, l’activité du cortex cingulaire antérieur (CCA) gauche, en comparaison avec les TS, était significativement supérieure seulement chez les adolescents souffrant de dTB – et non de dTU – durant la condition NoGo, et l’activité caudale gauche était plus élevée seulement chez les adolescents souffrant de dTU – et non de dTB – durant la condition NoGo. De plus, plusieurs régions neurales, dont le cortex préfrontal dorsolatéral (CPFDL), étaient positivement corrélées à un temps de réaction accru chez les adolescents souffrant de dTU – et non de dTB.
Conclusions:
Malgré certaines similitudes, les corrélats neuraux de la dépression sont différents dans la dTB et la dTU comparativement aux TS, et un meilleur recrutement des ressources du CCA durant l’inhibition de la réponse peut contribuer à distinguer la dTB.
Keywords: adolescence, dépression, trouble bipolaire, cingulaire antérieur, neuroimagerie
Introduction
Studies suggest that at least 20% of adolescents experience a major depressive episode before adulthood that is associated with significant morbidity and mortality (Brent & Birmaher, 2006). On the other hand, bipolar disorder (BD) is a recurrent and severe illness characterized by substantial functional impairment and suicide risk (Birmaher et al., 2009; Gore et al., 2011). The depressive episodes of BD (BDd) are the most common mood manifestation of the illness and are associated with the most burden (Birmaher et al., 2006; Geller, Tillman, Craney, & Bolhofner, 2004). It is critical to differentiate these two types of depression because they have different courses and require different treatment approaches (Chang, 2009). It is difficult, however, to clinically differentiate the symptoms of BDd from those of the depression of unipolar major depressive disorder (UDd) (Chang, 2009).
Abnormal activation in prefrontal and subcortical regions is postulated to underlie impaired cognitive control and impulsivity that are commonly reported in both adolescents (Blumberg et al., 2003; Chang et al., 2004; Forbes, 2011; Halari et al., 2009; Killgore, Gruber, & Yurgelun-Todd, 2007; Leibenluft et al., 2007; Pan et al., 2011; Passarotti, Sweeney, & Pavuluri, 2010; Pavuluri, Ellis, Wegbreit, Passarotti, & Stevens, 2012; Pavuluri, Passarotti, Harral, & Sweeney, 2010; Singh et al., 2010) and adults (Altshuler et al., 2005; Kaladjian et al., 2009; Langenecker et al., 2007; Townsend et al., 2012; Wagner et al., 2006) with BD and UDd. However, in contrast to the cognitive control imaging studies in adults with non-depressed BD (Altshuler et al., 2005; Kaladjian et al., 2009; Townsend et al., 2012), a couple of studies in adolescents with non-depressed BD reported increased cortical and subcortical activity (Blumberg et al., 2003; Chang et al., 2004; Singh et al., 2010), suggesting the possibilities of prefrontal hyperactivity at a young age as the primary abnormality or functional compensation involving greater recruitment of regulatory prefrontal cortical systems to process the information. Furthermore, similar imaging studies in adults (Langenecker et al., 2007; Wagner et al., 2006) and one study with adolescents (Yang et al., 2009) suggested increased cortical activity in UDd, but no study has investigated neural correlates of cognitive control in acutely depressed adolescents with BD. Identifying differential patterns of functional abnormalities in cognitive control neural systems in BDd relative to UDd and HC adolescents may help differentiate BD early in development, facilitate understanding of the state-specific neural substrates of depression, and provide insight into the similarities and differences of neurobiological and developmental etiology of BD versus UDd in adolescents (Diler, 2011).
In this cross-sectional functional MRI study, using a well-validated block-design Go/NoGo task in adolescents with BDd and UDd (Pan et al., 2011; Singh et al., 2010), we aimed to identify neural substrates of cognitive control in depressed adolescents with BDd versus depressed adolescents with UDd relative to healthy controls (HC). We hypothesized that adolescents with both BDd and UDd relative to HC would be successful in completing the Go/NoGo task; however, adolescents with both BDd and UDd relative to HC, and those with BDd relative to UDd, would recruit more neural resources and have higher prefrontal and subcortical neural activity during the NoGo response inhibition.
