Abstract
Background
Anti-tumor necrosis factor agents (anti-TNFs) have changed the course of rheumatoid arthritis (RA) for more than a decade. Use of these medications often results in remission, or at least low disease activity (LDA), but at substantial cost. It has been postulated that discontinuation of these medications among RA patients in remission or LDA may be possible without an associated increase in RA disease activity.
Objective
The goal of this systematic literature review is to summarize published articles regarding discontinuation of anti-TNFs in patients with RA.
Methods
A systematic literature review was conducted to identify English-language articles indexed in Pubmed from July 1999 through June 2013 reporting results regarding anti-TNF discontinuation in patients with RA. Study designs included observational longitudinal studies and clinical trials. Outcomes had to include one of the following: time to flare after anti-TNF discontinuation, failure to remain in remission, or LDA at the end of the study.
Results
Ten studies examined discontinuation of anti-TNF therapies in RA. Inclusion criteria varied significantly across studies in terms of disease activity status (remission or LDA) and duration of this disease status (1 year or 1 month) prior to discontinuation being attempted. Results from larger studies (e.g. > 100 patients) suggest that the proportion of patients who discontinued and did not have an increase in disease activity ranged between 24%-81%. In 2 studies that evaluated durability of LDA or remission after anti-TNF discontinuation, the mean time to relapse varied from 15 weeks to 14 months. In studies that analyzed radiographic data, once therapies were reinitiated after an increase in disease activity was detected, patients generally did not experience progression in structural damage.
Conclusion
Discontinuation of anti-TNF therapy is achievable for many RA patients who start in clinical remission or LDA. However, heterogeneous inclusion criteria and highly variable outcome definitions across studies make it difficult to efficiently summarize the literature on this topic or to conduct a meta-analysis. A dearth of evidence exists as to how to best predict which patients have the greatest likelihood to continue to do well after discontinuation of anti-TNF therapy.
Keywords: rheumatoid arthritis, anti-tumor necrosis factor discontinuation, rheumatoid arthritis remission
Introduction
The combination of anti-tumor necrosis factors (anti-TNFs) therapies (adalimumab, certolizumab, etanercept, golimumab, and infliximab) and non-biologic disease modifying anti-rheumatic drugs (DMARDs) like methotrexate (MTX) in patients with RA who have an inadequate or incomplete response to MTX has been shown to be efficacious in preventing progression of structural damage and functional deterioration 1-4. Despite the efficacy of these biological therapies for RA, their high cost 5 and safety issues (serious infections, malignancy, and other adverse events) 6-11 are among the concerns associated with prolonged use that may motivate physicians and patients to consider discontinuing anti-TNF therapy for RA patients who have been in sustained low disease activity (LDA) or remission. Given that the prevalence of RA is approximately 1.0% in the US 12, peak onset is in the 40s, and that anti-TNF drugs may cost up to ~$24,000 per year 5,13, there is an enormous expense associated with long term continuation of anti-TNF agents. For many RA patients under the current paradigm of care, lifelong treatment might be required.
Indeed, questions surrounding when, how, and in whom to discontinue anti-TNF therapy, were within the top 3 most important scientific gaps that needed to be answered in RA, as decided in a 2010 national consensus conference sponsored by the ACR 14. Our objective was to conduct a systematic review of the available literature on discontinuation of anti-TNF therapy in RA patients and associated features of study designs including eligibility criteria, outcome definitions, and outcomes of discontinuation.
Methods
Relevant studies were selected as part of this systematic review for patients with RA from July 1999 through June 2013. Details of the PubMed search that we conducted are outlined in the Appendix. All studies included must have reported results on any of the five commercially available anti-TNFs (adalimumab, etanercept, infliximab, golimumab, or certolizumab) for the treatment of RA only, and excluded other conditions for which these medications are also approved (i.e. Crohn's disease, Psoriatic arthritis, juvenile idiopathic arthritis). The inclusion criteria for the articles in this review consisted of observational longitudinal studies (prospective or retrospective), clinical trials or clinical trials’ long-term extensions that studied anti-TNF discontinuation. Case reports, review articles, anti-TNF treatment efficacy studies, discontinuation of anti-TNF studies due to ineffectiveness or side effects, and studies aimed to decrease the dose or dosing frequency of anti-TNF therapy, without at least one group that completely discontinued therapy, were excluded, as were studies regarding discontinuation of other types of biologics (abatacept, rituximab, tocilizumab) approved for RA. Studies that addressed only nonbiologic-DMARD drug-free remission or LDA were also eliminated. Two reviewers searched for these articles (INM and SS) and discordance was resolved by consensus among the reviewers (INM, SS and JRC).
