Abstract
Objective:
To test the hypothesis that neonatal sleep physiology reflects cerebral dysfunction, we compared neurologic examination scores to the proportions of recorded sleep/wake states, sleep depth, and sleep fragmentation in critically ill neonates.
Methods:
Newborn infants (≥35 weeks gestation) who required intensive care and were at risk for seizures were monitored with 8- to 12-hour polysomnograms (PSGs). For each infant, the distribution of sleep-wake states, entropy of the sequence of state transitions, and delta power from the EEG portion of the PSG were quantified. Standardized neurologic examination (Thompson) scores were calculated.
Results:
Twenty-eight infants participated (mean gestational age 39.0 ± 1.6 weeks). An increased fraction of quiet sleep correlated with worse neurologic examination scores (Spearman rho = 0.54, p = 0.003), but the proportion of active sleep did not (p > 0.1). Higher state entropy corresponded to better examination scores (rho = −0.43, p = 0.023). Decreased delta power during quiet sleep, but not the power at other frequencies, was also associated with worse examination scores (rho = −0.48, p = 0.009). These findings retained significance after adjustment for gestational age or postmenstrual age at the time of the PSG. Sleep stage transition probabilities were also related to examination scores.
Conclusions:
Among critically ill neonates at risk for CNS dysfunction, several features of recorded sleep—including analyses of sleep stages, depth, and fragmentation—showed associations with neurologic examination scores. Quantitative PSG analyses may add useful objective information to the traditional neurologic assessment of critically ill neonates.
Assessment of functional brain integrity is challenging for sick neonates, whose repertoires of signs of neurologic health or dysfunction are limited. The neonatal neurologic examination is based largely on behavioral observation and assessment of brainstem reflexes during wakefulness. Each day, however, newborns' brains actively create sleep for twice as many hours as wakefulness. Sleep as a complex and highly regulated neurologic function has barely been analyzed for neonates and is typically not systematically assessed by standard clinical neurologic evaluations.
Emerging evidence centered on sleep-disordered breathing suggests that abnormal sleep physiology later in infancy is associated with adverse neurologic and behavioral outcomes. As early as 6 months of age, seemingly healthy infants with frequent snoring, a sign of abnormal sleep, have lower cognitive development scores than those without parent-reported snoring.1 Symptoms of sleep-disordered breathing, even when isolated to the first year of life, are associated with subsequent adverse behavioral outcomes during elementary school ages.2 These data support the hypothesis that for infants and children, abnormal sleep could have a lasting negative impact on brain development.
Our objective was to test the hypothesis that disordered sleep in the neonatal period is associated with concurrent CNS dysfunction. We studied critically ill neonates whose brains, by virtue of their age and medical condition, may be particularly vulnerable to sleep disruption or eloquent in reflecting effects of dysregulated sleep. We used full polysomnography (PSG), a gold standard for characterization of sleep-wake states, in combination with quantitative analyses rarely applied in neonates, in an effort to identify new objective parameters that could shed light on neonatal sleep physiology and distinguish newborn infants at risk for suboptimal neurodevelopment.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of Michigan Institutional Review Board and a parent of each infant provided written informed consent. Term or near-term neonates (estimated gestational age ≥35 weeks) admitted to our neonatal intensive care unit (NICU) between March 2010 and March 2013 and judged clinically to be at risk for seizures, according to published guidelines,3 were candidates for participation. Exclusion criteria were prematurity, suspected or confirmed genetic syndrome that would affect neurodevelopment, and markedly abnormal EEG background, such as burst-suppression, that would preclude identification of sleep-wake cycling.