Methods
Participants
Thirty-two right-handed adolescents (assessed using the Edinburgh Handedness Inventory (Oldfield, 1971)) aged 12 to 17 years who had reached puberty (with a score ≥ 3 on Tanner’s Pubertal Development Scale (Marshall & Tanner, 1969)) were enrolled in the study; 12 with BDd (six BD-I and six BD-II, ten females, mean age= 15.5±1.2), ten with UDd (eight females, mean age= 15.9±1.1), and ten HC (eight females, mean age= 15.6±1.1). All depressed adolescents met (DSM-IV-TR (APA, 2000)) criteria for a diagnosis of major depressive episode (≥ 2 weeks), as determined by both parent and adolescent interviews with the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997). In addition, as determined by both parent and adolescent interviews with K-SADS-PL, BDd adolescents met strict DSM-IV (APA, 2000) criteria (e.g., episodicity) for BD-I or II. No first- or second-degree family history for BD was allowed for adolescents with UDd; however, we included depressed youth whose parents were reported as healthy (30% in BDd group versus 40% in UDd group) or having other forms of psychopathology. At the time of the scan, the Children’s Depression Rating Scale-Revised (Poznanski & Mokros, 1995) (CDRS-R) ≥ 40 and the Young Mania Rating Scale (Young, Biggs, Ziegler, & Meyer, 1978) (YMRS) <11 were required covering the past two weeks for all depressed adolescents. There were two adolescents in each BDd and UDd group experiencing their first depressive episode; others had >1 depressive episode in the past two years. There were five adolescents in BDd and four in UDd groups that had childhood-onset (age <12) mood disorder, five adolescents in BDd and four in UDd groups that had history of psychiatric hospitalization, and four adolescents in BDd and three in UDd groups that had history of suicide attempt. There were four inpatient adolescents in BDd and four inpatient adolescents in UDd groups, and no adolescents were subject to another research study. The above-mentioned rates were not statistically different (p<0.05) between adolescents with BDd and UDd. HC were not allowed to have any psychiatric/medical conditions or any first- or second-degree family psychiatric history. Urine screening was performed before the scanning to rule out pregnancy and substance abuse. We measured anxiety with a self-rated anxiety scale (SCARED) (Birmaher et al., 1997) and global functioning with the Clinical Global Impression (CGI)-Severity scores (Spearing, Post, Leverich, Brandt, & Nolen, 1997). The clinical findings between adolescents with BDd and UDd, respectively, were: 8.8±6.2 weeks versus 13.8±6.2 weeks, for the mean duration of current depressive episodes; 73.8±12.5 versus 65.8±13.3, for depression scores on the CDRS-R; 2.4±1.6 versus 2.2±1.4, for mania scores on YMRS; 3.3±18.2 versus 28.1±14.7, for anxiety scores on the child SCARED; and 5.3±0.96 versus 5±0.8, for the CGI-Severity. These scores were not statistically different (p<0.05) between adolescents with BDd and UDd (Table 1).
Table 1.
Demographic information and clinical variables
| BDd | UDd | HC | Overall significance | Pairwise comparisons | |||
|---|---|---|---|---|---|---|---|
| Mean age (years) | 15.5±1.2 | 15.9±1.1 | 15.6±1.2 | p=0.6 | - | ||
| Gender (females) | 80% | 80% | 80% | p=0.6 | - | ||
| Race (white) | 70% | 70% | 70% | p=1 | - | ||
| Family history of BD (%) | 90% | 0% | 0% | - | - | ||
| BD subtypes | 6 BD I, 6 BD II | N/A | N/A | - | - | ||
| Comorbid ADHD | 30% | 20% | 0% | p=0.2 | - | ||
| Comorbid anxiety disorders | 70% | 60% | 0% | p=0.001 | BDd >HC** | UDd > HC** | |
| Mean duration of the current depressive episode (weeks) | 8.8±6.2 | 13.8±6.2 | - | p=0.06 | - | ||
| Children’s Depression Rating Scale-revised (CDRS-R) | 73.8±12.5 | 65.8±13.3 | 19.1±1.8 | p=0.0001 | BDd >HC** | UDd > HC** | |
| Screen for Child Anxiety Related Emotional Disorders (SCARED) | 34.3±17.8 | 28.1±14.7 | 2.2±1.4 | p=0.0001 | BDd >HC** | UDd > HC** | |
| Young Mania Rating Scale (YMRS) | 3.3±18.2 | 2.2±1.1 | 0.8±0.8 | p=0.003 | BDd >HC** | UDd > HC** | |
| Mean of the Clinical Global Impression (CGI)- Severity | 5.3±0.96 | 5±0.8 | 1 | p=0.0001 | BDd >HC** | UDd > HC** | |
Significance level was set at p<0.05. Standard deviation is reported after ±. Statistical values, significance levels were shown in the table. Group differences were shown in bold in the table with the double asterisk (**) for a significance level of p<0.01.
BDd: bipolar disorder in depressed state, UDd: unipolar major depressive disorder in depressed state, HC: healthy controls, N/A: not available, ADHD: attention deficit hyperactivity disorder.
