Abstract
Objectives:
To determine whether smaller brain volumes in older women who had completed Women's Health Initiative (WHI)-assigned conjugated equine estrogen–based hormone therapy (HT), reported by WHI Memory Study (WHIMS)-MRI, correspond to a continuing increased rate of atrophy an average of 6.1 to 7.7 years later in WHIMS-MRI2.
Methods:
A total of 1,230 WHI participants were contacted: 797 (64.8%) consented, and 729 (59%) were rescanned an average of 4.7 years after the initial MRI scan. Mean annual rates of change in total brain volume, the primary outcome, and rates of change in ischemic lesion volumes, the secondary outcome, were compared between treatment groups using mixed-effect models with adjustment for trial, clinical site, age, intracranial volumes, and time between MRI measures.
Results:
Total brain volume decreased an average of 3.22 cm3/y in the active arm and 3.07 cm3/y in the placebo arm (p = 0.53). Total ischemic lesion volumes increased in both arms at a rate of 0.12 cm3/y (p = 0.88).
Conclusions:
Conjugated equine estrogen–based postmenopausal HT, previously assigned at WHI baseline, did not affect rates of decline in brain volumes or increases in brain lesion volumes during the 4.7 years between the initial and follow-up WHIMS-MRI studies. Smaller frontal lobe volumes were observed as persistent group differences among women assigned to active HT compared with placebo. Women with a history of cardiovascular disease treated with active HT, compared with placebo, had higher rates of accumulation in white matter lesion volume and total brain lesion volume. Further study may elucidate mechanisms that explain these findings.
Numerous cross-sectional and observational studies on humans and animal models have examined whether postmenopausal hormone therapy (HT) affects brain structure, with inconsistent results.1 This relationship was examined in the context of the MRI substudy of a large randomized placebo-controlled clinical trial: the Women's Health Initiative Memory Study (WHIMS)-MRI. Women aged 65 to 79 years treated for an average of 4.0 years with conjugated equine estrogen (CEE) with medroxyprogesterone acetate (MPA) or 5.6 years with CEE alone, compared with placebo, had smaller frontal lobe and hippocampal volumes on posttrial brain MRI.2 Because these findings were based on a single cross-sectional brain scan, it is unknown when the increased rate of atrophy occurred and whether it continued to increase, thereby signaling growing concerns about risks of cognitive impairment.
WHIMS-MRI2 conducted a second brain MRI in this cohort of women an average of 4.7 years after the initial scan. We report the principal findings from this longitudinal study: whether mean annual rates of change in total brain volume vary by prior random assignment to CEE-based HT. Secondarily, we examine whether rates of change in ischemic lesion volumes increase over time relative to prior treatment. The WHIMS-MRI2 protocol prespecified analyses to examine associations between on-trial HT exposure and 5 subgroup comparisons based on baseline age, global cognitive function, prior use of HT, history of cardiovascular disease, and CEE regimen. We report these findings and discuss their implications for identifying mechanisms by which HT may adversely affect brain health in older women.
METHODS
Design of the WHI HT trials and WHIMS.
The WHI HT trials evaluated postmenopausal HT and prevention of disease, with coronary heart disease as the primary outcome.3 Approximately 27,000 women aged 50 to 79 years enrolled into parallel randomized placebo-controlled trials of CEE 0.625 mg + MPA 2.5 mg/d for women with intact uteri (n = 16,608), or CEE alone (n = 10,739) for women with prior hysterectomy. Ancillary to the WHI HT trials, WHIMS examined the risk of all-cause dementia and global cognitive decline in 7,479 women without dementia aged 65 to 79 years at baseline and randomized to CEE + MPA (n = 16,608) or CEE alone (n = 10,739).
WHIMS-MRI studies.
The initial WHIMS-MRI study was conducted approximately 8 years after WHI randomization and an average of 3 years after termination of the WHIMS CEE + MPA trial or 1.4 years after the CEE alone trial.2,4,5 WHIMS-MRI2 was conducted 12.7 years post–WHI randomization and an average of 7.7 years after termination of the CEE + MPA trial or 6.1 years after the CEE alone trial. WHIMS-MRI2 scanning occurred an average of 4.7 years after the WHIMS-MRI initiation. Women in the WHIMS-MRI study who continued WHI follow-up were invited to join WHIMS-MRI2. WHIMS-MRI2 exclusion criteria included absolute contraindications and health-related factors and were identical to those previously reported for WHIMS-MRI.4,5
Standard protocol approvals, registrations, and patient consents.
ClinicalTrials.gov identifiers: NCT00000611 (WHIMS). Study protocols were approved by Institutional Review Boards at the WHIMS-MRI Coordinating Center, 14 WHIMS clinical centers, and the NIH. Written informed consent was obtained from every participant.
WHIMS-MRI studies scanning protocol.