Demographic and clinical information was collected and standardized neurologic examinations were performed by a pediatric neurologist (R.A.S.) on the day of the PSG. Examination findings were analyzed by computing Thompson scores.4 The Thompson score incorporates the major components of the neonatal neurologic examination (mental status, brainstem reflexes, movement, tone, tendon reflexes, and primitive reflexes), as well as the presence of clinically apparent seizures and assessment of the fontanelle. The Thompson score includes mental status, but is not otherwise designed to assess sleep-wake state characteristics. Low Thompson scores are associated with favorable neurodevelopmental prognosis among infants with hypoxic ischemic encephalopathy.4
PSG commenced once the infant was medically stable. The complexity of clinical care required by these infants did not permit standardized PSG start times, though nearly all (24 of 28) were recorded during the night. Each infant was monitored in the NICU with an 8- to 12-hour formal, attended, PSG. A sleep technologist was present at the infant's bedside to record detailed behavioral observations throughout the PSG. In addition to the 9-channel neonatal-montage EEG, other channels included bilateral electro-oculogram, chin surface EMG, chest and abdominal excursion (inductance plethysmography), nasal pressure, nasal/oral airflow (thermocouples), snoring sensor, oxygen saturation, ECG, bilateral anterior tibialis surface EMG, digital video, and transcutaneous CO2. PSGs were scored off-line by a single experienced, registered polysomnographic technologist, according to standard neonatal scoring rules,5 and reviewed by a board-certified sleep medicine physician. These individuals were blinded to the neurologic status of the infant.
For each infant, the proportion of each sleep-wake state was calculated. The entropy of the sequences of sleep-wake state transitions (combining active and quiet sleep into one “sleep” indicator) and sleep stage transitions (considering active and quiet sleep separately, but excluding indeterminate sleep) were calculated using the Walsh spectral entropy method.6,7 In this context, entropy measures the predictability of a temporal pattern. Lower entropy values suggest more regularity or uniformity in the temporal patterns of sleep stage transitions, while higher values indicate diminished regularity or predictability. This approach quantifies patterns of temporal regularity of a categorical time series without requiring numerical encoding (scaling) of the (categorical) sleep stages.
Power spectra from the EEG portion of the PSG were also quantified, using the Welch method for fast Fourier transform (FFT)8 (each 30-second epoch of EEG signal, sampled at 256 Hz, was divided into 8 sections with 50% overlap; each section was windowed with a Hamming window to reduce spectral leakage; and 8 modified periodograms were computed using the FFT and then averaged to reduce noise). The 30-second window was selected to correspond to the scored sleep epochs, which are 30 seconds in duration. The Spearman correlation of these results with standardized neurologic examination (Thompson) scores was calculated, adjusted for gestational age and for postmenstrual age at the time of the PSG.
Conditional stage transition probabilities, which characterize the chance that an infant in one sleep-wake state will transition to a specific, different, sleep-wake state, were also calculated and tested for correlation with Thompson scores. The estimate of the conditional probabilities for transitioning from one sleep-wake stage (stage “A”) to another (stage “B”) was computed by dividing the number of occurrences of stage “B” after stage “A” by the total number of transitions from stage “A” to any other stage.
All computations were performed in MATLAB (MathWorks, Natick, MA).