Our exclusion criteria included any contraindications for fMRI, psychosis, pervasive developmental disorders, eating disorders, learning disorders, BD Not Otherwise Specified, substance use, and mental retardation. Mental retardation (IQ< 80) was screened using the Verbal IQ of the Wechsler Abbreviated Scale of Intelligence (WASI) (Weschler, 1999). In addition, we excluded adolescents with UDd with history of medication-induced hypomania/mania and/or family history of BD (Miklowitz & Chang, 2008; Pavuluri, Birmaher, & Naylor, 2005). In adolescents with comorbid ADHD (two in BDd and one in UDd), we withdrew stimulant medications for at least 24 hours before the neuroimaging assessment to minimize potential confounding effects of these medications considering the possible effect of medications on dopaminergic systems and neuroimaging measures (e.g., normalization of neural findings) (Almeida, Versace, Hassel, Kupfer, & Phillips, 2010; Almeida et al., 2009; Hassel et al., 2008; Leibenluft et al., 2007; Nelson et al., 2007; Phillips, Travis, Fagiolini, & Kupfer, 2008; Rich et al., 2008). Our method was similar to other fMRI studies in youth that withheld stimulants before scanning (Leibenluft et al., 2007; Nelson et al., 2007; Rich et al., 2008). There were seven adolescents with BDd and six adolescents with UDd that had comorbid anxiety disorders. We included medicated (limited to three medications; nine in BDd and six in UDd) and unmedicated (three in BDd and four in UDd) depressed adolescents. There were two adolescents with mood stabilizers as monotherapy (e.g., lithium or antiepileptic mood stabilizer); two with atypical antipsychotic plus antidepressant medication combinations; three with mood stabilizers plus atypical antipsychotic medication combinations; two with mood stabilizers plus atypical antipsychotic plus antidepressant medication combinations in the BD group; and six with antidepressant monotherapy in the UDd group.
The University of Pittsburgh institutional review board approved the study protocol. Parents and adolescents provided informed written consent before the study.
Neuroimaging task
The Go/NoGo task consisted of a five-minute 38-second block-design paradigm in which participants were shown 120 letters. In Go trials, participants pressed a button to a visually presented letter stimulus. In NoGo trials, participants avoided response to a non-target letter stimulus (the letter V). Trials were segregated into two block types: condition A (Go) blocks, each with 20 Go trials, and condition B (NoGo) blocks, each with ten randomly distributed Go and ten NoGo trials. Seventy-five percent of the 120 trials over the entire task were therefore Go trials. Blocks were presented in the order of ABBABA, with 20-second interleaved rest periods (blank screen). Blocks began with a one-second black screen with the word Start and closed with a one-second fixation cross. Letter presentation for all trials comprised white letter stimuli being projected on a black background for 500 ms, with an interstimulus interval of 1000 ms, during which a crosshair was shown. During the 20-second interleaved rest periods, participants viewed a black screen with the word Rest presented centrally (Pan et al., 2011).
Functional imaging data acquisition
All neuroimaging data were collected at the Magnetic Resonance Research Center (MRRC), University of Pittsburgh, on a Trio 3.0 Tesla scanner (Siemens, AG). Anatomical images covering the entire brain were acquired using an axial 3D MPRAGE sequence, parallel to the AC–PC line (TE/TI/TR=3.29ms/900ms/2200ms, flip angle=9, isotropic 1 mm3 voxel, 192 axial slices, matrix size=256×192). Blood Oxygen Level Dependent (BOLD) functional images were acquired with a gradient-echo EPI sequence and cover 34 axial slices (3 mm thick, 0 mm gap) encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25 msec, field of view=205 mm, matrix=64 × 64). Before collection of fMRI data for each subject, a reference EPI scan was acquired and inspected for artifacts (e.g., ghosting), as well as for good signal across the entire volume of acquisition, including the medial temporal lobes.
Task performance
Task performance data were analyzed using one-way analyses of variance (ANOVAs) to examine the main effect of group on task performance accuracy – numbers of correct Go and NoGo responses, omissions (misses for Go stimuli), and commissions (incorrect button press for NoGo stimuli) – using SPSS 17 (SPSS, Inc., Chicago, IL).
Imaging analyses
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM5; London, United Kingdom). Data for each participant were first corrected for differences in acquisition time between slices; realigned using the first slice as a reference; and unwarped to correct static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. Movement cutoff was 2 mm or less. Data were co-registered with the participant’s anatomic image, segmented, normalized to a standard MNI template, resampled to 3×3×3 mm3 voxels, and spatially smoothed with a Gaussian kernel of 6 mm full width at half-maximum. A first-level fixed-effect model was constructed for the two blocks (Go and NoGo) entered as separate conditions in a block design in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. Trials were modeled using the canonical hemodynamic response function. The two conditions were entered as separate t-contrasts into second-level analyses. A second-level random-effects model was used for between-group comparison. Because the main focus of this study was to examine the extent to which the three groups were distinguished by patterns of neural activity during the response inhibition (NoGo) and motor control (Go) blocks, a 3-group (BDd, UDd, HC)-by-2-condition (NoGo versus Go blocks) ANOVA covarying for age was performed to examine the group by condition interaction on whole-brain activity during task performance given the potentially different relationships between age and neural activity in each group. First, a voxel-wise threshold of p<.05 was used for whole-brain analyses. Second, a cluster-level false-positive detection rate of p<.05 was maintained for whole-brain activity surviving the voxel-wise threshold of p<.05 using small volume correction (SVC) with a regional anatomic mask from the WFU Pickatlas for each whole-brain activity cluster ≥10 voxels, and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim (Pan et al., 2011). Peak BOLD signal changes were extracted from regions showing a significant group-by-condition interaction in the 3×2 analysis for each group for each condition. Post-hoc tests were performed on these extracted BOLD signal values to examine the extent to which pairwise between-group differences in activity contributed to the significant group-by-condition interactions in these analyses using independent t tests and appropriate statistical thresholds to control for multiple tests. In these post-hoc tests for regions showing a significant group-by-condition interaction in the 3×2 ANOVA, a significance threshold of p < (.05/6) .008 was employed to control for the three independent between-group pairwise tests for each of the two conditions in each region. In exploratory analyses, the potential relations between extracted BOLD signal from neural regions showed between-group differences in activity during each of the two block types of the task and depression severity, anxiety, ADHD, age, gender, and medication at the time of scanning. Pearson’s correlation and independent t tests were conducted as appropriate in SPSS 17.