Results
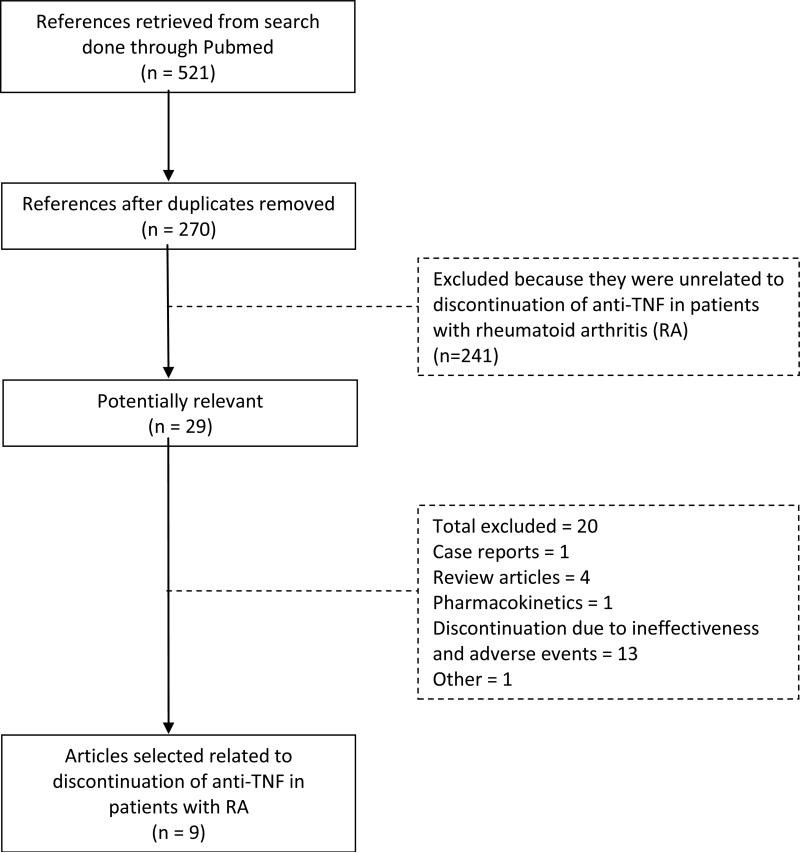
The initial search strategy retrieved 270 unique articles, which after exclusion of articles that did not meet inclusion criteria, yielded 29 potentially relevant studies (Figure 1). After hand reviewing these, there were a total of 9 relevant full-text studies included in the systematic review regarding discontinuation of anti-TNFs in RA patients. Of these, 6 evaluated the proportion of patients at the end of observation time that remained in LDA or remission, while 3 evaluated the durability of the initial disease status after anti-TNF discontinuation. Additionally, consultation by the authors with content experts yielded one additional clinical trial extension, presented as an abstract at the American College of Rheumatology 2011, which also met inclusion criteria and was included in this review. A summary of the study populations, endpoints and results of the 10 eligible studies retrieved appears in Table 1.
Figure 1.
Flow chart of literature search for articles regarding anti-tumor necrosis factor discontinuation in patients with rheumatoid arthritis within PubMed through June 2013
Table 1.
Published randomized trials and observational studies that discontinued anti-TNFs for RA patients in either low disease activity or remission
| Trial name (Reference) and number of patients who discontinued anti-TNF therapy | Study design | Disease activity criteria for discontinuation of anti-TNF therapy | Minimum Duration of LDA or remission prior to anti-TNF discontinuation | Study duration following anti-TNF discontinuation | Anti-TNF discontinued | % of patients in remission or LDA after anti-TNF discontinuation | Duration of remission/LDA off anti-TNF |
|---|---|---|---|---|---|---|---|
| Quinn 15 (N = 10) | Randomized trial/double blind | None† | N/A† | 12 months | Infliximab | 70%*** | N/A |
| BeSt 17 (N = 104)*¶ | Randomized trial/single blind | DAS 44 ≤ 2.4 | 6 months | ¥ | Infliximab | 24%*** | Median = 17 months (IQR 3−47) |
| Smolen 21 (N =197) | Randomized trial/blinded | DAS28 (ESR) ≤ 3.2 | 1 visit& | 12 months | Etanercept | 43%*** | N/A |
| HIT HARD 22 (N = 82) | Randomized trial/blinded | None† | N/A† | 6 months | Adalimumab | N/A†† | N/A |
| Nawata 23¶ (N = 9) | Observational study/prospective | DAS28 (ESR) < 2.6 | 6 months | ¥ | Infliximab | 5% | Mean = 14.2 months |
| Brocq 24¶ (N = 21) | Observational study/prospective | DAS28 (ESR) < 2.6 | 6 months | ¥ | Infliximab, etanercept or adalimumab | 25%** | Mean = 14.7 weeks |
| Tanaka 25 (N = 102) | Observational study/prospective | DAS28 (ESR) < 3.2 | 6 months | 12 months | Infliximab | 55%** | N/A |
| Van der Maas 26 (N = 51) | Observational study/prospective | DAS28 (ESR) < 3.2 | 6 months | 12 months | Infliximab | 16 %*** | N/A |
| Harigai 27 (N = 22) | Observational study/retrospecti ve | DAS28(CRP) < 2.7 | 1 time | 12 months | Adalimumab | 18%*** | N/A |
| OPTIMA29 (N =207) | Randomized trial/blinded | DAS (ESR) < 3.2 | 2 visits (1 month apart) | 12 months | Adalimumab | 81%** | N/A |
All patients discontinued anti-TNF after 6 months regardless of disease activity.
Outcome measured as sustained effect after 6 months adalimumab discontinuation.
Time to flare outcome.
This included a period of 24 weeks with DAS28 ≤ 3.2 and at week 36 of the open label study.
Follow up time varied.
Number of patients that met criteria for infliximab discontinuation in the 5 years of follow up and that were subsequently tapered off.
Remission or LDA at the end of observation time.
Patients that had persistent LDA and did not required anti-TNF retreatment during observation time.
