Abstract
Objective:
Electrographic seizures (ES) and electrographic status epilepticus (ESE) are common in children in the pediatric intensive care unit (PICU) with acute neurologic conditions. We aimed to determine whether ES or ESE was associated with worse long-term outcomes.
Methods:
Three hundred children with an acute neurologic condition and encephalopathy underwent clinically indicated EEG monitoring and were enrolled in a prospective observational study. We aimed to obtain follow-up data from 137 subjects who were neurodevelopmentally normal before PICU admission.
Results:
Follow-up data were collected for 60 of 137 subjects (44%) at a median of 2.7 years. Subjects with and without follow-up data were similar in clinical characteristics during the PICU admission. Among subjects with follow-up data, ES occurred in 12 subjects (20%) and ESE occurred in 14 subjects (23%). Multivariable analysis indicated that ESE was associated with an increased risk of unfavorable Glasgow Outcome Scale (Extended Pediatric Version) category (odds ratio 6.36, p = 0.01) and lower Pediatric Quality of Life Inventory scores (23 points lower, p = 0.001). Among subjects without prior epilepsy diagnoses ESE was associated with an increased risk of subsequently diagnosed epilepsy (odds ratio 13.3, p = 0.002). ES were not associated with worse outcomes.
Conclusions:
Among children with acute neurologic disorders who were reported to be neurodevelopmentally normal before PICU admission, ESE but not ES was associated with an increased risk of unfavorable global outcome, lower health-related quality of life scores, and an increased risk of subsequently diagnosed epilepsy even after adjusting for neurologic disorder category, EEG background category, and age.
Electrographic seizures (ES) and electrographic status epilepticus (ESE) have been reported in 10% to 40% of children in pediatric intensive care units (PICUs) who underwent clinically indicated continuous EEG (cEEG) monitoring.1–14 Thus, cEEG use is common and is increasing in PICUs.15,16 While cEEG is resource-intense,17 there is evidence that cEEG data often have an impact on clinical management.18 Furthermore, in addition to serving as biomarkers of brain injury and dysfunction, ES and ESE may also contribute to secondary brain injury and worse short-term outcome1,9,10,12,19–21 independently of acute encephalopathy etiology and critical illness severity.1,10,21 To date, studies have not evaluated whether there is an association between ES or ESE and long-term outcome. We performed a study of children in the PICU with acute encephalopathy who underwent cEEG to determine whether ES or ESE was associated with worse long-term outcome.
METHODS
Standard protocol approvals, registrations, and patient consents.
Informed written consent was obtained from the parents of patients undergoing clinically indicated cEEG monitoring for entry in the observational database. Subsequently, informed verbal consent was obtained from parents contacted for the follow-up long-term outcome study. This study was approved by the Children's Hospital of Philadelphia's Institutional Review Board.
Prospective observational database.
Infants and children treated in the PICU of a tertiary care hospital between July 2008 and September 2011 who underwent cEEG monitoring were enrolled in a prospective observational study. Neonates (younger than 1 month) were excluded. Clinical practice was to perform cEEG in patients with acute encephalopathy of any degree to identify ES and/or to determine whether abnormal movements or vital sign fluctuations of unknown etiology were seizures. EEGs were performed using a Grass-Telefactor (West Warwick, RI) video-EEG system with 21 gold-over-silver scalp surface electrodes positioned according to the international 10–20 system. cEEG monitoring duration was at least 24 hours when screening for ES or ESE or 72 hours for patients undergoing therapeutic hypothermia after cardiac arrest. The encephalography service interpreted the EEGs and the PICU and neurology consult services managed patient care. Prophylactic anticonvulsants were not routinely administered. Our clinical services aimed to terminate ES and ESE when identified, although no formal institutional management pathway was in place. The most frequently used anticonvulsants for ES and ESE in our PICU were phenobarbital, phenytoin-fosphenytoin, and levetiracetam.22
Clinical and cEEG data were prospectively collected. Clinical data consisted of age, sex, acute neurologic disorders, prior neurodevelopmental status, medications, intubation status, cEEG indication, cEEG findings including seizure occurrence and characteristics, hospital and PICU admission and discharge dates, and short-term outcome. Acute neurologic disorders were categorized as follows: 1) epilepsy-related; 2) acute structural (stroke, CNS inflammation or autoimmune disorder, traumatic brain injury, CNS infection, brain malformation, tumor/oncologic, and hypoxic-ischemic encephalopathy); and 3) acute nonstructural (sepsis, metabolic, pharmacologic sedation, toxin, paralytic administration). Patients who had both acute structural and nonstructural conditions (such as CNS infection and sepsis) were categorized as acute structural. Short-term functional outcome was assessed by assigning a Pediatric Cerebral Performance Category score. The categories are 1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma and vegetative state, and 6 = death.23 We have previously described the associations between ES and ESE and short-term outcome.21
EEG tracings were reviewed and interpreted by one pediatric encephalographer (N.S.A.) to define seizure categories (none, ES, or ESE) and EEG background categories. An ES was defined as an abnormal paroxysmal event that was different from the background, lasting longer than 10 seconds (or shorter if associated with a clinical change) with a temporal-spatial evolution in morphology, frequency, and amplitude, and with a plausible electrographic field. ESE was defined as either a single 30-minute ES or a series of recurrent independent ES totaling more than 30 minutes in any 1-hour period (50% seizure burden). Patients were scored as ESE if ESE occurred at any point during the recording. The ES and ESE distinction was used to evaluate the impact of seizure burden and is consistent with definitions used in previous studies.1,21 EEG background categories included 1) normal or sedated sleep, 2) slow and disorganized, 3) discontinuous or burst-suppression, and 4) attenuated and featureless.