Results
Task performance data
There was no significant effect of group on task performance accuracy for percentage of inaccurate Go responses (BDd: 13.5±7.9, UDd: 14.3±9, HC: 13.7±4.9; F2,31=0.53, p=.95) and percentage of inaccurate NoGo responses (BDd: 19.9±4.1, UDd: 14.8±8, HC: 16.2±6.8; F2,31=1.93, p=.16). There was no significant effect of group in reaction time in milliseconds for Go responses (BDd: 376.5±39.2, UDd: 384.9±27.1, HC: 387.9±55.5; F2,31=.22, p=.8) and for NoGo responses (BDd: 394.8±34.7, UDd: 408.9±13.5, HC: 404.8 ± 39.7; F2,31=.58, p=.56).
Neuroimaging data
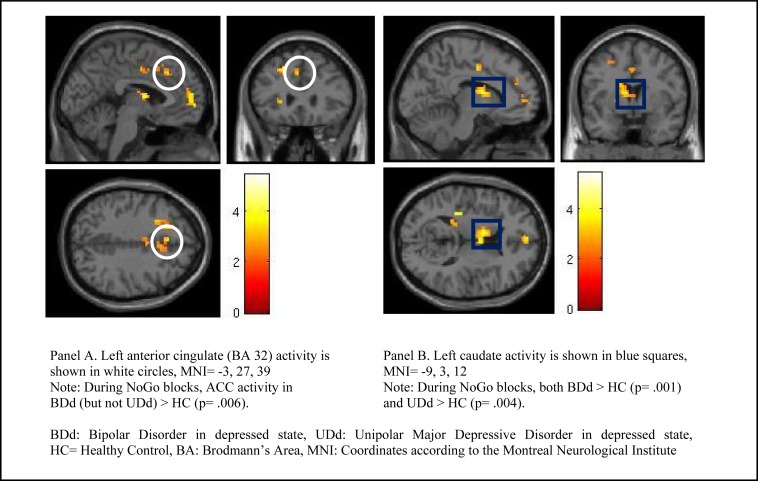
Group by condition analysis for the NoGo versus the Go condition by employing whole-brain analysis showed that several cortical regions (e.g., left supplemental motor area (BA 6), left dorsolateral prefrontal cortex (DLPFC, BA 9), left medial prefrontal cortex (BA 10), right insula, left anterior cingulate cortex (BA 32 and 33), right inferior parietal (BA 40), left superior temporal (BA 41), and left occipital (BA 17)) and subcortical (e.g., left caudate) regions were significantly different between BDd, UDd, and HC (Table 2). Pairwise comparisons showed that significance between the three groups was mainly due to increased neural activity in adolescents with both BDd and UDd relative to HC. More specially, adolescents with both BDd and UDd relative to HC had significantly higher neural activity in left superior temporal and left caudate during the NoGo condition. In addition, while the neural activity between adolescents with BDd and UDd was not significantly different, relative to HC, activity in left ACC (BA 32) during the NoGo condition was higher in adolescents with BDd but not with UDd, and activity in left caudate during the Go condition was higher in adolescents with UDd but not with BDd (Figure 1).
Table 2.
