Abstract
Objective:
To define the clinical spectrum and etiology of progressive myoclonic epilepsies (PMEs) in Italy using a database developed by the Genetics Commission of the Italian League against Epilepsy.
Methods:
We collected clinical and laboratory data from patients referred to 25 Italian epilepsy centers regardless of whether a positive causative factor was identified. PMEs of undetermined origins were grouped using 2-step cluster analysis.
Results:
We collected clinical data from 204 patients, including 77 with a diagnosis of Unverricht-Lundborg disease and 37 with a diagnosis of Lafora body disease; 31 patients had PMEs due to rarer genetic causes, mainly neuronal ceroid lipofuscinoses. Two more patients had celiac disease. Despite extensive investigation, we found no definitive etiology for 57 patients. Cluster analysis indicated that these patients could be grouped into 2 clusters defined by age at disease onset, age at myoclonus onset, previous psychomotor delay, seizure characteristics, photosensitivity, associated signs other than those included in the cardinal definition of PME, and pathologic MRI findings.
Conclusions:
Information concerning the distribution of different genetic causes of PMEs may provide a framework for an updated diagnostic workup. Phenotypes of the patients with PME of undetermined cause varied widely. The presence of separate clusters suggests that novel forms of PME are yet to be clinically and genetically characterized.
Progressive myoclonic epilepsies (PMEs) include phenotypes arising from various causes but all leading to myoclonic jerks (i.e., cortical reflex myoclonus) commonly associated with seizures and progressive neurologic impairment. Although the most commonly occurring PME phenotypes were identified many years ago and later attributed to specific genetic defects,1 other recently identified phenotypes are associated with novel mutations involving the PRICKLE1,2 SCARB2,3 and GOSR2 genes.4
PMEs usually present in late childhood or adolescence, which distinguishes them from epileptic encephalopathies that start with polymorphic seizures in early infancy,5,6 but adult-onset PMEs may be due to rare gene defects or immune or late degenerative disorders.7,8 As PMEs are rare diseases, little is known about the prevalence of the different forms of the disease. Moreover, despite the advances in molecular medicine, etiology remains undetermined in a substantial proportion of patients.
The aim of this study was to analyze the clinical, genetic, and laboratory characteristics of patients with PME from 25 epilepsy centers in Italy in order to define the distribution of the various types of PMEs with identified or undetermined causes. The Genetics Commission of the Italian League against Epilepsy (LICE) supported data collection.
METHODS
Data collection.
The study was proposed by the LICE Genetic Commission and started in January 2009.
Inclusion criteria.
Patients observed over the last 15 years (January 1998–December 2012) with worsening cortical reflex/action myoclonus involving multiple segments and followed up for at least 3 years were included in this study. Patients were included even in the absence of seizures other than myoclonic, and in the case of absence of ataxia or with minimal ataxia and mental decline.
Exclusion criteria.
Patients presenting with early-onset epileptic encephalopathies, focal or unilateral cortical myoclonus, or myoclonus of subcortical origin were excluded from this study.
Standard protocol approvals, registrations, and patient consents.
The Commission for Rare Diseases of the Lombardy region of Italy approved diagnostic procedures included in the protocol “Path Diagnostic Therapeutic Care.”
All patients or their parents/guardians signed an informed consent for all diagnostic procedures, participation in the study, and for the scientific use of their health record data. In addition, they signed separate consent forms for neuroradiologic examination and for genetic research on stored tissue samples.
The collected information included the following:
Geographic origin of the family, relevant family history, age at the time of the first neurologic sign, age at death or last observation, specific etiology if known.
Seizure scores based on the following categorization: no seizures other than myoclonic jerks = 0; one or a few tonic-clonic (TC) seizures at disease onset = 1; TC seizures throughout the course of the disease = 2; multiple seizure types (usually absences or tonic seizures) = 3.
Pathologic signs in addition to the cardinal PME phenotype5 (e.g., ocular or extrapyramidal signs, dysmorphisms) at disease onset or appearing during the disease course, and cognitive evaluation results (none = 0, mild = 1, moderate = 2, severe impairment = 3).