WHIMS-MRI completed brain scans on 1,403 women, of whom 674 had a single scan and 729 participated in both WHIMS-MRI studies, serving as the primary cohort for the current analyses. The WHIMS-MRI Quality Control Center2,5,6 developed scan acquisition and processing protocols for both WHIMS-MRI studies. Briefly,2 standard T1-weighted, T2-weighted, proton density–weighted, and fluid-attenuated inversion recovery scans were acquired. T1-weighted volumetric MRI scans were preprocessed to a standardized protocol for alignment, removal of extracranial material, and segmentation of brain into gray and white parenchyma and CSF. Regional volumetric measurements were obtained using an automated computer-based template warping method that summed the number of respective voxels within each anatomical region of interest (ROI). Intracranial volume was estimated as total cerebral hemispheric volumes plus the ventricular CSF. After histogram standardization and coregistration, ischemic lesion segmentation components of the algorithm were applied. A support vector machine classifier trained on expert-defined small-vessel ischemic disease lesions in 45 cases7 was used to classify small-vessel ischemic disease. The computer-assisted methodology was validated against manual segmentation by an experienced expert6 and used by other cohorts.7–10
Supratentorial brain tissue was classified as normal or abnormal (ischemic) gray or white matter and assigned to 1 of 92 anatomical ROIs of the cerebrum.6,7 ROIs were organized in an anatomically hierarchical system with 8 ROIs used for this analysis: total brain, frontal lobe, and hippocampus volumes; total lesion, white matter lesion, gray matter lesion, and basal ganglia lesion volumes; and ventricular CSF volumes.
MRI2 primary outcome measure.
Mean annual rates of change in total brain volume between the initial and second MRI scans were the primary outcome measure of WHIMS-MRI2. Total brain volume was chosen for the primary comparison because it was expected to provide the most stable estimate of change over time and to accumulate any effects of regional atrophy. Secondary measures included rates of change in regional brain volumes, total ischemic brain lesion volumes, and its constituents; ventricular volume was a tertiary outcome. Brain, lesion, and ventricular volumes were measured in cubic centimeters (cm3).
Statistical analysis.
Demographic and risk factor characteristics at WHI baseline were compared between 674 women who furnished one measure in the WHIMS-MRI study vs the 729 who were assessed at both time periods using χ2 tests. Likewise, similar comparisons were performed between those assigned to active drug vs placebo by trial and overall.
Longitudinal mixed-effects models were fitted for all 1,403 women who participated in the MRI studies to assess differences between treatment groups in brain volumes as well as changes over time, with adjustment for trial, visit, clinical site, age, intracranial volume, and time from randomization to MRI scan. A compound symmetry model was used for intrasubject correlations. The advantage of this model is that it used all available data at the time of the first MRI to incorporate within-person variation for estimation of mean differences in volumes. An interaction term for time between scans and treatment assignment was used to compare study arms. Formal tests of interactions were used to assess the consistency of treatment effects across subgroups of women defined by age, baseline modified Mini-Mental State Examination (3MSE) scores, prior HT, and history of cardiovascular disease.
Because the distributions of the ventricular and ischemic lesion volumes were highly skewed, a logarithmic transformation was used. A (2-tailed) critical value of 0.05 was used for the primary analysis. To examine potential bias resulting from loss to follow-up from the original MRI cohort, a propensity scores analysis was conducted.11
RESULTS
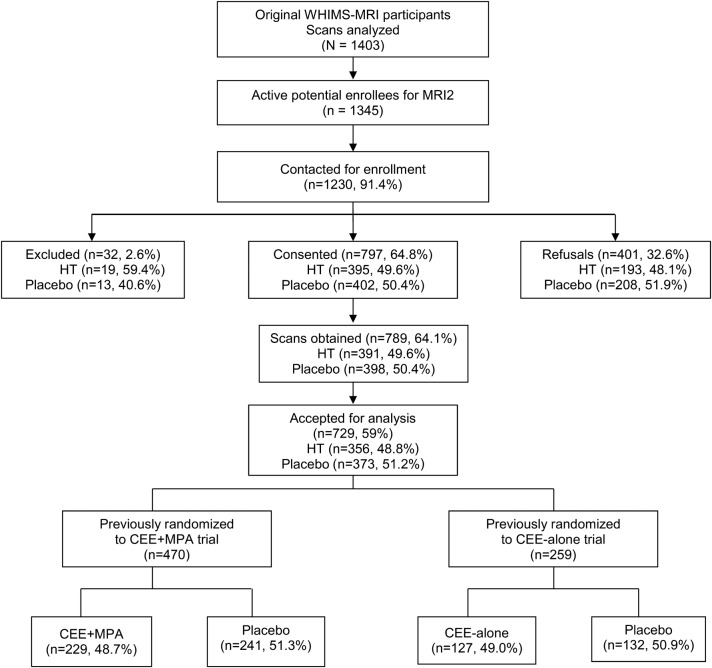
Of 1,403 WHIMS-MRI participants, 1,345 remained active in WHI and 1,230 (91.4%) were screened. Of these, 32 (2.6%) were ineligible, 401 (32.6%) refused to participate, 797 (64.8%) provided consent, and 729 (59%) had scans that met requirements for analysis (figure 1).