RESULTS
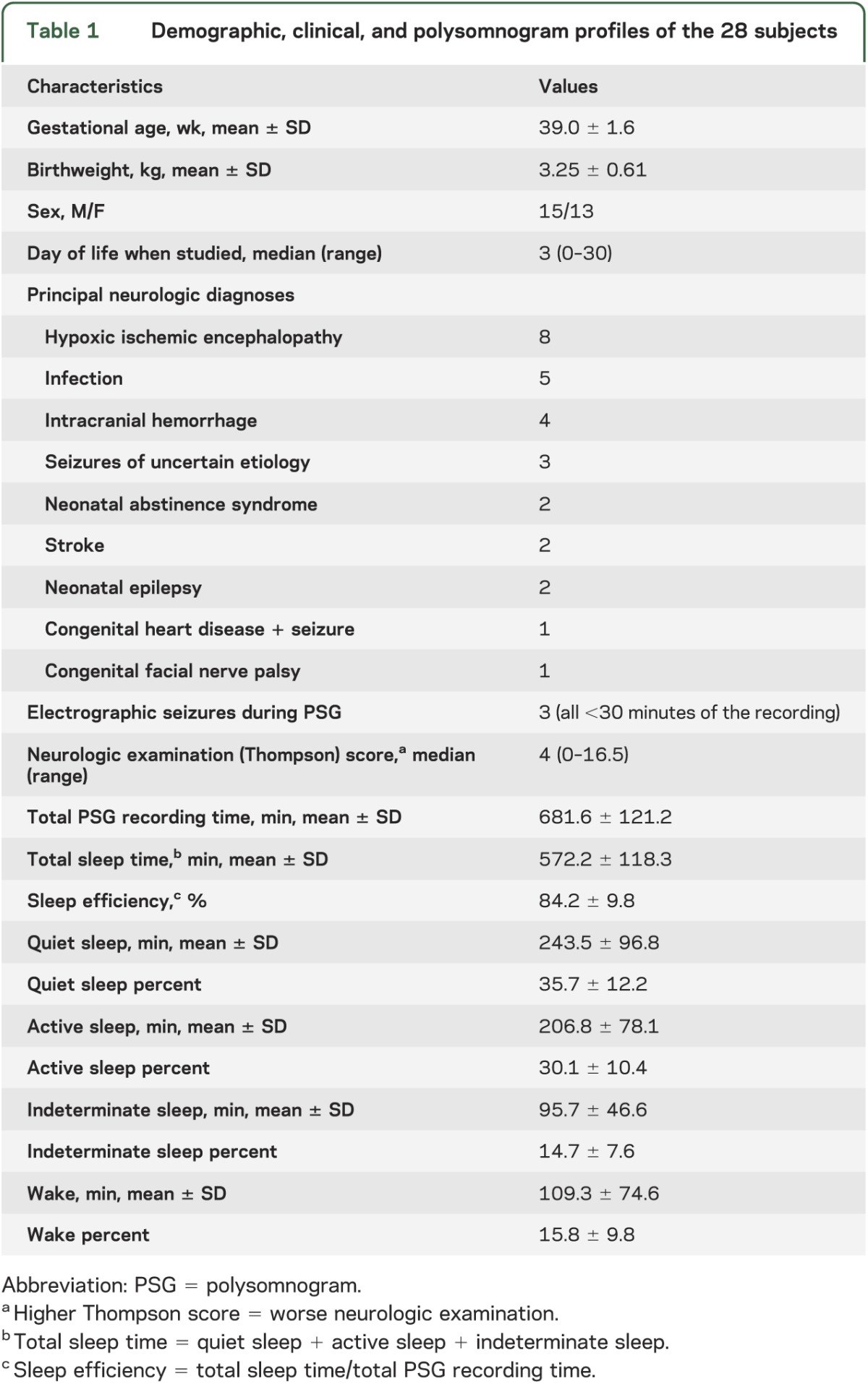
Twenty-eight newborn infants (15 male) were studied. Clinical and demographic characteristics are presented in table 1, as are summary statistics for the PSGs.
Table 1.
Demographic, clinical, and polysomnogram profiles of the 28 subjects
An increased fraction of quiet sleep correlated with worse neurologic examination scores (Spearman rho = 0.54, p = 0.003), but the proportion of active sleep did not (p > 0.1; table 2). An increased proportion of wakefulness showed a trend toward correlation with lower (more normal) neurologic examination scores (rho = −0.34, p = 0.053). The duration of individual sleep-wake state bouts (i.e., how long an infant remained in a particular state before transitioning to a different state) was not associated with examination scores (p > 0.1).
Table 2.