Long-term outcome study.
Using the 300 subjects enrolled in our prospective cEEG database, we conducted a secondary study assessing long-term outcome. We attempted to contact all subjects who survived to PICU discharge with at least 5 contact attempts (including weekend and evening calls) to all phone numbers included in the institutional medical record. A trained caller performed all of the structured phone interviews. Outcome data were obtained by proxy report (parents/guardians) and included the Glasgow Outcome Scale–Extended Pediatric Version (GOS-E Peds), the Pediatric Quality of Life Inventory (PedsQL), and an epilepsy questionnaire.
The GOS-E Peds is a global outcome measure addressing postinjury function. Seven domains (consciousness, independence inside the home, independence outside the home, school/work, social and leisure activity, family and friendships, and return to normal life) produce a category score. The GOS-E Peds has been shown to have high criterion-related validity and discriminant validity, has demonstrated sensitivity to injury severity,24 and is a suggested core global outcome measure by National Institute of Neurological Disorders and Stroke common data elements.25 GOS-E Peds scores were categorized as favorable (scores representing upper good recovery to lower moderate disability) or unfavorable (scores representing upper severe disability to vegetative state).
The PedsQL is a generic health-related quality-of-life instrument based on 4 functioning domains (physical, emotional, social, and school). It is valid and reliable in normal and patient populations,26,27 and it is a suggested core global outcome measure by National Institute of Neurological Disorders and Stroke common data elements.25
Among subjects who did not have an epilepsy-related diagnosis at the time of PICU care, we assessed whether subjects had developed subsequent epilepsy using a self-reported epilepsy questionnaire that has been validated for use in surveillance population-based surveys.28 The questionnaire wording was modified slightly to focus on children by changing the term “you” to “your child.” Subjects were classified as diagnosed epilepsy (“yes” to a question indicating a diagnosis of epilepsy) or possible epilepsy (“yes” to any epilepsy screening questions).