Figure 1. WHIMS-MRI2 CONSORT diagram.
CEE = conjugated equine estrogen; CONSORT = Consolidated Standards of Reporting Trials; HT = hormone therapy; MPA = medroxyprogesterone acetate; WHIMS = Women's Health Initiative Memory Study.
Table 1 shows baseline demographic and risk factor characteristics for women enrolled in the WHIMS-MRI and WHIMS-MRI2 studies. WHIMS-MRI2 women tended to be younger, were more likely to be white and have higher scores on the baseline 3MSE, but were less likely to have diabetes (all p < 0.01) or hypertension (p = 0.02) compared with WHIMS-MRI women.
Table 1.
Demographic and risk factor characteristics at the time of WHI enrollment by participation in WHIMS-MRI and WHIMS-MRI2 studies (N = 1,403)
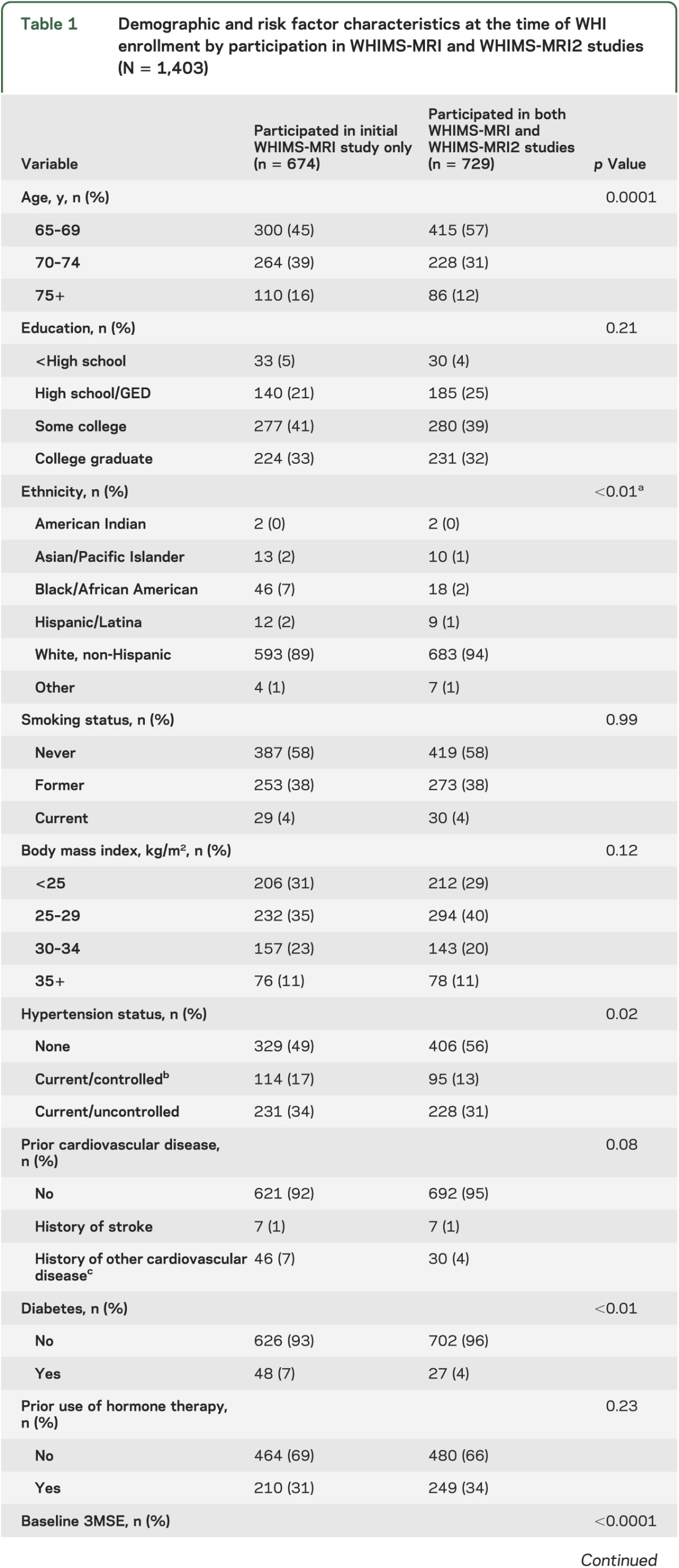
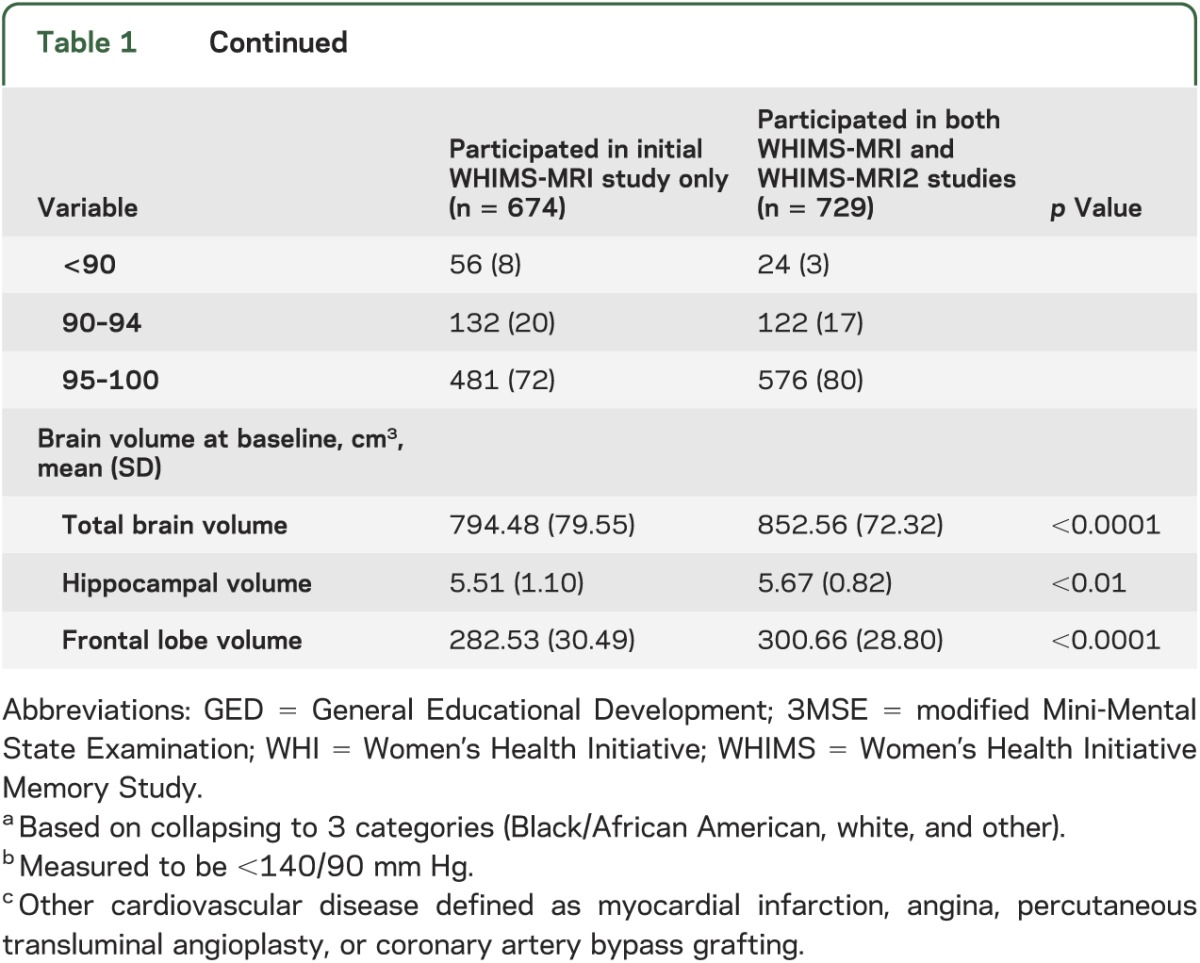
There were no differences among WHIMS-MRI2 women by WHI treatment assignment within or across the 2 WHIMS HT trials by age, education, ethnicity, smoking, body mass index, hypertension, cardiovascular disease, diabetes, prior use of HT, or 3MSE scores. Age at scanning ranged from 76 to 92 years (mean 82.8, SD 3.5).