Spearman correlations of quantitative polysomnographic data with neurologic examination (Thompson) scores for 28 neonates
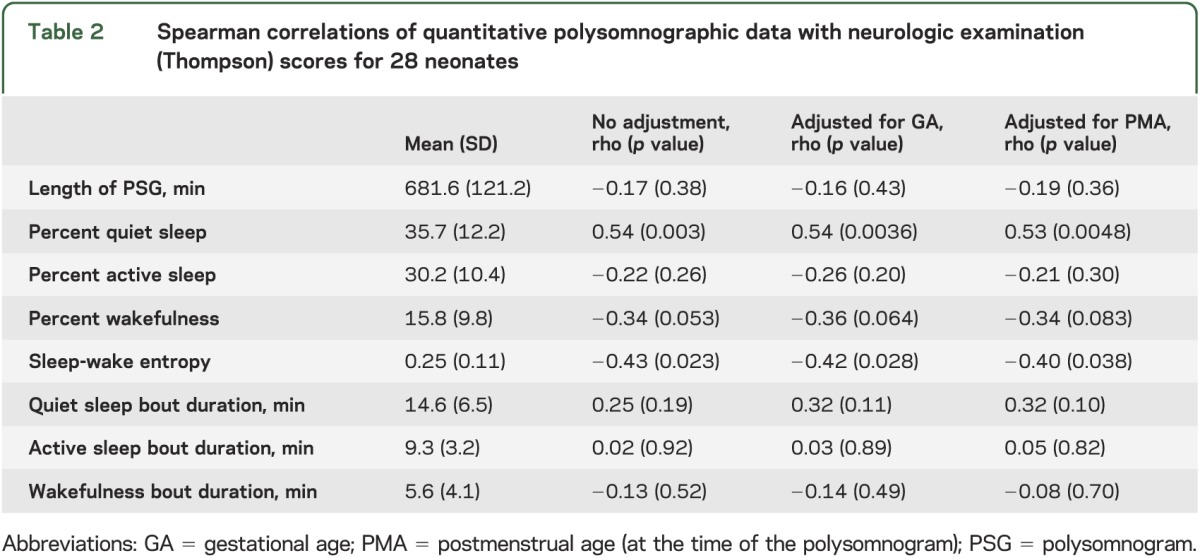
Higher sleep-wake state entropy was associated with lower (better) examination scores (rho = −0.43, p = 0.023), but specific sleep stage entropy (considering active and quiet sleep separately) did not reach significance (rho = −0.25, p= 0.19). Decreased EEG delta power during quiet sleep, but not the EEG power at other frequencies, was also associated with worse examination scores (table 3 and figure 1). These results remained significant after adjusting for gestational age at delivery or for postmenstrual age at the time of the PSG recording.
Table 3.
During quiet sleep, increased EEG delta power was associated with lower (more normal) Thompson scores, even after adjustment for gestational age or postmenstrual age at the time of the PSG

Figure 1. Lower delta power during quiet sleep is associated with worse neurologic examination scores.

On the EEG, lower delta frequency power (0.5–4 Hz) during quiet sleep was associated with worse neurologic examination (Thompson) scores (Spearman correlation p < 0.05, open circles). The EEG power at higher frequencies did not correlate with examination scores (p > 0.05, crosses).
Thirteen infants received phenobarbital for suspected or confirmed neonatal seizures, but only 3 had a seizure during the PSG recording (1 with 2, isolated, brief focal seizures; 1 with intermittent seizures during a 25-minute period; and 1 with a single 6-minute focal seizure). Logistic regression showed that treatment with phenobarbital was not associated with Thompson score (p = 0.71), fraction of quiet sleep (p = 0.18), EEG delta power during quiet sleep (p = 0.48), or sleep-wake state entropy (p = 0.89).
In univariate analyses, Thompson scores were associated with the fraction of quiet sleep (linear regression model r2 = 0.23, p = 0.01), the EEG delta power during quiet sleep (r2 = 0.20, p = 0.018), and sleep-wake state entropy (r2 = 0.19, p = 0.02). There was some colinearity between fraction of quiet sleep and delta power (Spearman rho = 0.64, p = 0.003) and between percent quiet sleep and sleep-wake state entropy (rho = −0.55, p = 0.003). Delta power was not correlated with sleep-wake state entropy (rho = 0.19, p = 0.34). A multiple linear regression model including all 3 of these variables explained more of the variance than did any one predictor alone (r2 = 0.32, p = 0.02).