Whole-brain activation results: 3×2 analysis of variance for study group (BDd, UDd, and HC adolescents) by condition (Go, NoGo) covarying for age and post-hoc tests between study groups
| MNI
|
Post-hoc tests
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain region | Side | BA | Cl | Alpha Sim | Alpha p | x | y | z | F | t | pairwise p | Block | Groups |
|
|
|
||||||||||||
| Cortical | |||||||||||||
| Supplemental motor area | L | 6 | 228 | 29 | 0.047 | −18 | 12 | 60 | |||||
| DLPFC | L | 9 | 82 | 17 | 0.04 | −6 | 48 | 33 | 5.71 | 2.4 | 0.026 | NoGo | BD>HC |
| Medial Frontal | L | 10 | 95 | 20 | 0.042 | −21 | 48 | 15 | 5.025 | 2.25 | 0.036 | NoGo | BD>HC |
| Right Insula | R | 43 | 18 | 0.041 | 54 | −33 | 27 | - | |||||
| ACC | L | 32 | 41 | 21 | 0.043 | −3 | 27 | 39 | 9.46 | 3.09 | 0.006* | NoGo | BD>HC |
| 6.66 | 2.59 | 0.017 | Go | BD>HC | |||||||||
| ACC | L | 33 | 50 | 16 | 0.039 | 0 | 18 | 18 | - | ||||
| Inferior parietal | R | 40 | 264 | 32 | 0.047 | 63 | −30 | 42 | - | ||||
| Superior temporal | L | 41 | 121 | 10 | 0.039 | −39 | −33 | 12 | 11.28 | 3.38 | 0.003* | NoGo | BD>HC |
| 11.06 | 3.35 | 0.004* | NoGo | UDd>HC | |||||||||
| Occipital | L | 19 | 417 | 12 | 0.045 | −39 | −66 | 12 | 7.002 | 2.66 | 0.015 | NoGo | BD>HC |
| 6.34 | 2.53 | 0.021 | NoGo | UDd>HC | |||||||||
| Subcortical | |||||||||||||
| Caudate | L | 78 | 12 | 0.05 | −9 | 3 | 12 | 7.58 | 2.77 | 0.012 | Go | BD>HC | |
| 13.39 | 3.69 | 0.001* | NoGo | BD>HC | |||||||||
| 12.41 | 3.55 | 0.002* | Go | UDd>HC | |||||||||
| 11.05 | 3.36 | 0.004* | NoGo | UDd>HC | |||||||||
Pairwise comparisons using t-tests for significant brain regions identified with ANOVA analysis (group by condition interaction) and significance level was identified as p < 0.008 (0.05/6) with an asterisk symbol under pairwise p values (corrected for multiple comparisons).
BA: Brodmann’s Area, CL: Cluster Size; AlphaSim: minimum cluster size for significance after AlphaSim correction, MNI: Coordinates according to the Montreal Neurological Institute, BDd: Bipolar Disorder in depressed state, UDd: Unipolar Major Depressive Disorder in depressed state, R=Right, L=Left, DLPFC: Dorsolateral Prefrontal Cortex, ACC: Anterior Cingulate Cortex.
Figure 1.
Left Anterior Cingulate (panel A) and Left Caudate (panel B) activity during the NoGo versus the Go condition (whole-brain analysis) in adolescents with BDd versus UDd relative to HC.
Exploratory analysis
There were no significant differences in neural activity and behavioral response (e.g., accuracy and reaction time) between BD I versus BD II depressed adolescents and between those with first or recurrent episode of depression in any regions showing functional abnormalities in adolescents with BD and UDd relative to HC. In adolescents with BDd, neural activity during both the Go (t=−3.27, p=0.008) and the NoGo (t=−3.42, p=0.007) conditions in the left caudate was higher in males than in females (p=0.01). There were no significant relationships between depression severity and behavioral response and magnitude of activity in any of those neural regions showing functional abnormalities relative to HC adolescents in adolescents either with BDd or UDd. Adolescents with BDd and UDd with, versus without, ADHD and with, versus without, anxiety disorders did not differ in behavioral response, depression scores, duration of depression, and neural activity in any of those regions showing significant functional abnormalities relative to HC adolescents. There were no significant differences in behavioral response and activity in any of the above significant neural regions in either group (adolescents with BDd and UDd) between those with (eight in BDd and six in UDd) versus those without psychotropic medication. When we analyzed association of neural activity and behavioral response and types of medications in adolescents with BDd, those who were on antipsychotic plus antidepressant medications (n=2) relative to those who were on no medication (n=3) had higher accuracy (87% versus 78%, t= 6.04, p= 0.009) and lower left occipital activity (t=3.34, p= 0.044) during the NoGo condition.
The correlation of behavioral response with neural activity was different in each study group: While there was no significant correlation in HC, in BDd adolescents, longer reaction time was positively correlated with higher left DLPFC activity during both the Go (r= .744, p= .006) and the NoGo (r= .632, p= .028) conditions. In contrast, in adolescents with UDd, longer reaction time was negatively correlated with neural activity in left DLPFC (Go condition; r= − .794, p= .006), left ACC (BA 32, Go condition; r= −.750, p= 0.013), and left caudate (Go condition; r= −. 788, p= .007 and NoGo condition; r= −. 680, p= .031).