Among 729 MRI2 women, 15 had incident mild cognitive impairment (MCI) or probable dementia before the second scan (10 MCI and 5 probable dementia). Of these, 3 MCI cases were identified during the time between the 2 scans. Eighteen incident strokes occurred before the second scan, and 6 of these occurred between the 2 MRI scans.
Primary and secondary outcome analyses.
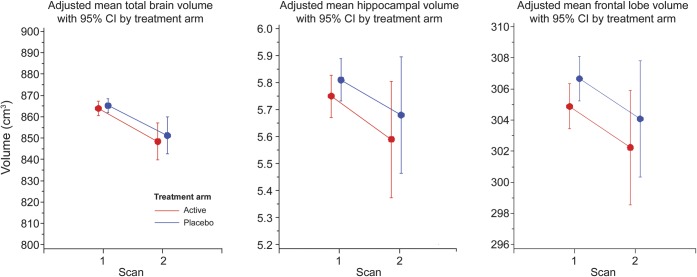
Total brain volume decreased 3.22 cm3 per year in the active arms and 3.07 cm3 in the placebo arms (p = 0.53). In contrast, the secondary outcome, total ischemic lesion volumes, increased in both arms at an annual rate of 0.12 cm3 (p = 0.88). We observed no differences in annual rates of change of any of the brain or lesion volumes by treatment arm (all p > 0.31) between the 2 WHIMS-MRI scans (table 2). Figure 2 shows fitted means for scans 1 and 2 for total brain, hippocampal, and frontal lobe volumes.