Analysis.
This study only included subjects who were reported to be neurodevelopmentally normal before PICU admission by parents and prior medical records when available.
Summary statistics are reported as medians and interquartile ranges for continuous data and counts and proportions for categorical data. Comparisons between subjects with and without follow-up data were examined using the χ2 test for categorical variables and the Wilcoxon rank-sum or Kruskal-Wallis test for continuous variables. Variables with p ≤ 0.2 in univariable analyses were eligible for inclusion in the multivariable models. Multivariable logistic regression was used to test the associations between seizure category and GOS-E Peds, and also between seizure category and subsequent epilepsy. Linear regression was used to test the association between seizure category and PedsQL. A priori covariates were chosen based on variables associated with unfavorable short-term outcome21 and included age, acute neurologic disorder category, and EEG background category. All statistics were performed using Stata 10.0 software (StataCorp, College Station, TX).
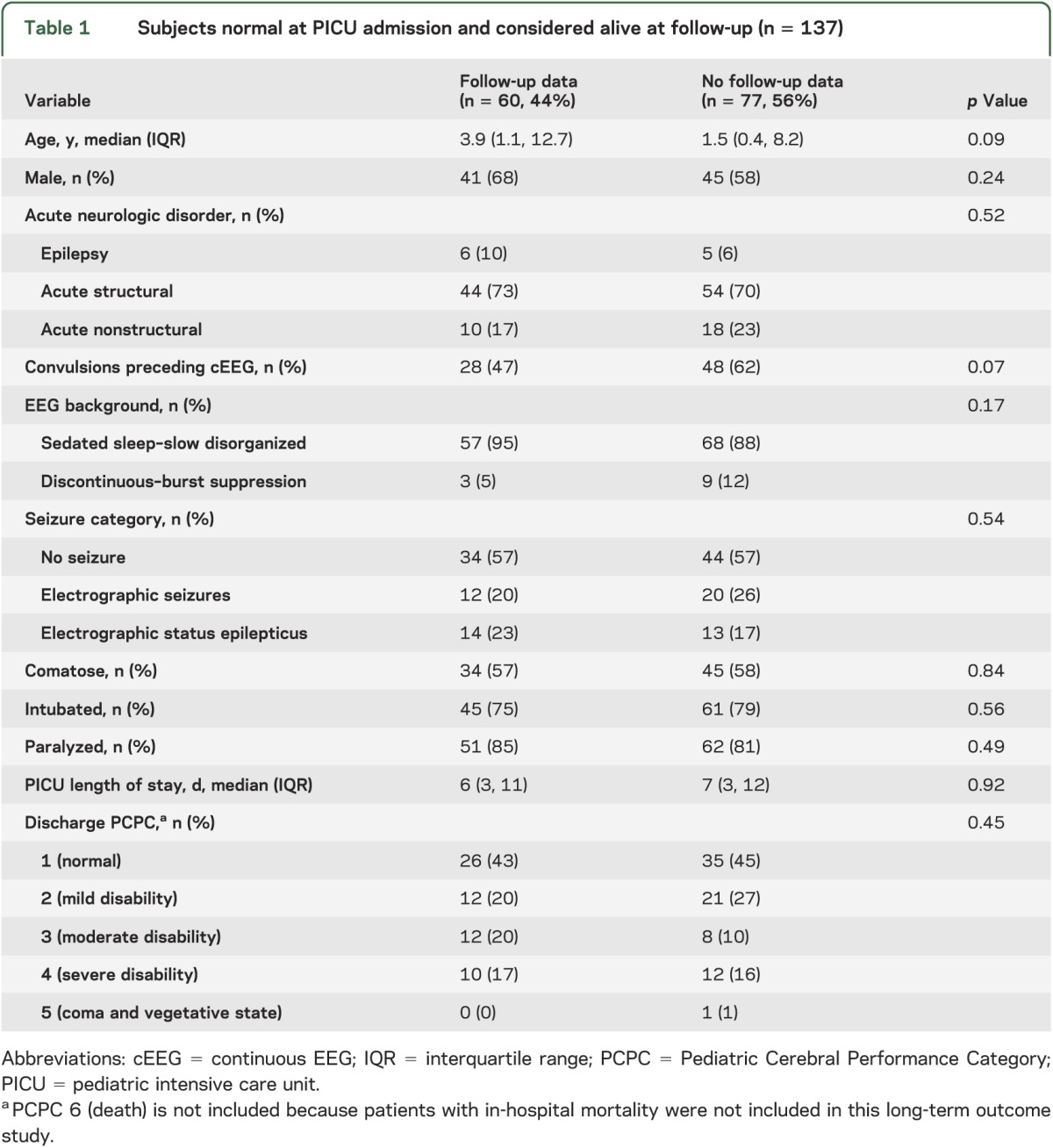
RESULTS
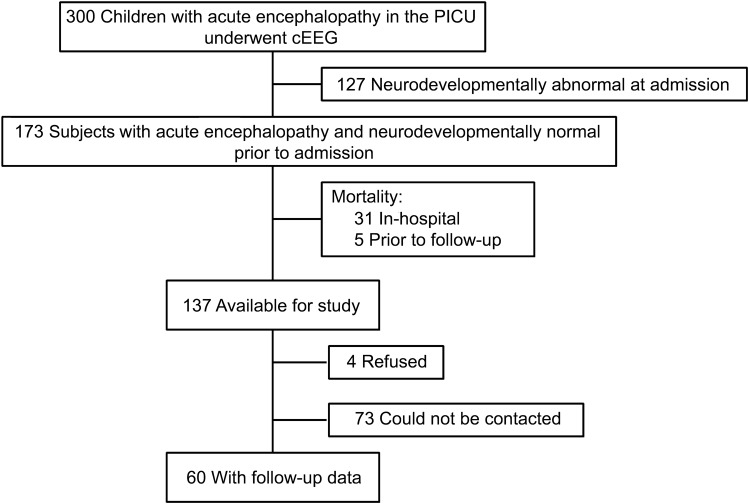
Three-hundred children with encephalopathy were enrolled in the acute care component of the study. One-hundred thirty-seven subjects were reported to be neurodevelopmentally normal at PICU admission by parents/guardians and survived to PICU discharge. Seventy-seven were not eligible for study inclusion (73 could not be contacted and 4 declined participation), resulting in a final sample of 60 study participants (figure). There were no significant differences in the acute care variables between the subjects with and without outcome data (table 1). Follow-up data were obtained a median of 2.6 years (1.5, 3.2) after PICU discharge. The median age at PICU admission was 3.9 years (1.1, 12.7) and 41 subjects (68%) were male.
Figure. Study flowchart.
cEEG = continuous EEG; PICU = pediatric intensive care unit.
Table 1.