Discussion
To our knowledge, this is the first study to examine similarities and differences of neural correlates of cognitive control in depressed adolescents with BDd versus those with UDd. Early diagnosis of BD, especially during the depressed state, is of vital importance considering the long delay and difficulty in distinguishing BD from the depression of UD and the high risk of morbidity and mortality associated with late diagnosis (Birmaher & Axelson, 2006). In addition, many children develop more severe forms of BD during follow-up (Axelson et al., 2011), while prefrontal maturation during adolescence is still in progress. There may be an opportunity to halt the progression of this neurodevelopmental illness, especially if the nature of the neural abnormalities can be identified (Fleck et al., 2010). Our results indicated that both BDd and UDd relative to HC showed significantly higher neural activity during the NoGo condition (e.g., left superior temporal and left caudate); however, there were dissociable patterns of neural activity underlying cognitive control in adolescents with BD versus adolescents with UDd relative to HC that can help distinguish neural substrates of depression in these two disorders. While no neural region was significantly different between BDd and UDd, in comparison to HC, left ACC (BA 32) activity was significantly higher during the NoGo condition only in BDd – not UDd – adolescents, and left caudate activity was higher during the Go condition only in UDd – not BDd – adolescents. Moreover, our findings of significant correlations between behavioral response and neural activity in adolescents with UDd (e.g., higher activity in left DLPFC (Go condition), left ACC (Go condition), and left caudate (Go and NoGo conditions) was correlated with shorter reaction time) suggested that adolescents with UDd recruited more of these neural regions to meet the demand of the cognitive task.
Our results of no behavioral difference in depressed groups relative to HC, but abnormal neural activity underlying cognitive control in cortical prefrontal/cingular (e.g., executing inhibitory control processes), temporal (e.g., attentional processing) and subcortical caudate regions (e.g., executing and stopping motor response), are similar to the majority of available studies in non-depressed BD and UDd children and adolescents (Blumberg et al., 2003; Chang et al., 2004; Forbes, 2011; Halari et al., 2009; Killgore et al., 2007; Leibenluft et al., 2007; Pan et al., 2011; Passarotti et al., 2010; Pavuluri et al., 2010; Singh et al., 2010). Increased activity of cortical and/or subcortical regions during response inhibition in this study in adolescents with BDd and UDd relative to HC was similar to the studies in adolescents with non-depressed BD (Blumberg et al., 2003; Chang et al., 2004; Singh et al., 2010) and UDd (Yang et al., 2009) and adults with UDd (Langenecker et al., 2007; Wagner et al., 2006). However, the direction of abnormalities was not consistent in other studies, and our results were in contrast to the majority of studies in euthymic or manic adults with BD that showed hypoactivity of prefrontal and subcortical neural activity during response inhibition (Altshuler et al., 2005; Kaladjian et al., 2009; Townsend et al., 2012) and to the few other fMRI studies in adolescents with BD (Leibenluft et al., 2007; Passarotti et al., 2010) and UDd (Halari et al., 2009). Although mean age between our groups did not differ, it is important to consider dissociable neural activity underlying motor inhibition between children and adults. For example, a recent study reported increased ACC activity in healthy youth compared to healthy adults when making errors, and decreased ACC activity in children with BD but increased ACC activity in adults with BD compared to age-matched healthy subjects during failed motor inhibition (Weathers et al., 2012). Several factors in addition to age may have contributed to the different findings in previous studies including a different clinical profile of the subjects (sex, acute mood state, and comorbidity), different tasks (e.g., response inhibition, interference) and different designs (e.g., block, event-related). Our cross-sectional study cannot answer whether cortical or subcortical hyperactivity came first in adolescents with BDd and UDd, and the question of whether patterns of increased activity during response inhibition in BDd adolescents progresses into hypoactivity of prefrontal-subcortical regions in adulthood remains unanswered. A recent treatment study in manic adolescents reported that left subgenual ACC activity during response inhibition was increased but subcortical amygdala activity did not change after divalproex treatment (Pavuluri et al., 2012), suggesting that some neural abnormalities in adolescents with BD can be mood-state dependent. Similar to our results, a recent study in adults, using a different task for working memory with emotional distractors (EFNBACK), suggested that dorsal ACC activity to high memory load with neutral face distractors can help distinguish BDd from UDd in female adults (Bertocci et al., 2011), although in that study UDd females had higher neural activity than BDd. Although the direction of change was not similar across previous studies, ACC is implicated in many studies and postulated as an important neural hub that has strong connections from lateral prefrontal activity as well as subcortical regions subserving several neural networks including cognitive control (Botvinik, Nystrom, Fissell, Carter, & Cohen, 1999; Johansen-Berg et al., 2008). Our findings of increased ACC (BA 32) activity relative to HC in adolescents with BDd but not in those with UDd add to the literature that the direction of abnormal ACC activity in depressed adolescents relative to HC can help distinguish BD from UDd.