One of the first clinical trials published was a double-blind clinical trial of 20 patients with early RA that were randomized to receive either MTX + infliximab (N = 10) or placebo + infliximab (N = 10) for 12 months15. Among these patients, discontinuation of infliximab began after 12 months of treatment in all the patients in the infliximab + MTX group, irrespective of disease activity. At that time, 7 out of 10 patients were able to discontinue infliximab and maintained the response for 12 months that they had obtained while on treatment with infliximab and MTX 15. There was 1 patient that never responded to treatment and 2 that had an increase in DAS28 (ESR) at least 32 weeks after the last infliximab infusion15.
The BeSt trial (a Dutch acronym for “Behandel Strategieën”, treatment strategies) 16 was a randomized controlled trial (N = 508) in which four different, DAS-steered, dynamic treatment strategies were compared. Two groups (group 1, sequential monotherapy, and group 2, step-up combination therapy) started with MTX monotherapy, with introduction of combination therapy with infliximab or prednisolone in case of insufficient response to at least three conventional DMARD. The other two groups started with combination therapy (group 3, initial combination therapy with MTX, SSZ and prednisolone (tapered from 60 to 7.5 mg/day), and group 4, initial combination therapy with MTX and infliximab (an anti-TNF) (N = 128)16. In a post-hoc analysis of the BeSt study, disease course after cessation of infliximab in early RA patients who achieved low disease activity (DAS ≤2.4) for 6 months was described in the 229 patients who were treated with methotrexate plus infliximab 17 at any point during the five-year follow up period. There were 77/120 (64%) from the initial infliximab treatment group (group 4) and 27/109 (25%, p < 0.001 vs. initial infliximab treatment group) from the delayed treatment group (groups 1-3) that met criteria for discontinuing infliximab17. Among the 104 patients that discontinued infliximab, 54 (out of which 43 were in the initial infliximab treatment group) remained with DAS44 ≤ 2.4, while 50 patients required retreatment with infliximab. The median duration in DAS44 ≤ 2.4 in the 50 patients that required retreatment was 17 months (interquartile range 3-47 months)17. Median damage progression was 0 both for patients who had an increase of the DAS44 to over 2.4 in the first year after cessation and patients whose DAS44 remained at 2.4 or less (p=0.56), although 14 patients had missing radiographic data17. Smoking (HR 2.1, 95% CI 1.1 to 4.2), treatment duration of 18 months or longer (HR 2.4, 1.1 to 5.4) and the presence of shared epitope (SE) (HR 3.7, 1.3 to 10.6) were independently associated with the need to re-introduce of infliximab. When anti-citrullinated protein antibodies (ACPA) status was used in the multivariate analysis instead of the SE (given correlation between these two, both were not included simultaneously), ACPA was not independently associated with the need to re-introduce infliximab. The authors of the BeSt trial and subsequent long-term and sub-analyses studies concluded that achieving tight control using DAS-driven goals led to prolonged lowering of disease activity and the ability to discontinue anti-TNF therapy, especially in patients with early RA17-20.
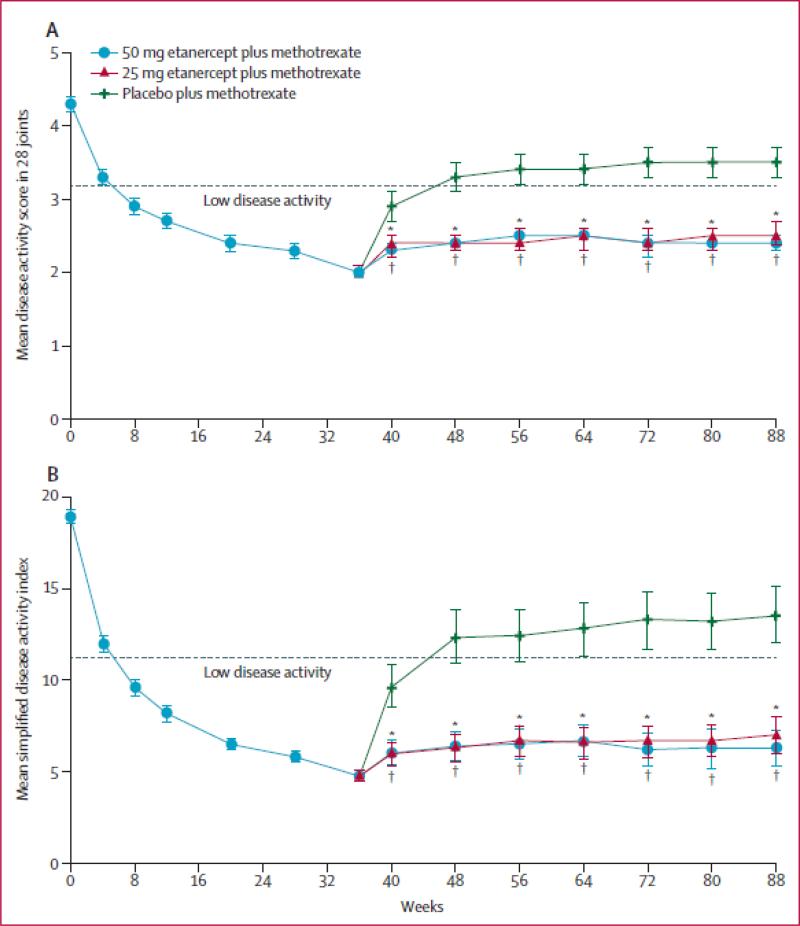
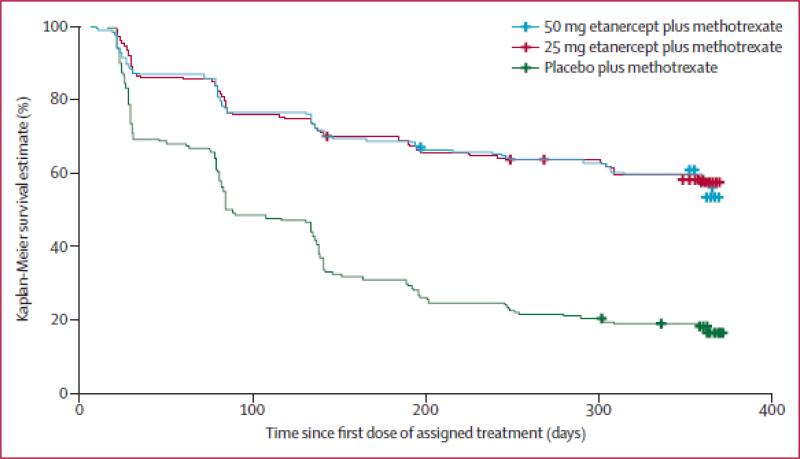