Age at the time of the onset of cortical myoclonus and myoclonus severity at the last observation (scored 1–5).9
Interictal/ictal EEG polyspikes and waves (PSW), central fast activity, and sensitivity to intermittent photic stimulation (IPS), neuroradiologic findings, positive biomarkers, and biochemical and morphologic findings supporting the diagnosis.
Diagnoses.
Diagnosis of Unverricht-Lundborg (EPM1) disease was based on evidence of homozygous or compound heterozygous CSTB gene mutations, whereas Lafora body (EPM2) disease diagnosis was based on evidence of mutations in the EPM2A or EPM2B (NHLRC1) gene or a skin biopsy positive for Lafora bodies. Diagnosis of neuronal ceroid lipofuscinosis (NCL) was based on morphologic findings of lipopigment deposits (granular osmiophilic, curvilinear, or fingerprints) in a skin/mucosa/cerebral biopsy. In most patients with NCL, we confirmed genetic mutations in one of the known NCL genes. Sialidoses were diagnosed on the basis of biochemical findings of defective neuraminidase activity in fibroblasts, while mitochondrial encephalopathies were confirmed on the basis of morphologic findings (ragged-red fibers in a muscle biopsy), mutations in mitochondrial genes, or defective respiratory chain complexes. Action myoclonus renal failure was diagnosed upon confirmation of SCARB2 gene mutations. Other diseases associated with a PME phenotype were diagnosed on the basis of specific biomolecular findings or lysosomal defects in lymphocytes or fibroblasts.
Patients classified as having “undetermined” PMEs were those who fulfilled the inclusion criteria, and for whom the extensive diagnostic workup failed to identify any underlying cause for PMEs.
We conducted statistical analysis using the Statistical Package for the Social Sciences (SPSS, version 17.0). This included 2-step cluster analysis of data from patients with undetermined PMEs based on categorical variables (positive family history, seizure characteristics, psychomotor delay preceding myoclonus onset, EEG paroxysms of PSW, photosensitivity, pathologic MRI findings, and associated signs other than those included in the cardinal definition of PME) and the continuous variables such as age at the time of disease and myoclonus onset. Assessment of differences between clusters included one-way analysis of variance for continuous data and χ2 test for categorical data.
RESULTS
As of December 2012, the database included 204 patients examined by 57 neurologists or pediatric neurologists working in 25 university or public hospital epilepsy centers and followed until the mean age of 33.3 ± 13.3 years; 26 patients had died (at a mean age of 27.7 ± 11.5 years) during the course of the follow-up.
Seventy-seven patients (37.7%) had EPM1 and CSTB gene mutations. The phenotype resulting from the homozygous expansion substantially overlapped the previously described phenotype,10,11 and consisted of prominent action-stimulus myoclonus and rare generalized seizures. The mean age at disease onset for patients with these mutations was 11.4 ± 3.2 years, whereas myoclonus appeared at a mean age of 13.5 ± 3.1 years (figure 1). Eight patients from 6 families with EPM1 due to compound heterozygosis with point or indel mutations on one allele had particular characteristics that included an earlier age at disease onset (7.8 ± 1.5 years), multiple seizure types, and cognitive impairment.12
Figure 1. Age at disease and myoclonus onset in patients with genetically identified progressive myoclonic epilepsies.

Gray segments of columns refer to the period before disease onset, yellow segments to the presentation of neurologic signs, and red segments to the presentation of myoclonus. The end of the columns defines the patients' ages at the time of the last observation. Columns representing EPM1 and EPM2 are based on mean values, whereas the other columns refer to individual patients. HD = Huntington disease; MERRF = myoclonic epilepsy with ragged-red fibers; NCL = neuronal ceroid lipofuscinosis; NPC = Niemann-Pick disease type C; SMA = spinal muscular atrophy.
Thirty-seven patients (18.1%) had EPM2 diagnosed in the presence of mutations in the NHLRC1 (n = 26, 70.3%) or EPM2A gene (n = 7; 18.9%) or positive skin biopsy (n = 4; 10.8%). The phenotype corresponded to previous descriptions.13,14 The mean age at disease onset was 12.6 ± 3.2 years, and the mean age at myoclonus onset was 15.1 ± 3.2 years. The course of the disease was severe in most cases, and 16 patients died 10.1 ± 5.7 years after disease onset; however, some patients had milder phenotypes with a protracted course lasting up to 24 years (figure 1).