Conditional stage transition probabilities for the 28 infants' PSGs differed somewhat according to neurologic examination score. Specifically, subjects with more abnormal (high) examination scores were more likely to transition from active sleep to quiet sleep than those with normal (low) examination scores (t test p = 0.02). The Spearman correlation of active to quiet sleep transition probability with Thompson score was significant (rho = 0.5, p = 0.006; figure 2). A bootstrap calculation of this Spearman correlation using 1,000 subsamples yielded rho = 0.5 with standard error = 0.005.
Figure 2. Sleep-wake stage transition probabilities for 28 critically ill neonates.
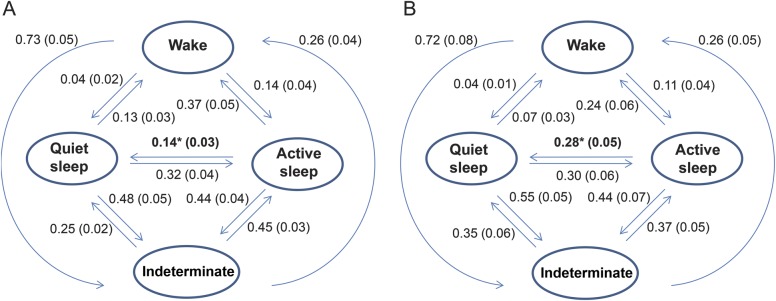
Sleep-wake stage transition probabilities for 28 critically ill neonates monitored with polysomnography. Those with Thompson scores at or below the group median (i.e., <5; n = 15) are represented in panel A, while those with more abnormal scores (higher than the median, i.e., ≥5; n = 13) are displayed in panel B. Numerical values in the figure are mean transition probabilities, with standard error in parentheses. Infants with higher (more abnormal) examination scores in comparison to those with lower (more normal) scores were more likely to transition from active sleep to quiet sleep (indicated with * in the figure; t test p = 0.02 for difference in mean transition probabilities). The Spearman correlation of active to quiet sleep transition probability with Thompson score was rho = 0.5, p = 0.006.
DISCUSSION
This study shows that among late preterm and term neonates with risk factors for seizures, several PSG measures, including the proportion of time spent in quiet sleep, EEG delta power during quiet sleep, sleep-wake state entropy, and the probability of transitioning from active to quiet sleep, are associated with neurologic examination scores. These novel findings combine to suggest a new hypothesis: generation of shorter periods of quiet sleep, with more intense slow wave (delta) EEG activity during those periods, may be markers of healthier neonatal neurologic function. Conversely, infants with compromised neurologic status may exhibit increased “pressure” to generate quiet sleep, as manifested by longer time in this state, lower sleep-wake state entropy, and tendency to terminate active sleep with quiet sleep, rather than wakefulness or indeterminate sleep.
PSG recording in neonates is technically challenging and not often undertaken in the NICU. Typically, clinical PSG interpretation for infants is heavily focused on the identification of sleep-disordered breathing (e.g., central or obstructive sleep apnea). However, the rich data recorded by PSG are amenable to much more refined analyses, as demonstrated here. Such analyses can provide objective and dynamic measures of neonatal brain function.