The PRESERVE trial (N = 834) evaluated patients with established RA to determine if LDA defined by DAS28 (ESR) ≤ 3.2 could be sustained with a reduced dose or withdrawal of etanercept (ETA) in RA patients who started with moderately active disease (DAS28 (ESR) >3.2 and ≤ 5.1), who were biologic naïve and were receiving stable doses of MTX 15-25mg/week 21. All patients during this study continued their previous dose of MTX 15-25 mg/week and also received 50 mg of ETA weekly for 36 weeks during an open label phase. At week 36, blinded randomization was performed for those patients who achieved LDA according to DAS28 (ESR) ≤ 3.2 (sustained from weeks 12 to 36 and at week 36) to 1) ETA 50 mg weekly + MTX; 2) ETA 25 mg weekly + MTX or 3) MTX + PBO. At week 88 (study completion), 83% of patients who had received at least one dose of ETA 50 mg/week and one or more DAS28 (ESR) evaluations were in LDA, compared with 43% who received placebo (mean difference 40.8%, 95% CI 32.5–49.1%; p<0.0001). Additionally, 79.1% of patients given ETA 25 mg/week were in LDA at week 88 (mean difference from placebo 35.9%, 27.0–44.8%; p<0·0001). Mean DAS28 (ESR) and simplified disease activity index (SDAI) deteriorated significantly in the placebo group compared with the ETA groups from week 40 onwards (Figure 2). The groups given either dose of ETA showed similar patterns of loss of response but maintained efficacy (i.e. persistent LDA) better than did the group given PBO (for both comparisons log-rank p<0.0001; Figure 3); this study was not statistically powered to identify any possible differences between the 2 etanercept dose groups 21.
Figure 2.
Mean (A) disease activity score in 28 joints and (B) simplified disease activity index among patients treated with either etanercept 50 mg weekly, 25 mg weekly or placebo, each of them on background methotrexate
Legend Figure 2: Patients from the modified intention-to-treat population in the double-blind period included. Last-observation-carried-forward analysis. Bars show 95% CIs. *50 mg etanercept plus methotrexate versus placebo plus methotrexate p<0·0001. †25 mg etanercept plus methotrexate versus placebo plus methotrexate p<0·0001.
Figure 3.
Time to loss of efficacy (i.e. persistent LDA) among patients treated with either etanercept 50 mg weekly, 25 mg weekly or placebo, each of them on background methotrexate
Legend Figure 3: Patients in this analysis were randomized after achieving disease activity score in 28 joints with erythrocyte sedimentation rate of ≤ 3.2 at week 36 of treatment with methotrexate and 50 mg or 25 mg of etanercept. Crosses indicate censoring.
The HIT HARD (High Induction Therapy with Anti-Rheumatic Drugs) study (N = 82) investigated whether or not early treatment with anti-TNF in very early RA as induction therapy led to a long lasting effect that could be maintained when the biologic was withdrawn 22. In this study, treatment with ADA + MTX or PBO + MTX was blindly assigned for 6 months, after which ADA and PBO were withdrawn regardless of disease activity. At week 24, treatment with ADA + MTX compared with PBO + MTX resulted in a statistically significant difference between the groups in DAS28 (ESR) scores (0.53 (95% CI 0.13 to 0.93; p=0.009) and a difference in HAQ of 0.26 units (p=0.001). The mean disease activity at the time of ADA cessation (week 24) was DAS28 (ESR) = 3.0 for the ADA + MTX group. The primary outcome, DAS28 (ESR) at 48 weeks, (i.e. another 24 weeks after ADA discontinuation) showed no significant difference in DAS28 (ESR) scores between the groups ((DAS28 (ESR) difference of 0.21 (95% CI −0.3 to 0.7; p=0.41))22.