Subjects normal at PICU admission and considered alive at follow-up (n = 137)
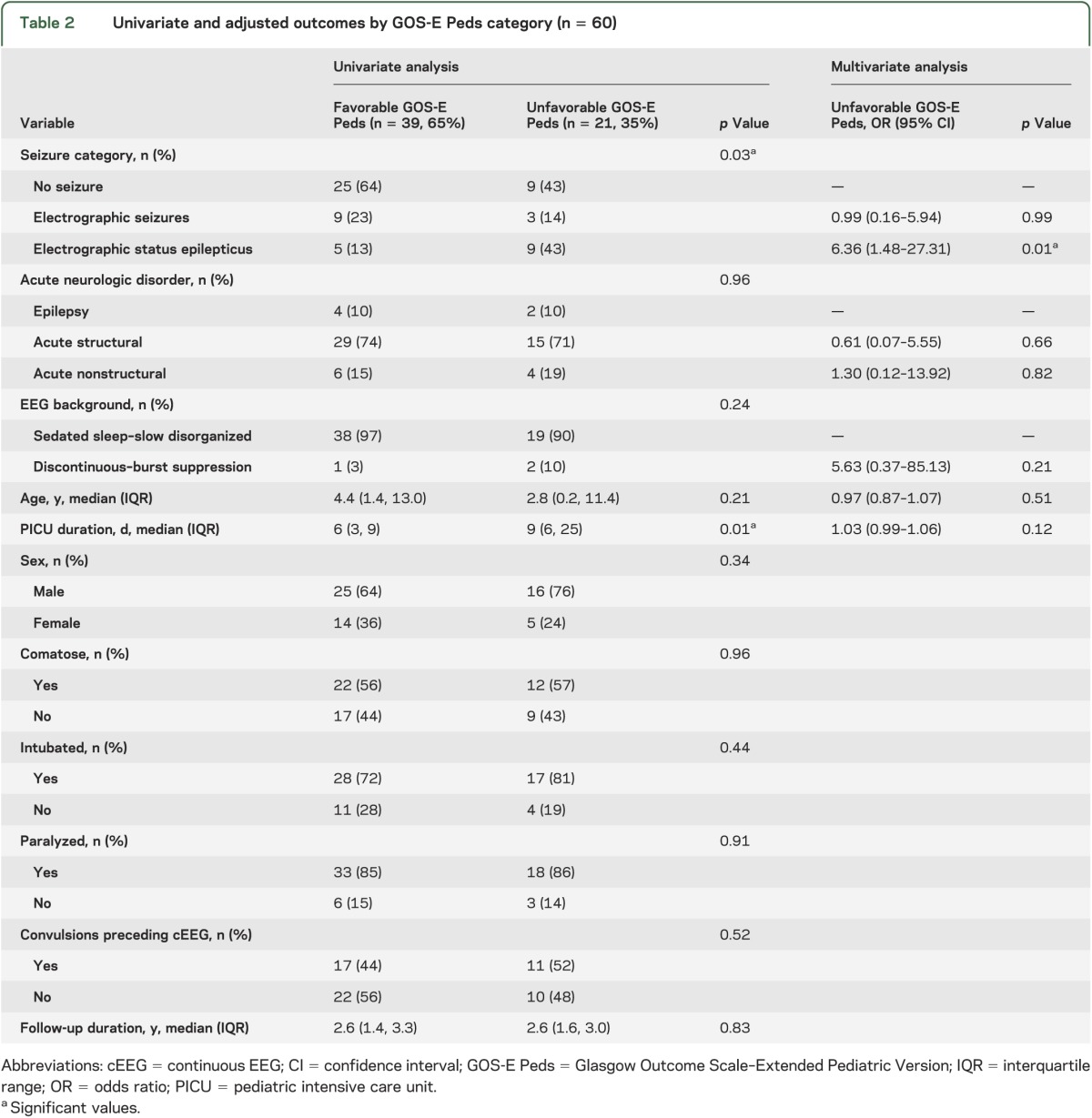
GOS-E Peds scores were upper good recovery for 24 subjects (40%), lower good recovery for 11 subjects (18%), upper moderate disability for 2 subjects (3%), lower moderate disability for 2 subjects (3%), upper severe disability for 8 subjects (13%), and lower severe disability for 13 subjects (22%). Thus, unfavorable outcome by GOS-E Peds occurred for 21 subjects (35%). On univariable analysis, seizure category and PICU duration were associated with GOS-E Peds category (table 2). After controlling for EEG background, acute neurologic disorder, age, and PICU duration, ESE was associated with unfavorable GOS-E Peds (odds ratio [OR] 6.36; 95% confidence interval [CI] 1.48, 27.31; p = 0.01). However, ES were not associated with unfavorable GOS-E Peds (OR 0.99; 95% CI 0.16, 5.94; p = 0.99) (table 2).
Table 2.