Most EPM1 and EPM2 patients came from southern Italy and very few from central Italy; a cluster of EPM1 families came from northwestern Italy.
Thirty-one patients (15.2%) had PMEs due to a variety of genetic disorders, each affecting just a few patients: this included NCL in 12 cases, myoclonic epilepsy with ragged-red fibers (MERRF) in 5, PME with or without renal failure due to mutations in the SCARB2 gene in 4, type I or II sialidosis in 3, Gaucher disease in 2, and type C Niemann-Pick disease in 1. Three patients had a PME phenotype due to juvenile-onset Huntington disease, and one as a peculiar phenotype of spinal muscular atrophy. Two more patients had a PME phenotype associated with celiac disease. Some of the patients or small subgroups have been previously described in detail.15–21
Figure 1 shows ages at disease and myoclonus onset and the duration of follow-up. The age at disease onset of the 5 patients with mitochondrial disorders varied widely from 7 to 50 years. They also had signs other than the cardinal PME symptoms, including hypoacusia (n = 3), loss of visual acuity (n = 1), myopathy (n = 1), and neuropathy (n = 1). Four had the classical MERRF mutation and showed typical maternally inherited transmission; the fifth had a respiratory complex III defect, but no identified genetic abnormalities.
The 12 patients with PME due to NCL (the largest group of patients with genetically determined PME after EPM1 and EPM2) included 8 patients with childhood or adolescent onset (6.8 ± 3.1 years) and a long delay before the appearance of typical cortical myoclonus, although myoclonus was actually the first sign in the 4 patients with an adult onset (32.8 ± 19.1 years). Skin (n = 10), rectal (n = 1), or brain (n = 1) biopsy always revealed typical lipofuscin inclusions in the different cell types. Two patients with infantile onset had retinopathy and most showed severe mental decline. Five patients (including the 4 with adult onset) had mutations in the CLN6 gene, and 2 had CLN5 or CLN2 mutations; the remaining 5 patients did not have any mutation in any of the investigated genes.
Patients with undetermined PMEs.
The age at disease onset for the 57 patients in whom the causative factor remained unidentified varied widely from 1 to 38 years. In 19 patients (33.3%), the appearance of cortical myoclonus marked the onset of the neurologic disorder alone (n = 8) or with cerebellar signs (n = 3) or seizures (n = 8); in the remaining 38, evidence of cortical myoclonus appeared years after cerebellar signs (n = 3), TC seizures (n = 18), or cognitive dysfunction as seen in psychomotor delay (n = 17) (table e-1 on the Neurology® Web site at www.neurology.org).
Six patients had associated non-neurologic signs, including mildly dysmorphic features, livedo reticularis, cataracts, or femoral head necrosis in 2 siblings. Eleven patients showed neurologic signs not included in the cardinal PME phenotype5,6: impaired visual acuity (n = 2, associated with retinopathy in 1), mild extrapyramidal signs (n = 6, occurring throughout the disease course), and axonal neuropathy (n = 3). Most patients (77.2%) underwent a skin biopsy, which showed unclassified stored material in 7 cases; 70.2% underwent a muscle biopsy, which showed mild morphologic changes insufficient to support definite myopathy in 2 cases and nonspecific lipid accumulation in 2.
MRI revealed mild atrophic changes involving the cerebral hemispheres in 1 case, the cerebellum in 11 cases, both the cerebellum and cerebral hemisphere in 12 cases, and nonspecific white matter T2 hyperintensities in 3 cases.
Diagnostic procedures aimed at detecting mitochondrial disorders by means of muscle biopsy and mtDNA evaluations were used in 84.2% of cases, probably because of the known variability of these disorders, but all were negative.
