Abstract
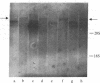
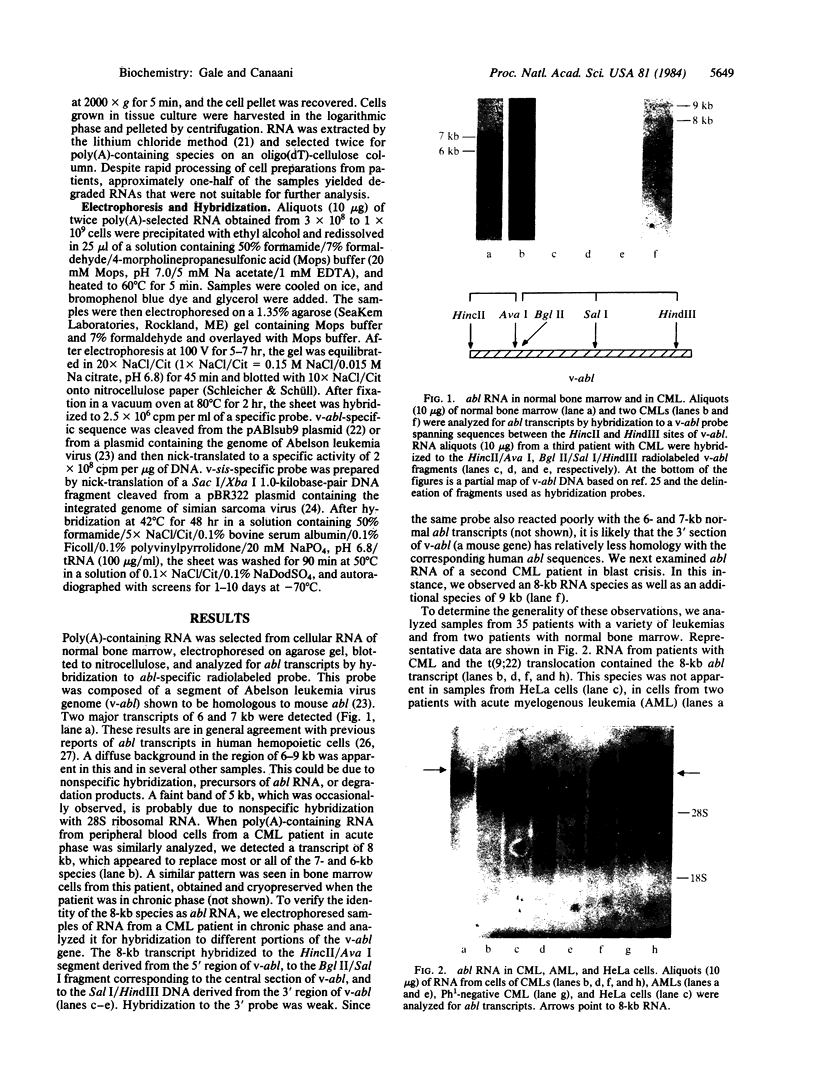
Chronic myelogenous leukemia (CML) is a clonal hematologic malignancy characterized by a reciprocal translocation between chromosomes 9 and 22 [t(9;22)] in greater than 90% of cases. This translocation results in a short chromosome 22, termed the Philadelphia (Ph1 or 22q-) chromosome. Recently, the cellular oncogenes abl and sis were mapped to human chromosomes 9 and 22, respectively. Moreover, abl was shown to be translocated from chromosome 9 to 22 and sis from chromosome 22 to 9 in CML patients with t(9;22). These findings raised the possibility that one or both of these oncogenes is activated and directly involved in the development of the disease. We analyzed expression of the abl and sis oncogenes in leukemic cells from CML patients with t(9;22). We found that sis is not expressed but that abl is transcribed into an 8-kilobase RNA. This abl RNA is also present in two leukemic cell lines (EM2 and K562), which were derived from CML patients and contain the t(9;22). This 8-kilobase RNA is not detected in normal cells, in other human leukemias without t(9;22), or in human cell lines that lack t(9;22). The consistent presence of this abl RNA transcript in CML with t(9;22) suggests that it is a consequence of abl translocation and that it plays a role in the development of this leukemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bartram C. R., de Klein A., Hagemeijer A., Grosveld G., Heisterkamp N., Groffen J. Localization of the human c-sis oncogene in Ph1-positive and Ph1-negative chronic myelocytic leukemia by in situ hybridization. Blood. 1984 Jan;63(1):223–225. [PubMed] [Google Scholar]
- Bartram C. R., de Klein A., Hagemeijer A., van Agthoven T., Geurts van Kessel A., Bootsma D., Grosveld G., Ferguson-Smith M. A., Davies T., Stone M. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Gale R. P., Steiner-Saltz D., Berrebi A., Aghai E., Januszewicz E. Altered transcription of an oncogene in chronic myeloid leukaemia. Lancet. 1984 Mar 17;1(8377):593–595. doi: 10.1016/s0140-6736(84)90997-8. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gallo R. C., Giallongo A., Croce C. M. Chromosomal localization of the human homolog (c-sis) of the simian sarcoma virus onc gene. Science. 1982 Nov 12;218(4573):686–688. doi: 10.1126/science.6291150. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Retroviral transforming genes in normal cells? Nature. 1983 Jul 21;304(5923):219–226. doi: 10.1038/304219a0. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
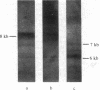
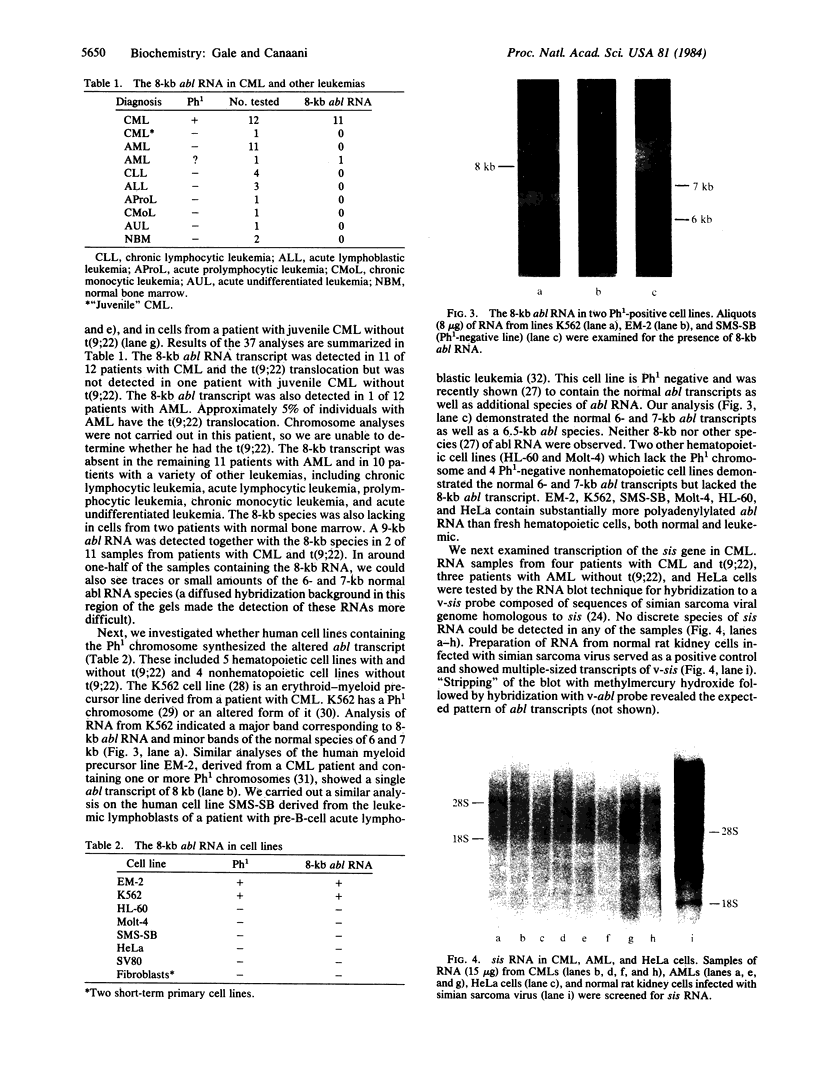
- Francis G. E., Michalevicz R., Wickremasinghe R. G. Chronic myeloid leukaemia and the Philadelphia translocation: do the c-sis oncogene and platelet-derived growth factor provide the link? Leuk Res. 1983;7(6):817–820. doi: 10.1016/0145-2126(83)90076-0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Davisson P. B., Gans P. J., Moloney W. C. In vitro induction of continuous acute promyelocytic leukemia cell lines by Friend or Abelson murine leukemia virus. Blood. 1979 May;53(5):987–1001. [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Stephenson J. R., van Kessel A. G., de Klein A., Grosveld G., Bootsma D. c-sis is translocated from chromosome 22 to chromosome 9 in chronic myelocytic leukemia. J Exp Med. 1983 Jul 1;158(1):9–15. doi: 10.1084/jem.158.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Chronic myelogenous leukemia--new concepts (first of two parts). N Engl J Med. 1981 May 14;304(20):1201–1209. doi: 10.1056/NEJM198105143042004. [DOI] [PubMed] [Google Scholar]
- Lewis J. P., Watson-Williams E. J., Lazerson J., Jenks H. M. Chronic myelogenous leukemia and genetic events at 9q34. Hematol Oncol. 1983 Jul-Sep;1(3):269–274. doi: 10.1002/hon.2900010309. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mitelman F., Levan G. Clustering of aberrations to specific chromosomes in human neoplasms. III. Incidence and geographic distribution of chromosome aberrations in 856 cases. Hereditas. 1978;89(2):207–232. doi: 10.1111/j.1601-5223.1978.tb01277.x. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C., HUNGERFORD D. A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960 Jul;25:85–109. [PubMed] [Google Scholar]