Table 2.
Estimated mean (standard error) total, regional, and ischemic lesion brain volumes (cubic centimeters)a and annual rates of changeb by treatment assignment (based on mixed-model repeated-measures analyses of covariance)
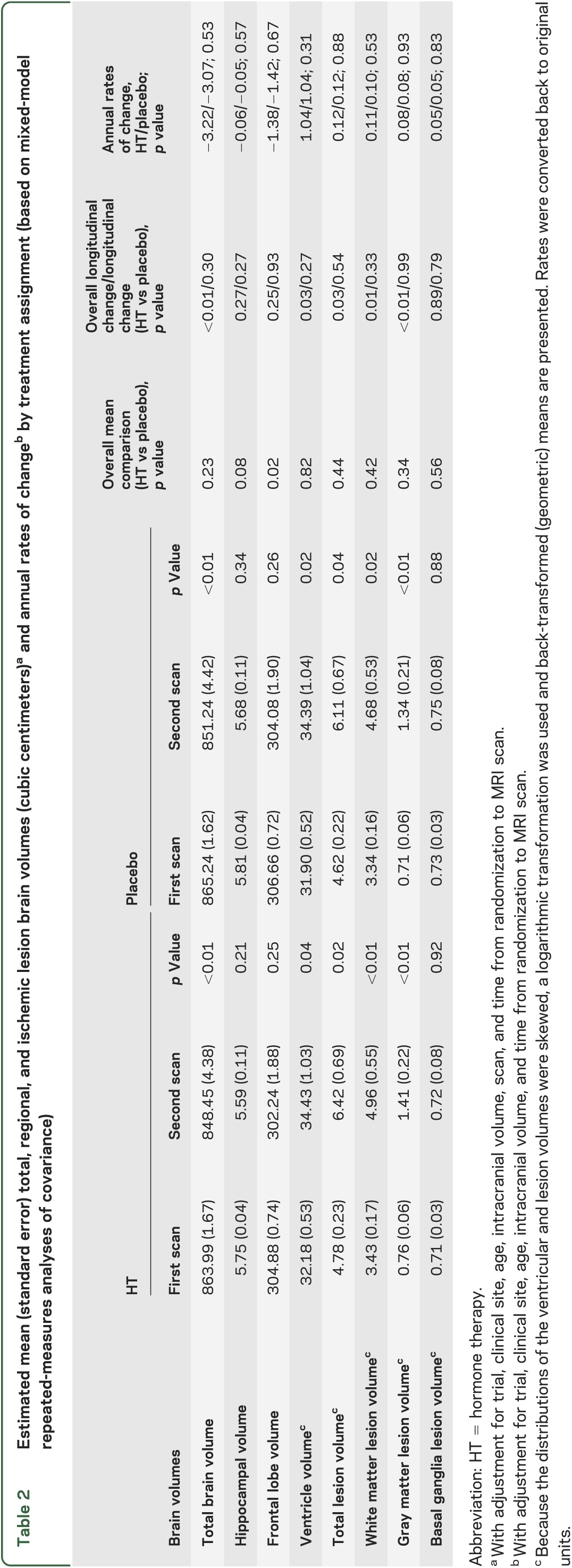
Figure 2. WHIMS-MRI2 adjusted mean brain volumes with 95% CI by treatment arm.
CI = confidence interval; WHIMS = Women's Health Initiative Memory Study.
Table 2 also presents adjusted mean total and regional brain and ischemic lesion volumes for the first and second WHIMS-MRI scans. We observed longitudinal declines in all measures of brain volumes. Mean total brain volumes declined within arms (both <0.01), but there were no differences by treatment assignment overall (p = 0.23).
Marked differences in brain volumes by treatment occurred only in the frontal lobes. Women assigned to HT had smaller frontal lobe volumes overall compared with those assigned to placebo (p = 0.02). Similarly, women assigned to HT, compared with placebo, showed a trend toward smaller hippocampal brain volumes (p = 0.08).
Ventricular volumes were larger at the second scan within the HT (p = 0.04) and placebo (p = 0.02) groups, but there was no difference overall by treatment assignment (p = 0.82). Increases in ventricular volumes were significant overall (p = 0.03) with no differences by treatment (p = 0.27).
Similarly, lesion volumes increased significantly across scans within treatment arms, except those in the basal ganglia, but there were no treatment-related differences in overall lesion volumes. Likewise, increases in lesion volumes over time were significant, except those in the basal ganglia, but did not differ by treatment assignment.
Subgroup analyses.