There was one observational study of Japanese patients with established RA that were treated with infliximab and MTX (> 6 mg/week which is common in Japan)23. This study evaluated 52 patients with DAS28 (ESR) < 2.6 (i.e. DAS28 (ESR) remission) for 6 months and thus qualified for infliximab discontinuation. In this study, prednisone, non-steroidal anti-inflammatory drugs and DMARD other than MTX were discontinued before infliximab was discontinued. Nine patients were able to maintain DAS28 (ESR) < 2.6 for up to 29 months, with a mean of 14.2 months duration. The MTX dose of all the patients that discontinued infliximab was 8 mg/week. Suppression of joint destruction was able to be maintained with MTX alone.
An observational study determined the time to relapse after anti-TNF discontinuation in RA patients in remission24. In this study, 21 RA patients achieved remission, defined by DAS28 (ESR) < 2.6, for at least 6 months without nonsteroidal inflammatory drugs or more than 5 mg of prednisone per day. Concomitant DMARD therapy was allowable. These individuals were taken off the anti-TNF, with 14 patients on etanercept, 5 on adalimumab and 2 on infliximab. Patients had established RA, with mean duration of remission as defined above on anti-TNF therapy of 19.2 months. Of the 21 patients, 14 were on concomitant DMARDs. The patients were followed every month, and flare was defined as a DAS28 (ESR) > 3.2, in which case anti-TNF therapy was reinitiated. Excluding one patient who died 1 month after anti-TNF was discontinued, 9 out of 20 patients (45%) remained in remission after 6 months of discontinuation of anti-TNF, and 5 out of 20 (25%) after 12 months. The mean time to flare was 14.7 weeks. An interesting comparison made in this study was that the 5 relapse-free patients after 12 months of discontinuation of anti-TNF had a longer cumulative time on anti-TNF therapy before anti-TNF withdrawal compared to the 15 that relapsed after 12 months (56 months vs. 35 months, P = 0.012) and a longer time in remission on anti-TNF therapy (35 months vs.14.5 months, P = 0.04). Among these 5 patients, 4 did not have radiographic progression. After reinitiating anti-TNF in the 15 patients that flared, all recaptured remission, the majority (13 patients) of them within 2 months. This study did not find any difference in relapse-free group and relapsing group in terms of presence of anti-CCP. A notable design feature of this study was its monthly visits that allowed for prompt reinstitution of biologic therapy in patients that relapsed, which could explain why patients were able in achieving remission again so quickly.
Another observational study evaluated the possibility of discontinuing anti-TNF medication in 114 RA patients with established RA (mean disease duration of 5.9 years) treated with infliximab + MTX who had achieved LDA defined by DAS28ESR < 3.2 constantly for more than 24 weeks25. The primary end point was DAS28ESR <3.2 at week 52 after infliximab was discontinued, and yearly progression of modified total Sharp score (mTSS) <0.5 (structural remission) for that same year25. Secondary end points were DAS28ESR <2.6 (clinical remission) for 1 year25. Patients were restarted on infliximab if they had DAS28ESR ≥ 3.2 at week 52 or at any point after infliximab cessation where DAS28ESR ≥ 3.2. Out of 114 patients, 12 patients did not complete the 1 year follow up, leaving 102 patients with data for the entire year of follow-up. Of these, 56 out of 102 patients (55%) were in LDA according to DAS28 (ESR) < 3.2 at week 52 of discontinuing infliximab and there were 44 patients (43%) that reached DAS28 (ESR) < 2.6 at week 52. Of these individuals, 46 patients reinitiated infliximab; 29 patients restarted within the 52 weeks of follow up, and 17 patients had a DAS28 (ESR) > 3.2 at week 52 and infliximab was restarted at that point, for a total of 46 patients who had to reinitiate infliximab25. Regarding radiographic progression, only 49 patients out of 102 had both hand and feet X-ray data available and evaluable to be analyzed for structural damage (baseline and at 52 weeks). The yearly mean progression of mTSS (ΔmTSS) was not significantly different between two groups (0.3 vs. 1.6 for those that maintained LDA vs. those that did not; p = 0.109) while the yearly progression of mTSS < 0.5 was 67% of patients in the LDA maintenance vs. 44% in the LDA failures after 1 year (p = 0.216). Among the factors that were associated with remaining in LDA after infliximab discontinuation were shorter disease duration, lower mTSS score and lower DAS28 (ESR) at the time when infliximab was discontinued25.
In a small (N = 51) longitudinal study of RA patients who were treated with MTX and infliximab and achieved LDA according to DAS28 (ESR) < 3.2 for at least 6 months, infliximab was discontinued or dose reduced26. This study showed that after 1 year of discontinuing infliximab, 16% (95% CI 6 to 26%) was able to remain without it; another 45% were able to be maintained on LDA at a reduced infliximab dose (95% CI 31% to 59%). This study also evaluated the probability of not requiring dose re-escalation. Dose re-escalation was based on a flare definition that consisted of an increase in DAS28 (ESR) of 1.2 units compared with baseline on two subsequent visits with at least 2 weeks in between visits if DAS28 ESR was < 3.2; after a threshold of DAS28 (ESR) > 3.2 was reached, an increase of 0.6 in DAS28 (ESR) was used as a criteria for re-escalation. After infliximab dose reduction to 75% or 50% of the initial infliximab dose, it was observed that the probability of avoidance of dose re-escalation was approximately 75% for both reduced dose groups after 200 days. The probability of avoidance of dose re-escalation was < 50% when the dose was reduced to 25%, and when the medication was discontinued, this probability was approximately 25% after 200 days.