Univariate and adjusted outcomes by GOS-E Peds category (n = 60)
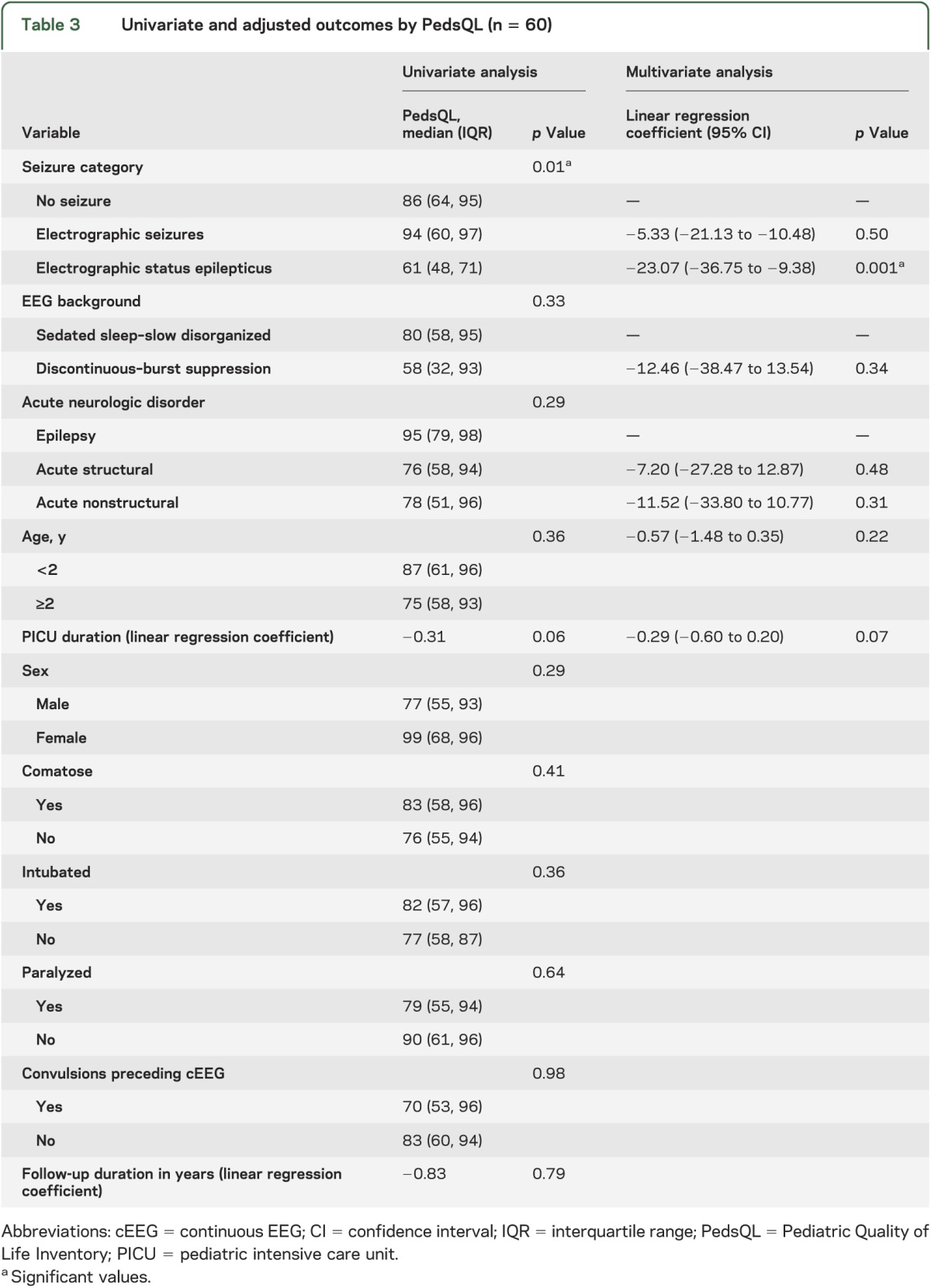
The median (interquartile range) PedsQL scores were 86 (64, 95) for subjects without seizures, 94 (60, 97) for subjects with ES, and 62 (48, 71) for subjects with ESE (p = 0.01). On univariable analysis, only seizure category was associated with lower PedsQL scores (table 3). After controlling for EEG background, acute neurologic disorder, age, and PICU duration, ESE was associated with lower PedsQL scores (23.07 points lower, p = 0.001) while ES were not associated with lower PedsQL scores (table 3).
Table 3.
Univariate and adjusted outcomes by PedsQL (n = 60)
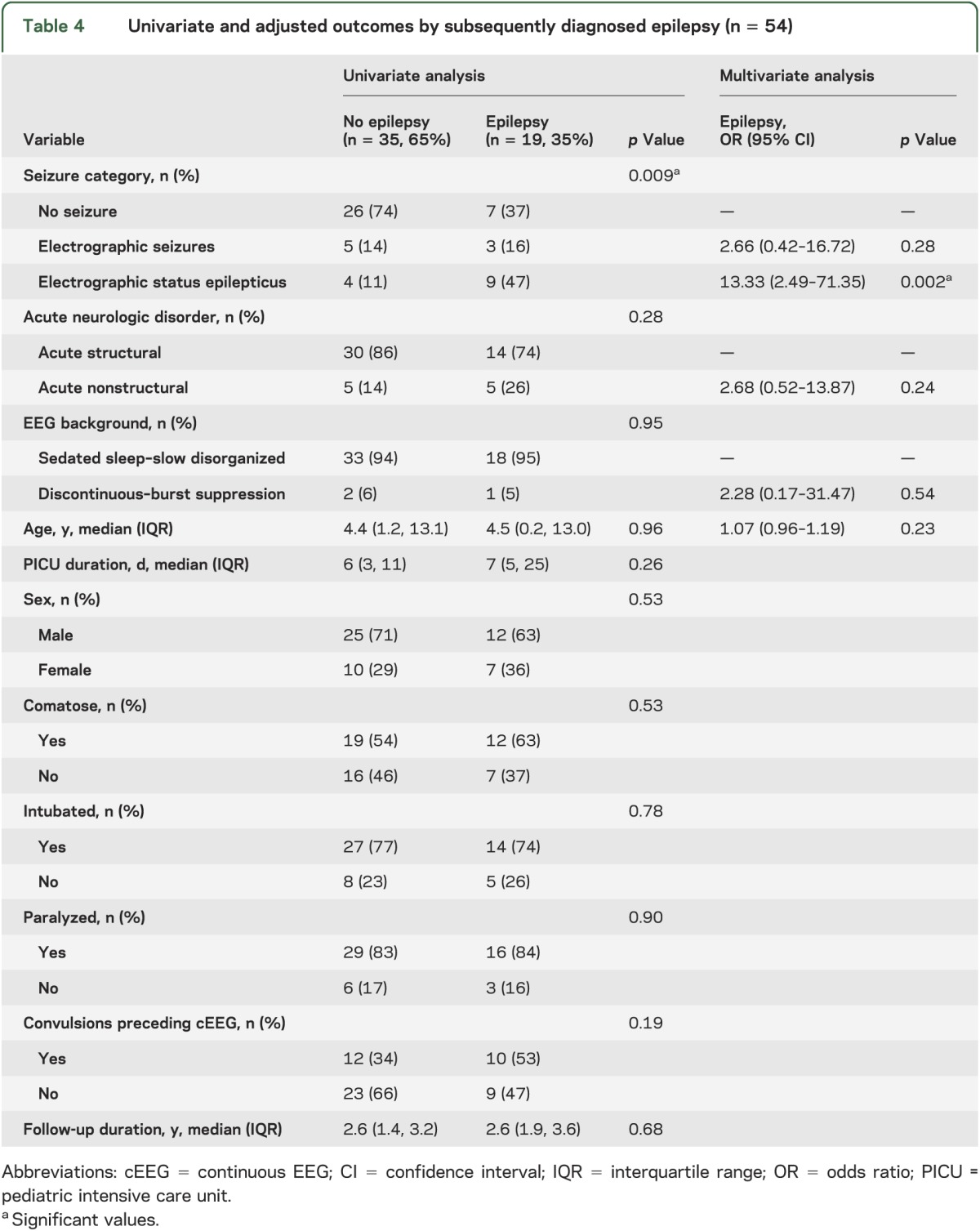
Subsequent epilepsy was assessed among the 54 subjects who did not have epilepsy diagnoses before PICU admission. New physician-diagnosed epilepsy occurred in 19 subjects, of whom 7 subjects (37%) had no seizures, 3 subjects (16%) had ES, and 9 subjects (47%) had ESE. On univariable analysis, only seizure category was associated with an increased risk of subsequently diagnosed epilepsy (table 4). After controlling for EEG background, acute neurologic disorder, and age, ESE was associated with diagnosed epilepsy at follow-up (OR 13.33; 95% CI 2.49, 71.35; p = 0.002) while ES were not associated with diagnosed epilepsy at follow-up (OR 2.66; 95% CI 0.42, 16.72; p = 0.28) (table 4). Possible epilepsy at follow-up was present in 19 of 33 (58%) without seizures, 6 of 8 with ES (75%), and 12 of 13 with ESE (92%). After controlling for EEG background, acute neurologic disorder, and age, ESE was associated with possible epilepsy at follow-up (OR 13.41; 95% CI 1.37, 131.40; p = 0.026) while ES were not associated with possible epilepsy at follow-up (OR 1.85; 95% CI 0.25, 13.69; p = 0.55).
Table 4.
Univariate and adjusted outcomes by subsequently diagnosed epilepsy (n = 54)
DISCUSSION
This observational study of cEEG-monitored children in the PICU with outcome assessment at a median of 2.6 years showed that when compared with patients without seizures, ESE was associated with unfavorable global outcome (GOS-E Peds), lower health-related quality of life (PedsQL), and an increased risk of developing subsequent epilepsy after controlling for acute neurologic disorder category, EEG background category, and age. When compared with patients without seizures, ES were not associated with worse outcomes.
ES have been reported in 10% to 40% of children in PICUs who underwent cEEG monitoring.1–14 The largest study to date was a multicenter retrospective study of 550 consecutive children who underwent cEEG monitoring. ES occurred in 30%, and was classified as ESE in 38%.1 Those data are consistent with single-center data we have reported from the current observational study database.14,21 This study used a subset of subjects from the database (neurodevelopmentally normal on PICU admission and alive for follow-up) and identified ES or ESE in 43% of 60 subjects.