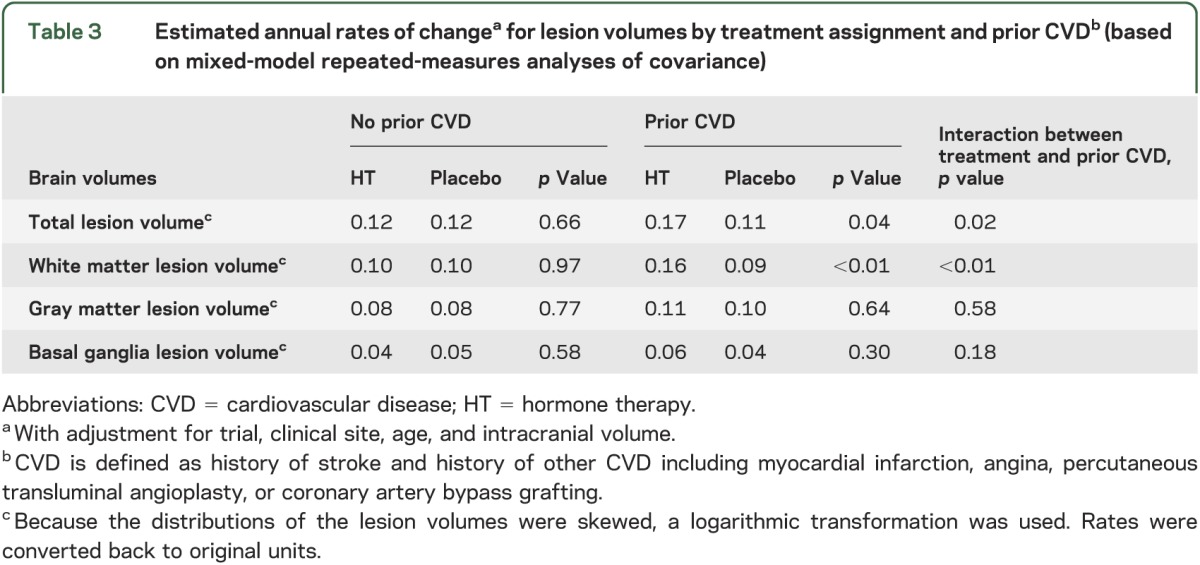
Treatment-related effects on rates of change did not differ across subgroups of women defined by age, 3MSE scores, prior HT, and CEE regimen. However, table 3 shows that women with a history of cardiovascular disease treated with active HT had greater increases in white matter lesion volume (p < 0.01) and total brain lesion volume (p = 0.02).
Table 3.
Estimated annual rates of changea for lesion volumes by treatment assignment and prior CVDb (based on mixed-model repeated-measures analyses of covariance)
There was also an interaction between 3MSE by HT on brain volumes. Decrements in 2 measures of brain volumes were associated with active HT in women with the lowest baseline 3MSE scores. For hippocampal volume, the fitted mean differences (standard error) by 3MSE were as follows: <90 = −0.48 (0.17) cm3; 90–94 = −0.16 (0.10) cm3; and 95–100 = −0.03 (0.05) cm3; p = 0.02 for arm by 3MSE interaction. Similarly, for total brain volume, the fitted mean differences (standard error) by 3MSE were as follows: <90 = −14.23 (6.81) cm3; 90–94 = 7.66 (3.80) cm3; and 95–100 = −0.14 (1.85) cm3; p = 0.05 for arm by 3MSE interaction.
DISCUSSION
As our principal finding, WHIMS-MRI2 found no HT-associated acceleration in brain atrophy during the 4.7 years between the WHIMS-MRI scans. As expected in a cohort in which ages ranged from 76 to 92 years, we observed longitudinal declines in brain volumes, but they were not related to treatment. Secondarily, we found increased total brain ischemic lesion load that was unrelated to treatment, a finding that is consistent with previous reports from the WHIMS-MRI study.5
We also observed smaller frontal lobe volumes (p = 0.02) and a trend toward smaller hippocampal volumes (p = 0.08) in women assigned to HT overall compared with placebo. These findings represent persistent and somewhat attenuated group effects of active HT relative to those observed in the initial WHIMS-MRI study in 2009.2 Although previous studies demonstrate that there are clinically significant effects in the women most vulnerable to the adverse effects of HT, i.e., those with lower cognitive function at baseline,2 the clinical significance of the persistent changes in frontal lobe volume has yet to be determined.