The primary endpoint of the retrospective BRIGHT study (Biologics-free RemIssion and low disease activity after stoppinG adalimumab in Japanese patients with rHeumatoid arThritis)27 examined patients that completed the open extension of a double-blind, placebo-controlled trial of ADA monotherapy in Japan and who had LDA (DAS28-CRP < 2.7) at the last administration of ADA in the extension trials. Determination of whether to discontinue ADA and when to reinitiate ADA again was made by the treating rheumatologist, and criteria defining disease flare that required ADA to be restarted was not pre-specified. Of the 46 patients that completed the BRIGHT study and who were in LDA at the last administration of ADA, 22 patients then discontinued ADA; 8 of these were reinitiated on ADA or a different biologic while 4 (18%) maintain LDA at every visit through week 52. Among the remaining 10 patients, 6 had missing data to calculate DAS (CRP) while the other 4 had disease activity fluctuate between DAS28 (CRP) < 2.7 and DAS28 (CRP) ≥ 2.7, though these patients were not reinitiated on ADA. The discretionary nature of when and whether to reinitiate ADA is a limitation of this retrospective study given that this decision was made by the treating rheumatologist. Additionally, the patients that achieved the primary endpoint had longer RA disease duration, used glucocorticoids more frequently, and had higher titers of rheumatoid factor.
The OPTIMA 28 (Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab) long-term extension study 29 was a trial presented in abstract form at the ACR annual meeting in 2011. Results of this study showed that 81% of those patients that discontinued ADA remained in LDA based on DAS28 (ESR) < 3.2 after 1 year and 91% of the patients that continued ADA stayed in LDA (p = 0.04). According to the ACR/European League Against Rheumatism (EULAR) provisional remission criteria of SDAI ≤ 3.3 30, 51% of patients who discontinued ADA and continued only MTX remained in remission one year later, while 62% of those that continued MTX + ADA remained in remission, a difference of 11% (p-value = 0.10) in the proportion of patients remaining in remission between those that continued ADA versus those that discontinued 29. There were 84% versus 92% of the patients continued in LDA (SDAI ≤ 11), an 8% difference between those that discontinued or continued ADA (p = 0.07) 29. Importantly, patients were not required to have attained remission before ADA therapy was withdrawn, only LDA, and they only had to achieve LDA at 2 visits spaced one month apart. This relatively liberal inclusion criterion increased the size of patient population who can potentially withdraw anti-TNF but probably contributed to a higher failure rate than would be expected with more rigorous inclusion criteria (e.g. clinical remission for 12 months before discontinuation).
Discussion
This systematic review summarized the published literature that investigated the inclusion criteria, outcome definitions, and results of anti-TNF cessation in patients with RA that were in either LDA or clinical remission. The majority of these studies consisted of long-term extension clinical trials of efficacy studies of anti-TNF biologics for RA patients who were anti-TNF naïve or in some instances, DMARD and biologic naïve. There were several other observational studies that included established RA patients. Despite dramatic heterogeneity in the study methods, inclusion criteria, outcome definitions, duration of the study, and specific medication discontinued, approximately 30-80% of patients that discontinued anti-TNF were able to remain in the favorable disease activity state (remission or LDA) for at least 1 year and in many cases, much longer. Among patients that had an increase in their disease activity, the majority of these patients returned to the previous disease status and did not accumulate structural damage after anti-TNF therapy was reinstituted17,20. None of the studies that evaluated discontinuation of anti-TNFs analyzed withdrawal of golimumab or certolizumab, the 2 newest anti-TNFs.
Except for the PRESERVE trial, the clinical trials discussed here were powered to determine efficacy of the anti-TNF and/or other therapies, not to determine the success of anti-TNF cessation once LDA or remission had been achieved. PRESERVE 21 was the only study powered to determine differences between continuation of ETA 50 mg or 25 mg a week vs. discontinuation of ETA, although was not powered to determine differences between the 2 ETA dose groups. The ‘add on’ nature to these studies may decrease their generalizability, and none were designed to test variations in inclusion criteria to examine which patients might be more optimal to discontinue and retain control of disease activity. The observational studies had an additional limitation in that most had very small sample sizes. The associated lack of power of these studies is relevant since no significant differences were observed between the groups that discontinued the anti-TNF vs. those that continued. However, irrespective of the low statistical power to detect differences, the observed outcomes between those who discontinued anti-TNF therapy and those that did not were generally of small magnitude and little clinical relevance.
There were significant heterogeneity across studies, one of which was that the inclusion criteria in these studies varied in terms of the instrument used to measure disease activity (DAS28 (ESR) or DAS28CRP or DAS44) and the disease activity required prior to anti-TNF discontinuation (remission or LDA). The duration of either LDA or remission prior to anti-TNF discontinuation (1 month or 6 months) was also variable across studies. Given this heterogeneity, our review was not able to inform the optimal duration of anti-TNF treatment, remission or LDA disease activity prior to successful discontinuation of anti-TNF. Additionally, while there were studies that defined flare and specified a treatment protocol if patients had a flare, the majority of the studies did not outline this clearly. Another difference across studies was the outcome; some studies analyzed duration of either remission or LDA, other studies analyzed the proportion of patients in remission only once, at the end of the study, and in other studies it was ambiguous. It also was not clear if patients were allowed to receive rescue medications (e.g. steroids) at only one point or several times during the trial, and whether they could continue in the study if they had an increase in disease activity. Lastly, radiographic progression was not assessed in every study, and the studies that did have x-ray data were only assessed in a portion of the patients. As an important component for future studies, even if successful cessation of anti-TNF seems feasible from a clinical standpoint, evidence to confirm lack of progressive structural damage seems of particular importance to include in any prospective study.