Because ES and ESE are common in critically ill children, there is an increasing interest in determining whether identifying and managing these seizures could serve as a neuroprotective strategy. EEG monitoring use is increasing, largely based on the presumption that identifying and managing seizures could improve outcome. A survey addressing cEEG use at 61 large North American pediatric hospitals reported a 30% increase in the number of critically ill patients undergoing cEEG from 2010 to 2011.16 Both physician surveys15 and observational studies22 indicate that anticonvulsants are administered when seizures are identified. A recent Neurocritical Care Society guideline recommended that cEEG should be used to identify nonconvulsive status epilepticus in many comatose patients, and that treatment should continue rapidly until both clinical and EEG-only seizures cease.29
Increasing cEEG use is logical if ES or ESE are associated with worse outcome, and if identifying and managing ES or ESE is associated with improved outcome. To date, the extent to which these seizures are biomarkers of more severe brain injury and/or independently lead to secondary brain injury has not been established. Our data add to this literature by providing an initial exploration of the impact of ES and ESE on long-term outcomes. Our findings are consistent with prior short-term outcome studies that have reported associations between ES or ESE and worse short-term outcome1,9,10,12,19–21 even after accounting for potential confounders related to acute encephalopathy etiology and critical illness severity.1,10,21 In a multicenter retrospective study of 550 children who underwent clinically indicated cEEG, ESE but not ES were associated with an increased mortality risk on multivariable analysis including EEG background category and neurologic disorder category.1 Similarly, our 200-subject, single-center, prospective study found that ESE, but not ES, was associated with an increased risk of in-hospital mortality and worsening Pediatric Cerebral Performance Category score at discharge after controlling for seizure category, age, acute neurologic disorder, prior neurodevelopmental status, and EEG background category.21 Another study of 204 comatose neonates and children found that ES were associated with a higher risk of unfavorable short-term neurologic outcome after controlling for age, etiology, pediatric index of mortality score, Adelaide coma score, and EEG background category.10 A retrospective study of 237 children with convulsive or nonconvulsive status epilepticus (defined as more than 10 minutes of seizures) found that epilepsy and neurologic deficits were more common in children with refractory than aborted status epilepticus, and longer seizure durations predicted unfavorable outcome.19 However, these data were obtained in children with both convulsive and nonconvulsive status, and thus may not be directly comparable to our data.
Several studies have reported worse outcome in critically ill adults with acute seizures,30–32 and several studies have suggested mechanisms by which ES could yield secondary brain injury, including elevated intracranial pressure and lactate/pyruvate ratios during ES in adults with traumatic brain injury,33 development of hippocampal atrophy ipsilateral to an acute ES focus in adults with traumatic brain injury,34 and regional hyperperfusion concordant with the ES focus in adults with epilepsy.35 The current findings indicate that additional research to determine whether seizure identification and management are associated with improved neurodevelopmental outcome would be beneficial.
Epilepsy occurs after many types of brain injury. In a study of 105 adults with clinically evident acute symptomatic seizures due to heterogeneous etiologies, 12% of subjects had experienced an unprovoked seizure within 2 years of their acute symptomatic seizure.36 Similarly, a study of adults noted that at 10-year follow-up, the risk of unprovoked seizures was higher among adults who had experienced clinically evident acute symptomatic status epilepticus than those who experienced acute symptomatic seizures after controlling for age, sex, and cause.37 Our data are consistent with those findings because ESE was associated with an increased risk of subsequent diagnosed epilepsy. Management strategies that effectively reduce critically ill children's exposure to a high acute symptomatic seizure burden could reduce the possible epileptogenic potential of these seizures.
This study has limitations. Follow-up data were only obtained for 44% of subjects from the original cohort. Fortunately, acute care data were available for all subjects. Although there was a trend in which fewer patients with follow-up data had convulsive seizures preceding cEEG, we did not identify differences between subjects with and without follow-up data, suggesting that the follow-up cohort may be representative of the entire cohort. cEEG was initiated only when considered clinically indicated, and this determination may have varied among clinicians. Subjects needed to consent for enrollment in the original observational study and a few patients refused consent. cEEG was continued for a clinically determined duration so some patients scored as having no seizures may have experienced seizures after cEEG was discontinued. We used phone-administered outcome assessments and not formal neuropsychological evaluation. We screened for epilepsy using a brief phone questionnaire and not screening by a neurologist. A prospective longitudinal study including protocoled cEEG indications, standardized cEEG durations, active methods of subject retention, and formal neuropsychological assessments would yield an improved understanding of long-term outcome. We stratified seizure burden as ES and ESE, and ESE could involve prolonged or multiple brief seizures. The optimal method for stratifying seizure burden is unknown and most likely the seizure burden sufficient to produce secondary brain injury varies based on age, acute encephalopathy etiology, and seizure anatomical distribution and characteristics. This study only included subjects who were neurodevelopmentally normal upon PICU admission, which limits the generalizability of these data. Importantly, our clinical practice involved managing ES and ESE when identified, but subjects with ESE still had worse outcome. This could indicate that efforts to identify and manage ESE do not improve outcome. However, our anticonvulsant management was highly varied22 and not based on any data guiding the optimal management of ES and ESE. Possibly, optimized seizure identification and management strategies would yield improved outcomes.
Our data indicate that among children in the PICU with acute encephalopathy, ESE is associated with unfavorable long-term global outcome, lower health-related quality of life, and a higher occurrence of subsequently diagnosed epilepsy, while ES are not associated with worse outcomes. The current data suggest that identifying and managing every ES may not be needed while identifying and managing ESE might reduce secondary brain injury and serve as a neuroprotective strategy. Additionally, these data suggest that children who experience ESE may require closer follow-up because they are at risk of more substantial impairments in functional outcome and quality of life, and they may be at increased risk of developing subsequent epilepsy. Further studies are needed with larger cohorts, more complete follow-up, and more detailed outcome measures. Additionally, efficacy trials are needed to determine which optimized management approaches aimed at identifying and terminating ES and ESE are associated with improved long-term outcomes.