Depression scores in our study were not correlated with any neural activity in each depressed group. The only published fMRI study available in BDd adolescents reported positive correlation of DLPFC activity with depression scores (Chang, Wagner, Garrett, Howe, & Reiss, 2008), but earlier cognitive control studies in adolescents with UDd reported discrepant findings about the correlation of depression scores with neural findings (positive correlation with ACC (Killgore et al., 2007; Yang et al., 2009) and left DLPFC (Killgore et al., 2007) and negative correlation with medial prefrontal cortex and right DLPFC (Killgore et al., 2007)). These conflicting findings may reflect failure or increased efforts of prefrontal regions to compensate for increased subcortical regions while attempting to maintain behavioral response in adolescents. Each depressed group relative to HC had increased left caudate activity during the NoGo condition, but only UDd and not BDd adolescents showed increased left caudate activity during the Go condition of motor control relative to HC. Subcortical regions are important regions in executing or ending the motor response; however, impairment in cognitive processing between groups may be related to the cortical-subcortical network rather than abnormalities in individual regions, as our correlation analysis suggested that the DLPFC may be effective in UDd adolescents, but not in BDd adolescents, in improving accuracy of behavioral responses. The prefrontal cortex, especially DLPFC, is identified as the neural region underlying effortful regulation of cognitive processes, and its maturation for enhanced connectivity is delayed during healthy maturation compared to other cortical regions, and its neurodevelopmental trajectory is possibly disrupted in BD (Fleck et al., 2010). Although similar deficits were reported in both children and adults with bipolar disorder compared to healthy subjects during successful motor inhibition (e.g., decreased neural activity in the nucleus accumbens and ventral prefrontal cortex), the direction of neural abnormalities in ACC during failed motor inhibition was different in children (e.g., hypoactivation) and adults (e.g., hyperactivation) compared to health controls, suggesting the need for longitudinal studies to characterize developmental trajectories of response inhibition and motor control in youth with depression (Weathers et al., 2012). Although our whole-brain analysis could not identify significant correlation of neural regions with accuracy of behavioral response in BDd, future studies should apply brain network approach and connectivity analysis to better map the cognitive abnormalities and their neural substrates in UDd versus BDd adolescents.
Limitations
There were limitations to this study. This is a cross-sectional study with a small sample size, and we recruited predominantly female adolescents who had low ADHD comorbidity (10 to 20%) relative to some pediatric BD studies (Axelson et al., 2006), and they were allowed to be on (non-stimulant) psychotropic medications during scanning. Available studies suggest that medication may have normalizing, rather than confounding, effects upon abnormal neural activity in adolescents with BD (Almeida et al., 2010; Almeida et al., 2009; Hassel et al., 2008; Leibenluft et al., 2007; Nelson et al., 2007; Phillips et al., 2008; Rich et al., 2008). In our study, there were no significant differences in activity in any of the above significant neural regions in each study group between those with versus without psychotropic medication. However, we found lower left occipital activity during the NoGo experiment in BDd adolescents in those who were on atypical antipsychotic medication plus antidepressant combination relative to those BDd adolescents on no medication, suggesting the possibility of type II error (e.g., the normalization effect of this medication combination on neural abnormality in BDd versus HC), rather than type I error. Although event-related designs and analyses might be able to extract individual cognitive processes subserved by activated brain regions, we used block-design Go/NoGo task, similar to studies in adolescents with UDd (Pan et al., 2011) and euthymic BD (Singh et al., 2010), to examine a combination of cognitive control processes that includes response inhibition in addition to sustained attention, target detection, and rule maintenance over a sustained period of time to capitalize on a higher proportion of NoGo trials (Pan et al., 2011; Singh et al., 2010).
Conclusions
Both adolescent groups with BDd and UDd shared similar neural correlates of depression (e.g., higher left superior temporal and left caudate activity) during response inhibition, but our study provides the first results of dissociable patterns of neural activity underlying cognitive control in adolescents with BDd versus UDd relative to HC, suggesting that increased ACC activity during response inhibition can help distinguish BDd adolescents. However, we still know little about state versus trait abnormalities and how the disruption in neural maturation progresses over time (Strakowski et al., 2012), and we need larger longitudinal fMRI studies mapping developmental trajectories of cognitive neural networks in adolescents with BD versus UDd.
Acknowledgments/Conflicts of Interest
This study was supported by the American Academy of Child and Adolescent Psychiatry (AACAP) Ryan Licht Sang Foundation Quest for the Test Bipolar Disorder Award. Dr. Birmaher receives royalties from Random House Inc and Lippincott Williams and Wilkins. Other authors reported no conflict of interest. Dr. Ladouceur (K01 MH083001) and Dr. Pan (K23 MH082884) receive funding from NIMH.