Based upon the results of this review, it seems more feasible to discontinue anti-TNF in patients with earlier RA, those given more aggressive treatment, and who more quickly achieve LDA or remission (e.g. within the first 6 months of treatment), which may be a proxies for a deeper depth of remission. On the other hand, there also seems to be a meaningfully large patient population with established RA that can either decrease the dose of anti-TNF 21 or completely discontinue without clinical worsening of disease activity, major progression of structural damage or worsening of functional status25. Nevertheless, characterization of the best set of predictors or patient subgroups that can successfully discontinue is lacking across all studies, and small sample size in most would not even allow for this kind of analysis.
While a robust set of predictors of successful maintenance of remission or LDA after ant-TNF discontinuation is not yet available, a small observational study (not included in this review, because its primary outcome was to identify predictors of successful anti-TNF discontinuation rather than examine likelihood of success against a control group that did not discontinue) suggested that lack of power Doppler signal on musculoskeletal ultrasound may be useful to predict successful discontinuation of anti-TNF 31. Among the studies included in this review only one evaluated ultrasound, but the sample size was small (n = 12) and final conclusions on how this modality might predict persistence of remission are unclear24. Within the studies included in this review, several specific factors were identified as potential predictors of successful anti-TNF discontinuation including early RA 25,32, DAS28 (ESR) < 2.225 compared to DAS28 (ESR) > 2.225 (p < 0.001) at time of anti-TNF withdrawal 25, early aggressive combined treatment 17,18,20, and high functional status (HAQ score < 0.20) as defined by Health Assessment Questionnaire-Disability Index (HAQ-DI) 25. Serologic status is often a major determinant of RA severity and treatment refractoriness; however among the studies included in this review, only one study 17 performed an analysis to determine if serologic status was independently associated with increase in risk of worsening after anti-TNF discontinuation, and it did not find such an association. Another 2 studies24,27 found no difference in the proportion of serologic status between the patients that relapsed and those that did not. The small amount of information in the studies regarding serologic status and possibility to successfully discontinue anti-TNF therapy is therefore limited.
In conclusion, discontinuation of anti-TNF biologics in patients with RA without increasing disease activity seems possible, especially among patients with earlier RA treated with more aggressive combination treatment. There is a subgroup of patients with established RA who can successfully discontinue, but at present specific predictors for this population do not exist. Reassuringly for future studies, among patients that discontinue anti-TNF and that had an increase in their disease activity, 70%-87% returned to either LDA or remission once anti-TNF was reinitiated17,24-26. This data suggest that if an attempt to discontinue anti-TNF fails, return to the previous good control of disease state is attainable for most. Structural progression seems low for those patients who start in low disease activity or remission and discontinue anti-TNF therapy regardless. There is no data in the studies that analyzed anti-TNF discontinuation on golimumab and certolizumab; however it seems reasonable to infer that successful discontinuation of these 2 biologics could be similar to the other anti-TNFs given that their relatively similar mechanism of action. Future studies with carefully described inclusion criteria and outcome definitions are warranted in order to obtain valid results for the important topic of anti-TNF discontinuation and can potentially lead to establishing clinical practice guidelines for biologic withdrawal. Definitive conclusions from the data summarized in this report from both trials and observational studies cannot be drawn given their multiple limitations and heterogeneity in patient populations and outcomes. This evidence gap supports ongoing need to consider new and well-powered prospective trial(s) of biologic withdrawal in RA patients who have achieved at least LDA if not remission.
Acknowledgments
Dr. Curtis: is supported by the Agency for Healthcare Research & Quality (R01 HS018517).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Conflict of interest:
Dr. Navarro-Millán: Nothing to disclose
Dr. Sattui: Nothing to disclose
References
- 1.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER Study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatooid arthritis who have not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 2.Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 3.Kavanaugh A, St. Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric antitumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol. 2000;27:841–850. [PubMed] [Google Scholar]
- 4.Keystone E, Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthrits: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–3329. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 5.Ollendorf DA, Klingman D, Hazard E, Ray S. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthrits in a managed care population. Clin Ther. 2009;31(4):825–835. doi: 10.1016/j.clinthera.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Al-Niaimi F. Adalimumab-induced lupus erythematosus. Eur J Dermatol. 2009;31(4):825–835. doi: 10.1684/ejd.2009.0668. [DOI] [PubMed] [Google Scholar]
- 7.Cairns AP, Duncan MK, Hinder AE, Taggart AJ. New onset systemic lupus erythematosus in a patient receiving entanercept for rheumatoid arthritis. Ann Rheum Dis. 2002;61:1031–1032. doi: 10.1136/ard.61.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan N, Edwards ET, Cupps TR, et al. Demyelination occuring during anti-tumor necrosis factor alpha therpay for inflammatory arthritis. Arthritis Rheum. 2001;44:2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Bongartz T, A.J. S, swetting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of seriou infections and malignancies. J Am Med Assoc. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Mease P, Dijkmans BA, et al. Adalimumab safety and mortality rates from globall clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;12:1863–1869. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis JR, Xi J, Patkar N, Xie A, Saag KG, Martin C. Drug-specific and time-dependent risks of bacterial infection among patients with rheumatoid arthritis who were exposed to tumor necrosis factor alpha antagonists. Arthritis and rheumatism. 2007 Dec;56(12):4226–4227. doi: 10.1002/art.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel S, Crowson C, O'Fallon W. The epidemiology of rheumatoid arthritis in Rochester, Minnesota 1955-1985. Arthritis Rheum. 1999;1999(42):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.IMS [5/21/2012];2007 Top Therapeutic Classes by U.S. Sales. 2007 :1. http://www.imshealth.com/imshealth/Global/Content/Document/Top-Line%20Industry%20Data/2007%20Top%20Therapeutic%20Classes%20by%20Sales.pdf.