GLOSSARY
- cEEG
continuous EEG
- CI
confidence interval
- ES
electrographic seizures
- ESE
electrographic status epilepticus
- GOS-E Peds
Glasgow Outcome Scale–Extended Pediatric Version
- OR
odds ratio
- PedsQL
Pediatric Quality of Life Inventory
- PICU
pediatric intensive care unit
AUTHOR CONTRIBUTIONS
Katherine Wagenman: acquisition of data, critical revision of the manuscript. Taylor Blake: study concept and design, analysis and interpretation, critical revision of the manuscript. Sarah Sanchez: acquisition of data, critical revision of the manuscript. Dr. Shultheis, Dr. Radcliffe, Dr. Berg, and Dr. Dlugos: study concept and design, analysis and interpretation, critical revision of the manuscript. Dr. Topjian: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript. Dr. Abend: study concept and design, acquisition of data, analysis and interpretation, drafting of initial version of the manuscript, critical revision of the manuscript, study supervision.
STUDY FUNDING
Supported by NIH (K23NS076550) to Dr. Abend and the Clinical & Translational Research Center (grant UL1RR024134).
DISCLOSURE
K. Wagenman, T. Blake, S. Sanchez, and M. Schultheis report no disclosures. J. Radcliffe receives funding from the Clinical & Translational Research Center (grant UL1RR024134). R. Berg reports no disclosures. D. Dlugos receives research funding from NIH (1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276) and has received payment for medical-legal review work. A. Topjian receives research funding from NIH (K12HD047349 and U01HL094345). N. Abend receives research funding from NIH (K23NS076550), receives royalties from Demos Medical Publishing, and has received payment for medical-legal review work. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013;81:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol 2005;32:162–165 [DOI] [PubMed] [Google Scholar]
- 3.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750–1755 [DOI] [PubMed] [Google Scholar]
- 4.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol 2007;37:165–170 [DOI] [PubMed] [Google Scholar]
- 5.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 2006;47:1504–1509 [DOI] [PubMed] [Google Scholar]
- 6.Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia 2010;51:1198–1204 [DOI] [PubMed] [Google Scholar]
- 7.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009;72:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia 2011;52:1130–1136 [DOI] [PubMed] [Google Scholar]
- 9.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics 2012;129:e748–e755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med 2012;38:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango JI, Deibert CP, Brown D, Bell M, Dvorchik I, Adelson PD. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst 2012;28:1925–1929 [DOI] [PubMed] [Google Scholar]
- 12.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care 2012;17:31–38 [DOI] [PubMed] [Google Scholar]
- 13.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia 2011;52:1973–1978 [DOI] [PubMed] [Google Scholar]
- 14.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology 2011;76:1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care 2010;12:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. EEG monitoring in critically ill children: indications and strategies. Pediatr Neurol 2012;46:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abend NS, Topjian AA, Gutierrez-Colina AM, Donnelly M, Clancy RR, Dlugos DJ. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care 2011;15:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia 2008;49:615–625 [DOI] [PubMed] [Google Scholar]
- 20.Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child 2012;97:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013;41:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abend NS, Sanchez SM, Berg RA, Dlugos DJ, Topjian AA. Treatment of electrographic seizures and status epilepticus in critically ill children: a single center experience. Seizure 2013;22:467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616–2620 [DOI] [PubMed] [Google Scholar]
- 24.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma 2012;29:1126–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCauley SR, Wilde EA, Anderson VA, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma 2012;29:678–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–812 [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the Pediatric Quality of Life Inventory. Med Care 1999;37:126–139 [DOI] [PubMed] [Google Scholar]
- 28.Brooks DR, Avetisyan R, Jarrett KM, et al. Validation of self-reported epilepsy for purposes of community surveillance. Epilepsy Behav 2012;23:57–63 [DOI] [PubMed] [Google Scholar]
- 29.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23 [DOI] [PubMed] [Google Scholar]
- 30.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996;47:83–89 [DOI] [PubMed] [Google Scholar]
- 31.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009;37:2051–2056 [DOI] [PubMed] [Google Scholar]
- 32.Carrera E, Claassen J, Oddo M, Emerson RG, Mayer SA, Hirsch LJ. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch Neurol 2008;65:1612–1618 [DOI] [PubMed] [Google Scholar]
- 33.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007;35:2830–2836 [PMC free article] [PubMed] [Google Scholar]
- 34.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology 2010;75:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauf M, Slotboom J, Nirkko A, von Bredow F, Ozdoba C, Wiest R. Cortical regional hyperperfusion in nonconvulsive status epilepticus measured by dynamic brain perfusion CT. AJNR Am J Neuroradiol 2009;30:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung H, Man CB, Hui AC, Kwan P, Wong KS. Prognosticating acute symptomatic seizures using two different seizure outcomes. Epilepsia 2010;51:1570–1579 [DOI] [PubMed] [Google Scholar]
- 37.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol 1998;44:908–912 [DOI] [PubMed] [Google Scholar]