The literature is mixed regarding the effects of postmenopausal HT on brain volumes. Two studies of neuroprotective effects of HT (CEE or estradiol) on aging brain regions reported that HT current-users (mean age 58.9 years [SD 6.4] treated for 10.5 years [SD 9.3]) had larger mean hippocampal volumes compared with similar-age never-users and significantly older past-users.12,13 Within the current-users, however, a negative correlation between treatment duration and hippocampal volumes led the authors to conclude that the neuroprotective role of HT on hippocampal volume is duration-dependent in aging women.12,13 This suggests that at some point, HT may be toxic rather than protective to the aging brain. These findings are consistent with the bioenergetic mechanism,14 which we discuss below, and support the WHIMS-MRI finding that women 65 years and older treated with active CEE-based HT had smaller hippocampal and frontal lobe volumes.2
We reported that women with prior cardiovascular disease who were assigned to active HT had higher rates of accumulated white matter lesion volume and total brain lesion volume during the interval between the first and second MRI scan (table 3.) These findings relate to earlier WHI reports that women assigned to CEE + MPA, compared with placebo, had a 41% increase in stroke over 5.2 years,15 and CEE with and without MPA was associated with an increased risk of clinical stroke.16,17
In addition, WHIMS-MRI2 women with the lowest 3MSE scores at WHI enrollment who were treated with active HT had the largest decrements in hippocampal (p = 0.02) and total brain volumes (p = 0.05) between the 2 MRI measures. These findings are interesting, not only because they corroborate the prior WHIMS-MRI report,2 but they also show that despite the relatively younger and healthier WHIMS-MRI2 cohort, a subset of women who were most vulnerable cognitively had the smallest hippocampal and total brain volumes on longitudinal MRI.
The findings we report and prior results from the WHIMS trials shed light on potential mechanisms underlying the adverse effects of CEE-based therapies in older women. First, these therapies result in deficits in cognitive function, increases in incident cognitive impairment, and decrements in brain volumes that occur during the first few years of therapy and persist long after therapy ends, without triggering additional posttreatment acceleration of declines.18 Second, the increased risk of cognitive impairment appears to be mediated by increased atrophy.19 Third, effects on brain structure appear to be diffuse, not targeting circumscribed brain regions.20 Fourth, while ischemic lesion volumes within WHIMS women are linked to cognitive deficits,21 small-vessel disease does not appear to be the mechanism through which CEE-based therapies exert their predominant effect.5 The lack of an effect on ischemic lesions is consistent with the absence of an effect on the nearby retinal microvasculature,22 but inconsistent with the increased rate of stroke associated with CEE therapy.16 Fifth, extensive analyses have found no subgroups among older WHIMS women for whom benefits are observed; however, relatively greater adverse effects appear to be present for women with lower levels of cognitive function at baseline. Finally, the overall effects of CEE alone vs CEE + MPA therapy on the brain are much more similar than different, despite the marked differences in risk factors between the cohorts in these 2 parallel trials, suggesting that the primary agent in the effects we observe is CEE.
How does CEE adversely affect older women's cognitive health? We discuss a single mechanism that is consistent with our findings above, and relates to the role that estrogen may have in regulating mitochondrial function and bioenergetics. Proponents of this approach argue that as the brain ages, it shifts from “glucose-driven” bioenergetics, which are enhanced by estrogen, toward less-efficient ketone-based pathways, which may be downregulated by estrogen therapy.14,23,24 This results in a state of energy deprivation in the brain that may lead to diffuse degeneration and atrophy. This mechanism is consistent with an acute, but persistent, decrement in brain volume that may be larger among older women who have existing energy dysregulation. If this is the case, the WHIMS findings may not generalize to younger, healthier women. Results from the KEEPS (Kronos Early Estrogen Prevention Study) trial conducted in younger women will soon be available to assess the impact of HT on cognition in women treated closer to the menopausal transition.
We note recently published findings on cognitive function from women who enrolled in the WHI at ages 50 to 54 years.25 For these women, there was no long-term effect of HT on cognition, either positive or negative. Another area of work is related to estrogen receptor binding that may explain how neuroprotective effects of estrogen may erode with aging26,27; however, these do not explain why CEE therapy may adversely affect brain volumes in older women.
Advantages of this study include random assignment to treatment, a large sample of older women, detailed demographic and risk factor data, and longitudinal standardized brain MRI scans with strict quality assurance. It addresses whether CEE-based HT affects longitudinal structural brain changes in the context of the randomized placebo-controlled WHI HT trials and the WHIMS trials of incident probable dementia.
Our sample may have underrepresented participants most at risk for MRI pathology because of selective attrition over a 12-year period, and somewhat attenuated HT-associated brain volume decrements may reflect the healthier follow-up cohort. Also, approximately one-third of the WHIMS participants who were eligible for WHIMS-MRI2 refused to participate. Other MRI studies have reported similar patterns of differential enrollment such that resulting cohorts are at relatively lower risk of neuropathology compared with the targeted population.4,28,29
Supplementary Material
GLOSSARY
- CEE
conjugated equine estrogen
- HT
hormone therapy
- MCI
mild cognitive impairment
- MPA
medroxyprogesterone acetate
- 3MSE
modified Mini-Mental State Examination
- ROI
region of interest
- WHI
Women's Health Initiative
- WHIMS
Women's Health Initiative Memory Study
Footnotes
Supplemental data at www.neurology.org
Editorial, page 380
AUTHOR CONTRIBUTIONS
Laura H. Coker: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Mark A. Espeland: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, obtaining funding. Patricia E. Hogan: analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Susan M. Resnick: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. R. Nick Bryan: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision. Jennifer G. Robinson: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Joseph S. Goveas: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Christos Davatzikos: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision. Lewis H. Kuller: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Jeff D. Williamson: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data. Cheryl D. Bushnell: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. Sally A. Shumaker: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
Supported by the National Heart, Lung, and Blood Institute, NIH.