- 14.American College of Rheumatology Rheumatoid Arthritis Clinical Trial Investigators Ad Hoc Task F American College of Rheumatology Clinical Trial Priorities and Design Conference, July 22-23, 2010. Arthritis Rheum. 2011 Aug;63(8):2151–2156. doi: 10.1002/art.30402. [DOI] [PubMed] [Google Scholar]
- 15.Quinn MA, Conaghan PG, O'Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2005 Jan;52(1):27–35. doi: 10.1002/art.20712. [DOI] [PubMed] [Google Scholar]
- 16.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis and rheumatism. 2005 Nov;52(11):3381–3390. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 17.van den Broek M, Klarenbeek NB, Dirven L, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Annals of the rheumatic diseases. 2011 Aug;70(8):1389–1394. doi: 10.1136/ard.2010.147751. [DOI] [PubMed] [Google Scholar]
- 18.van der Kooij SM, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Drug-free remission, functioning and radiographic damage after 4 years of response-driven treatment in patients with recent-onset rheumatoid arthritis. Annals of the rheumatic diseases. 2009 Jun;68(6):914–921. doi: 10.1136/ard.2008.092254. [DOI] [PubMed] [Google Scholar]
- 19.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Annals of internal medicine. 2007 Mar 20;146(6):406–415. doi: 10.7326/0003-4819-146-6-200703200-00005. [DOI] [PubMed] [Google Scholar]
- 20.Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Annals of the rheumatic diseases. 2011 Feb;70(2):315–319. doi: 10.1136/ard.2010.136556. [DOI] [PubMed] [Google Scholar]
- 21.Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013 Mar 16;381(9870):918–929. doi: 10.1016/S0140-6736(12)61811-X. [DOI] [PubMed] [Google Scholar]
- 22.Detert J, Bastian H, Listing J, et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Annals of the rheumatic diseases. 2013 Jun;72(6):844–850. doi: 10.1136/annrheumdis-2012-201612. [DOI] [PubMed] [Google Scholar]
- 23.Nawata M, Saito K, Nakayamada S, Tanaka Y. Discontinuation of infliximab in rheumatoid arthritis patients in clinical remission. Modern rheumatology / the Japan Rheumatism Association. 2008;18(5):460–464. doi: 10.1007/s10165-008-0089-1. [DOI] [PubMed] [Google Scholar]
- 24.Brocq O, Millasseau E, Albert C, et al. Effect of discontinuing TNFalpha antagonist therapy in patients with remission of rheumatoid arthritis. Joint, bone, spine : revue du rhumatisme. 2009 Jul;76(4):350–355. doi: 10.1016/j.jbspin.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Annals of the rheumatic diseases. 2010 Jul;69(7):1286–1291. doi: 10.1136/ard.2009.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Maas A, Kievit W, van den Bemt BJ, van den Hoogen FH, van Riel PL, den Broeder AA. Down-titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Annals of the rheumatic diseases. 2012 Nov;71(11):1849–1854. doi: 10.1136/annrheumdis-2011-200945. [DOI] [PubMed] [Google Scholar]
- 27.Harigai M, Takeuchi T, Tanaka Y, Matsubara T, Yamanaka H, Miyasaka N. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Modern rheumatology / the Japan Rheumatism Association. 2012 Nov;22(6):814–822. doi: 10.1007/s10165-011-0586-5. [DOI] [PubMed] [Google Scholar]
- 28.Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Annals of the rheumatic diseases. 2013 Jan;72(1):64–71. doi: 10.1136/annrheumdis-2011-201247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanaugh A EP, Fleischmann R, van Vollenhoven RF, Pavelka K, Durez P, et al. Withdrawal of Adalimumab in Early Rheumatoid Arthritis Patients Who Attained Stable Low Disease Activity with Adalimumab Plus Methotrexate: Results of a Phase 4, Double-Blind, Placebo-Controlled Trial. Arthritis and rheumatism. 2011;63(S10):65–66. [Google Scholar]
- 30.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Annals of the rheumatic diseases. 2011 Mar;70(3):404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 31.Saleem B, Brown AK, Quinn M, et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012 Jan 31; doi: 10.1136/annrheumdis-2011-200548. [DOI] [PubMed] [Google Scholar]
- 32.Saleem B, Keen H, Goeb V, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Annals of the rheumatic diseases. 2010 Sep;69(9):1636–1642. doi: 10.1136/ard.2009.117341. [DOI] [PubMed] [Google Scholar]