We demonstrate that the fraction of quiet sleep and the EEG delta power during quiet sleep are interrelated. Other authors have reported differences in EEG delta power among healthy preterm vs term newborns (across several sleep stages)9 and between small for gestational age vs appropriate for gestational age term neonates without overt cerebral dysfunction (without mention of sleep stage).10 Increased fraction of quiet sleep in the first days of life was associated with lower motor development scores at 6 months of age in a study of healthy term newborns.11 Similarly, increased time in quiet sleep has been described among newborns with suspected hypoxic ischemic encephalopathy, compared with healthy controls, prior to the era of treatment with therapeutic hypothermia.12 Here, we expand these observations to sick neonates with a variety of risk factors for cerebral dysfunction, including 8 infants with hypoxic ischemic encephalopathy who were treated with therapeutic hypothermia. We also add sleep-wake state entropy and sleep stage transition probability as objective measures of CNS function, which may distinguish those with cerebral dysfunction from those with normal neurologic status. Others have described additional quantitative analyses of amplitude-integrated EEG, including approximate entropy, that may distinguish between neonates with moderate vs severe hypoxic ischemic encephalopathy, but previous authors did not relate these findings to sleep-wake cycling.13 An advantage of our methodology is that analyzing the variation of the sleep stages, as opposed to the raw EEG, provides a metric from data that are typically available for clinically indicated PSGs (i.e., the sleep stage scoring file). This might enable more rapid clinical translation of our work.
Measures of entropy are appealing for the analysis of sleep-related data, as they are designed to quantify the complexity and predictability of periodic patterns.14,15 We demonstrated that infants with low sleep-wake entropy had worse neurologic examination scores. We speculate that low sleep-wake state entropy reflects an important loss of physiologic variability. Other forms of diminished physiologic variability have been reported as markers of critical illness for neonates. For example, reduced heart rate variability was associated with increased morbidity in preterm infants,16 and decreased systemic regional oxygen saturation variability (measured with near-infrared spectroscopy) was associated with worse short-term outcome in neonates with hypoxic ischemic encephalopathy.17
Among older infants and children, abnormal sleep physiology, identified by symptoms of sleep-disordered breathing, is associated with adverse long-term neurobehavioral outcomes.1,2 In a population-based study, symptoms of sleep-disordered breathing in infancy, even after subsequent resolution for several years, was associated with a 40% increased odds of behavioral morbidity (especially hyperactivity) at ages 4 and 7 years.2 In multivariate analyses, the impact of sleep-disordered breathing was greater than several traditionally recognized social risk factors for adverse behavioral outcomes, including mother's education, maternal smoking, and paternal employment. These data suggest that for older infants and children, abnormal sleep is a biomarker of cerebral dysfunction and, equally important, may be a contributing factor to suboptimal neurodevelopmental outcomes. For newborns, at a critical stage of brain development, sleep physiology may provide similarly robust biomarkers of neurologic function. Given the neonatal brain's exquisite vulnerability to injury, detrimental effects of sleep disruption could be even more potent.
A study of low-risk preterm infants, without significant intraventricular hemorrhage or other relevant comorbidities, corroborates this hypothesis. Based on 4 hours of behavioral observation of sleep-wake stages, without formal PSG, infants with abnormal sleep regulation, compared to infants with mature behavioral state organization, subsequently scored lower on early childhood measures of cognitive, verbal, and executive functioning.18 These findings were independent from birthweight, illness severity, and gestational age. Combined with our data, these observations suggest that quantitative assessment of neonatal sleep dysregulation could provide worthwhile, objective measures of risk for adverse neurodevelopmental outcomes. Well-powered studies with long-term follow-up are needed, to determine whether neonatal sleep dysregulation is an indicator of concurrent cerebral dysfunction (poor sleep generated by an already abnormal brain), a mediator of long-term brain development (poor sleep contributing directly to aberrant development), or a more complex combination of these 2 scenarios.