DISCLOSURE
L. Coker is funded by NIH grants DK092241, HC-09-05, U01HL096814-01, N01-WH-4-4221, and HHSN268201100004C. M. Espeland and P. Hogan report no disclosures. S. Resnick reports no disclosures. Her spouse has research grants and contracts managed through Johns Hopkins University with the following: Amgen, Avid Pharmaceuticals/Eli Lilly, Biotie, Intracellular, Johnson & Johnson, Lundbeck, and Roche Pharmaceuticals. He has consulted and received travel reimbursement and honoraria from Amgen and Concert Pharmaceuticals. R. Bryan reports no disclosures. J. Robinson receives support from research grants to the University of Iowa from Amarin, Amgen, Daiichi-Sankyo, Esperion, Genentech/Hoffmann-La Roche, GlaxoSmithKline, Merck, and Zinfandel/Takeda. J. Goveas receives support from the Alzheimer's Association New Investigator Research Grant NIRG-11-204070, Advancing Healthier Wisconsin Endowment for Research, Extendicare Foundation, and nonsalary support from the Clinical and Translational Science Award Program of the National Center for Research Resources (1UL1RR031973).C. Davatzikos, L. Kuller, and J. Williamson report no disclosures. C. Bushnell received research salary support funding from NIH/National Institute of Neurological Disorders and Stroke KO2 NS058760. Nonsalary supported funding is from a World Federation of Neurology/World Stroke Organization pilot grant, North Carolina Stroke Care Collaborative Quality Improvement grant, Bugher Foundation/American Stroke Association Program Project Grant, and pilot funds from the Wake Forest University Translational Science Center and Department of Neurology. S. Shumaker reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wnuk A, Korol DL, Erickson KI. Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas 2012;73:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnick SR, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 2009;72:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13:S5–S17 [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo SA, Felton D, Andrews LA, et al. Enrollment in a brain magnetic resonance study: results from the Women's Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI). Acad Radiol 2007;14:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coker LH, Hogan PE, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology 2009;72:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol 2008;15:300–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure in people with type 2 diabetes (ACCORD MIND): a randomized open-label study. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 2009;72:1906–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Ground J. Automatic segmentation of different-sized white matter lesions by voxel probability estimation. Med Image Anal 2004;8:205–215 [DOI] [PubMed] [Google Scholar]
- 10.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 2002;21:1421–1439 [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 12.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users, and men: a possible window of opportunity effect. Neurobiol Aging 2008;29:95–101 [DOI] [PubMed] [Google Scholar]
- 13.Lord C, Engert V, Lupien SJ, Pruessner JC. Effect of sex and estrogen therapy on the aging brain: a voxel-based morphometry study. Menopause 2010;17:846–851 [DOI] [PubMed] [Google Scholar]
- 14.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci 2008;31:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333 [DOI] [PubMed] [Google Scholar]
- 16.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. JAMA 2003;289:2673–2684 [DOI] [PubMed] [Google Scholar]
- 17.Women's Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA 2004;291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 18.Espeland MA, Brunner RL, Hogan PA, et al. Long term effects of conjugated equine estrogen therapies on domain-specific cognitive function: results from the Women's Health Initiative Study of Cognitive Aging (WHISCA) Extension. J Am Geriatr Soc 2010;58:1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espeland MA, Tindle HA, Bushnell CA, et al. ; Women's Health Initiative Memory Study Brain volumes, cognitive impairment, and conjugated equine estrogens. J Gerontol A Biol Sci Med Sci 2009;64:1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casanova R, Espeland MA, Goveas J, et al. Application of machine learning methods to describe the effects of conjugated equine estrogens therapy on region-specific brain volumes. Magn Reson Imaging 2011;29:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tooze JA, Gaussoin SA, Resnick SM, et al. ; Women's Health Initiative Memory Study A uniform approach to modeling risk factor relationships for ischemic lesion prevalence and extent: the Women's Health Initiative Magnetic Resonance Imaging Study. Neuroepidemiology 2010;34:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haan M, Espeland MA, Klein BE, et al. Cognitive function and retinal and ischemic brain changes: the Women's Health Initiative. Neurology 2012;78:942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao J, Rettberg JR, Klosinski LP, Cardenas E, Brinton RD. Shift in brain metabolism in late onset Alzheimer's disease: implications for biomarkers and therapeutic interventions. Mol Aspects Med 2011;32:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao J, Brinton RD. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer's disease. Adv Pharmacol 2012;64:327–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espeland MA, Shumaker SA, Leng I, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med 2013;173:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang QG, Han D, Wang RM, et al. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci USA 2011;108:E617–E624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ/P13-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging 2011;32:1949–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGroot JC, De Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry 2000;57:1071–1076 [DOI] [PubMed] [Google Scholar]
- 29.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke 2002;33:26–30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.