- Ozanne B., Wheeler T., Zack J., Smith G., Dale B. Transforming gene of a human leukaemia cell is unrelated to the expressed tumour virus related gene of the cell. Nature. 1982 Oct 21;299(5885):744–747. doi: 10.1038/299744a0. [DOI] [PubMed] [Google Scholar]
- Potter M., Sklar M. D., Rowe W. P. Rapid viral induction of plasmacytomas in pristane-primed BALB-c mice. Science. 1973 Nov 9;182(4112):592–594. doi: 10.1126/science.182.4112.592. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Identification of the constant chromosome regions involved in human hematologic malignant disease. Science. 1982 May 14;216(4547):749–751. doi: 10.1126/science.7079737. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Testa J. R. Chromosome abnormalities in malignant hematologic diseases. Adv Cancer Res. 1982;36:103–148. doi: 10.1016/s0065-230x(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A. Chromosomes and causation of human cancer and leukemia: XL. The Ph1 and other translocations in CML. Cancer. 1980 Nov 15;46(10):2221–2226. doi: 10.1002/1097-0142(19801115)46:10<2221::aid-cncr2820461019>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Selden J. R., Emanuel B. S., Wang E., Cannizzaro L., Palumbo A., Erikson J., Nowell P. C., Rovera G., Croce C. M. Amplified C lambda and c-abl genes are on the same marker chromosome in K562 leukemia cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7289–7292. doi: 10.1073/pnas.80.23.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Dev V. G., Shannon W. A., Jr Characterization of a novel human pre-B leukemia cell line. J Immunol. 1981 Feb;126(2):596–602. [PubMed] [Google Scholar]
- Swan D. C., McBride O. W., Robbins K. C., Keithley D. A., Reddy E. P., Aaronson S. A. Chromosomal mapping of the simian sarcoma virus onc gene analogue in human cells. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4691–4695. doi: 10.1073/pnas.79.15.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]