Our study has some limitations. This research involved intensive evaluation of each critically ill infant, and therefore necessarily focused on a somewhat limited sample size, as have previous studies on this patient population.19,20 Recording formal PSGs in normal healthy control infants would be instructive, as factors that affect sleep-wake cycling may differ between sick and healthy infants. However, practical barriers to recruitment of healthy newborns for 8 to 12 hours of intensive monitoring are significant. Furthermore, characterization of sleep regulation in critically ill neonates provides data directly relevant to this important patient group. Longitudinal follow-up of these infants is ongoing, to validate quantitative neonatal sleep measures as mediators of long-term neurodevelopmental outcomes. Whether quantitative sleep state analyses will provide clinically useful prognostic markers that provide value beyond the clinical examination, neuroimaging, and EEG findings for at-risk neonates remains to be determined. Analyses of PSG, such as the approach we describe here, potentially could provide quantitative measures of efficacy for neurotherapeutic interventions. For example, EEG-sleep complexity appears to be improved by a skin-to-skin (“kangaroo care”) intervention for preterm infants.21
Strengths of this study include the use of full, attended PSG performed at the NICU bedside of 28 critically ill neonates. Standardized PSG scoring by a single experienced technologist and review by a board-certified sleep medicine physician was strengthened by blinding these individuals to the neurologic status of the subjects. A unique aspect of our work is the focus on quantitative PSG measures that could be replicated at other sites with a level of reliability that exceeds what can be achieved by human visual scoring.
The use of real-time parameters recorded at the NICU bedside, such as sleep-wake cycling data, as opposed to static measures (e.g., MRI of the brain), offers a potential advantage, both clinically and scientifically. Quantitative PSG analyses may also add useful objective information to the traditional neurologic assessment of critically ill neonates. In this initial sample of neonates at risk for brain dysfunction, an increased proportion of quiet sleep, diminished EEG delta power during quiet sleep, and decreased sleep-wake entropy were associated with more abnormal neurologic examination scores, while sleep stage transition probabilities differed between those with nearly normal examination scores and those with more abnormal scores. A greater understanding of the sources of subtle variations in sleep-wake cycling, and their potential neurodevelopmental implications, could provide the rationale for testing novel interventions designed to optimize neonatal sleep regulation, with a goal of ultimately improving neurodevelopmental outcome.
GLOSSARY
- FFT
fast Fourier transform
- NICU
neonatal intensive care unit
- PSG
polysomnography
AUTHOR CONTRIBUTIONS
Dr. Shellhaas designed the study, interpreted the data, and drafted the manuscript. Dr. Burns analyzed the data, assisted in writing the manuscript, and revised the manuscript. Dr. Barks assisted in the study design and data interpretation and revised the manuscript. Dr. Chervin assisted in the study design and data interpretation and revised the manuscript.
STUDY FUNDING
Supported by the Child Neurology Foundation's Shields Fellowship Award, the University of Michigan's Janette Ferrantino Investigator Award, NICHD (5K23HD068402), and NHLBI (HL105999).
DISCLOSURE
R. Shellhaas receives research funding from the NIH, the Child Neurology Foundation, and intramural grants from the University of Michigan's Department of Pediatrics and Communicable Diseases. She serves on the editorial boards of Pediatric Neurology and Journal of Child Neurology. J. Burns receives research funding from the NIH via subcontract from the University of Michigan. He is named in patents owned by the University of Michigan and Michigan Technological University for data processing algorithms to assess patients for sleep disorders. J. Barks receives research funding from the NIH. R. Chervin has conducted research funded by the NIH and the Fox Foundation; was involved with unrestricted educational gifts to the University of Michigan from Philips Respironics and Fisher Paykel; serves on boards of directors for the American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, Association of Professional Sleep Societies, and International Pediatric Sleep Association; has been a member of advisory boards for the nonprofit Sweet Dreamzzz and the NHLBI; and serves as a section editor for UpToDate, Inc., and a book editor for Cambridge University Press. He is named in patents, or patents pending, owned by the University of Michigan, for devices and drugs designed to assess and treat patients for sleep disorders. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Piteo AM, Kennedy JD, Roberts RM, et al. Snoring and cognitive development in infancy. Sleep Med 2011;12:981–987 [DOI] [PubMed] [Google Scholar]
- 2.Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics 2012;129:e857–e865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shellhaas RA, Chang T, Tsuchida T, et al. The American clinical neurophysiology society's guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 2011;28:611–617 [DOI] [PubMed] [Google Scholar]
- 4.Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 1997;86:757–761 [DOI] [PubMed] [Google Scholar]
- 5.Anders T, Emde R, Parmalee A. A Manual of Standardized Terminology, Techniques and Criteria for the Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles: UCLA Brain Information Services; 1971 [Google Scholar]
- 6.Kirsch MR, Monahan K, Weng J, Redline S, Loparo KA. Entropy-based measures for quantifying sleep-stage transition dynamics: relationship to sleep fragmentation and daytime sleepiness. IEEE Trans Biomed Eng 2012;59:787–796 [DOI] [PubMed] [Google Scholar]
- 7.Walsh JL. A closed set of normal orthogonal functions. Am J Mathematics 1923;45:5–24 [Google Scholar]
- 8.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoustics 1967;AU-15:70–73 [Google Scholar]
- 9.Scher MS, Sun M, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Comparisons of EEG sleep state-specific spectral values between healthy full-term and preterm infants at comparable postconceptional ages. Sleep 1994;17:47–51 [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir OMA, Ergin H, Sahiner T. Electrophysiological assessment of the brain function in term SGA infants. Brain Res 2009;1270:33–38 [DOI] [PubMed] [Google Scholar]
- 11.Freudigman KA, Thoman EB. Infant sleep during the first postnatal day: an opportunity for assessment of vulnerability. Pediatrics 1993;92:373–379 [PubMed] [Google Scholar]
- 12.Scher MS, Steppe DA, Beggarly ME, Salerno DG, Banks DL. Neonatal EEG-sleep disruption mimicking hypoxic-ischemic encephalopathy after intrapartum asphyxia. Sleep Med 2002;3:411–415 [DOI] [PubMed] [Google Scholar]
- 13.Burnsed J, Quigg M, Zanelli S, Goodkin HP. Clinical severity, rather than body temperature, during the rewarming phase of therapeutic hypothermia affect quantitative EEG in neonates with hypoxic ischemic encephalopathy. J Clin Neurophysiol 2011;28:10–14 [DOI] [PubMed] [Google Scholar]
- 14.Jamasebi R, Redline S, Patel SR, Loparo KA. Entropy-based measures of EEG arousals as biomarkers for sleep dynamics: applications to hypertension. Sleep 2008;31:935–943 [PMC free article] [PubMed] [Google Scholar]
- 15.Stoffer DA, Scher MS, Richardson GA, Day NL, Coble PA. A Walsh-Fourier analysis of the effects of moderate maternal alcohol consumption on neonatal sleep-state cycling. J Am Stat Assoc 1988;83:954–963 [Google Scholar]
- 16.Saria S, Rajani AK, Gould J, Koller D, Penn AA. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med 2010;2:48ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shellhaas RA, Thelen BJ, Bapuraj JR, et al. Limited short-term prognostic utility of cerebral NIRS during neonatal therapeutic hypothermia. Neurology 2013;81:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisman O, Magori-Cohen R, Louzoun Y, Eidelman AI, Feldman R. Sleep-wake transitions in premature neonates predict early development. Pediatrics 2011;128:706–714 [DOI] [PubMed] [Google Scholar]
- 19.Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics 2011;128:910–916 [DOI] [PubMed] [Google Scholar]
- 20.Kuhn P, Zores C, Pebayle T, et al. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ratio threshold of 5 to 10 dBA. Pediatr Res 2012;71:386–392 [DOI] [PubMed] [Google Scholar]
- 21.Kaffashi F, Scher MS, Ludington-Hoe SM, Loparo KA. An analysis of the kangaroo care intervention using neonatal EEG complexity: a preliminary study. Clin Neurophysiol 2013;124:238–246 [DOI] [PubMed] [Google Scholar]