References
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: A state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67(5):414–421. doi: 10.1016/j.biopsych.2009.09.027. S0006-3223(09)01154-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66(5):451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Cohen MS. Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58(10):763–769. doi: 10.1016/j.biopsych.2005.09.012. S0006-3223(05)01208-4 [pii] [DOI] [PubMed] [Google Scholar]
- American Psychological Association . Diagnostic and Statistical Manual of Mental Disorders, Disorders, 4th ed. Text Revision (DSM-IV-TR) Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- Axelson DA, Birmaher B, Strober MA, Goldstein BI, Ha W, Gill MK, Keller MB. Course of subthreshold bipolar disorder in youth: Diagnostic progression from bipolar disorder not otherwise specified. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(10):1001–1016. e1003. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR, Phillips ML. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychological Medicine. 2011:1–12. doi: 10.1017/S003329171100242X. S003329171100242X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: A review of the existing literature. Development and Psychopathology. 2006;18(4):1023–1035. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry. 2009;166(7):795–804. doi: 10.1176/appi.ajp.2009.08101569. appi.ajp.2009.08101569 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63(2):175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: Preliminary observations from functional MRI. American Journal of Psychiatry. 2003;160(7):1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Botvinik M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brent DA, Birmaher B. Treatment-resistant depression in adolescents: Recognition and management. Child and Adolescent Psychiatric Clinics of North America. 2006;15(4):1015–1034. x. doi: 10.1016/j.chc.2006.05.001. S1056-4993(06)00035-6 [pii] [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61(8):781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang K. Challenges in the diagnosis and treatment of pediatric bipolar depression. Dialogues in Clinical Neuroscience. 2009;11(1):73–80. doi: 10.31887/DCNS.2009.11.1/kchang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disorders. 2008;10(3):426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Diler RS. Neuroimaging can help identify biomarkers of early onset bipolar disorder. Bulletin of Clinical Psychopharmacology. 2011;22(1):1–4. [Google Scholar]
- Fleck DE, Cerullo MA, Nandagopal J, Adler CM, Patel NC, Strakowski SM, Delbello M. Neurodevelopment in Bipolar Disorder: A Neuroimaging Perspective. In: Miklowitz DJ, Cicchetti D, editors. Understanding Bipolar Disorder: A Developmental Psychopathology Perspective. New York, NY: The Guilford Press; 2010. pp. 259–281. [Google Scholar]
- Forbes EE. fMRI studies of reward processing in adolescent depression. Neuropsychopharmacology. 2011;36(1):372–373. doi: 10.1038/npp.2010.164. npp2010164 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Archives of General Psychiatry. 2004;61(5):459–467. doi: 10.1001/archpsyc.61.5.459. [see comment] [DOI] [PubMed] [Google Scholar]
- Gore FM, Bloem PJN, Patton GC, Ferguson J, Joseph V, Coffey C, Mathers CD. Global burden of disease in young people aged 10–24 years: A systematic analysis. The Lancet. 2011;377(9783):2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. JCPP1972 [pii] [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: No associations with psychotropic medication load. Bipolar Disorders. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. bhm167 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: An event-related fMRI study. Psychiatry Research. 2009;173(1):45–51. doi: 10.1016/j.pscychresns.2008.08.003. S0925-4927(08)00122-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data [see comments] Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neuroscience Letters. 2007;416(1):43–48. doi: 10.1016/j.neulet.2007.01.081. S0304-3940(07)00100-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007;62(11):1272–1280. doi: 10.1016/j.biopsych.2007.02.019. S0006-3223(07)00154-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Chang KD. Prevention of bipolar disorder in at-risk children: Theoretical assumptions and empirical foundations. Development & Psychopathology. 2008;20(3):881–897. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, Leibenluft E. Brain systems underlying response flexibility in healthy and bipolar adolescents: An event-related fMRI study. Bipolar Disorders. 2007;9(8):810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edingurgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(6):602–611. e603. doi: 10.1016/j.jaac.2011.03.018. S0890-8567(11)00271-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Research. 2010;181(1):36–43. doi: 10.1016/j.pscychresns.2009.07.002. S0925-4927(09)00167-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: A review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Ellis JA, Wegbreit E, Passarotti AM, Stevens MC. Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behavioral Brain Research. 2012;226(2):493–503. doi: 10.1016/j.bbr.2011.10.003. S0166-4328(11)00721-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. Journal of Clinical Psychiatry. 2010;71(11):1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Mokros H. Children’s Depression Rating Scale-Revised. Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: Impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Mazaika P, Garrett A, Adleman N, Kelley R, Reiss A. Neural correlates of response inhibition in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20(1):15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Research. 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, Townsend JD. The functional neuroanatomy of bipolar disorder: A consensus model. Bipolar Disorders. 2012;14(4):313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend JD, Bookheimer SY, Foland-Ross LC, Moody TD, Eisenberger NI, Fischer JS, Altshuler LL. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disorders. 2012;14(4):442–450. doi: 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Schlosser RG. Cortical inefficiency in patients with unipolar depression: An event-related FMRI study with the Stroop task. Biological Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. S0006-3223(05)01434-4 [pii] [DOI] [PubMed] [Google Scholar]
- Weathers JD, Stringaris A, Deveney CM, Brotman MA, Zarate CA, Jr, Connolly ME, Leibenluft E. A developmental study of the neural circuitry mediating motor inhibition in bipolar disorder. American Journal of Psychiatry. 2012;169(6):633–641. doi: 10.1176/appi.ajp.2012.11081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Association; 1999. [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20(4):440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]