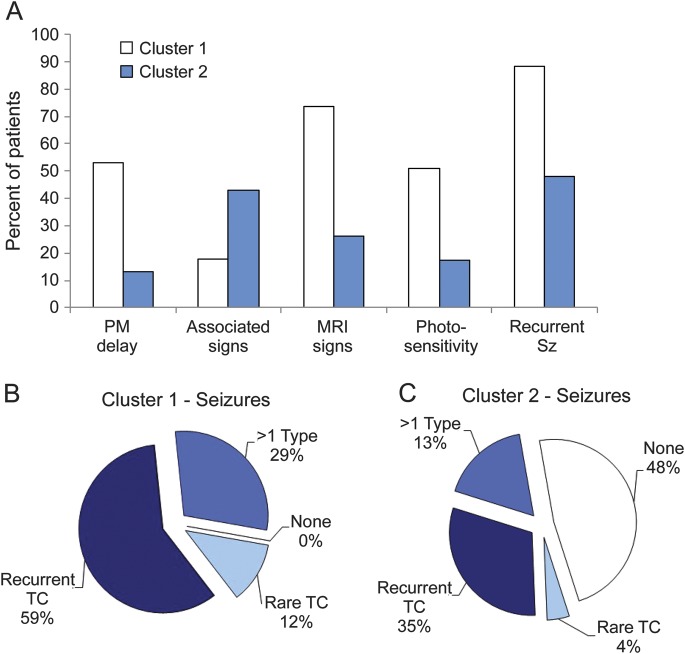
Two-step cluster analysis showed that patients with PME of undetermined cause fell into 2 clusters: one of 34 subjects (59.6%, cluster 1) and the other of 23 subjects (40.4%, cluster 2). These clusters were different in terms of age at disease and myoclonus onset (figure 2). Patients in cluster 1 (figure 2, A and B) had earlier onset of both disease (8.0 ± 6.9 years vs 18.7 ± 10.7 years, F = 20.9, p < 0.001) and myoclonus (13.7 ± 6.7 years vs 23.3 ± 9.1; F = 20.1, p < 0.001), while patients in cluster 2 (figure 2, C and D) often had adult onset.
Figure 2. Age at disease and myoclonus onset in patients with undetermined progressive myoclonic epilepsies belonging to clusters 1 and 2.
Note that most of the patients in cluster 1 experienced early onset of disease and myoclonus (A, B), whereas those in cluster 2 experienced more variable but predominantly late onset (C, D) of neurologic symptoms and myoclonus.
Figure 3A highlights the fact that clinical characteristics differ between the clusters: the presence of psychomotor delay (χ2 = 9.4, p = 0.002), EEG photosensitivity (χ2 = 15.5, p < 0.001), associated signs (χ2 = 5.9, p = 0.01), and pathologic brain MRI findings (χ2 = 12.4, p < 0.001). Among patients in cluster 1, 12 (35.3%) had cerebellar atrophy, 9 (26.4%) had cerebral and cerebellar atrophy, 4 (11.7%) had cerebral atrophy or white matter changes. Among patients in cluster 2, 3 (13.4%) had cerebellar atrophy, 2 cerebral and cerebellar atrophy (13.4%), and 1 had white matter changes.
Figure 3. Characteristics distinguishing patients with undetermined progressive myoclonic epilepsies.
Cluster 1 included more patients in whom psychomotor (PM) delay preceded the appearance of cortical myoclonus, EEG paroxysms evoked by intermittent photic stimulation, and atrophic changes in the cerebral cortex or cerebellum, whereas cluster 2 included a larger number of patients with associated signs exceeding the typical progressive myoclonic epilepsies presentation (A). Recurrent and polymorphic seizures were more common in cluster 1 (B), whereas almost half of the patients in cluster 2 had no seizures (other than myoclonic) (C). Sz = seizures; TC = tonic-clonic.
All patients in cluster 1 had seizures that included other than myoclonic seizures, which recurred during the follow-up period in 88% of the cluster, despite rational treatments; seizures were polymorphic (including TC and absence seizures with or without myoclonic or atonic components) in 29.4% of those patients (figure 3B). Among patients in cluster 2, only 47% (figure 3C) had seizures other than myoclonic (χ2 = 20.3; p = 0.001).
The duration of follow-up in patients with undermined PMEs was on average 18.8 years (±10.8 years). At the time of last observation, the myoclonus score was higher in cluster 1 patients (3.8 ± 1.1 vs 2.9 ± 1.2; Student t test p = 0.003). There was no difference in cognitive level at the time of the last observation; cognitive impairment was recorded in 76.6% of the patients in cluster 1 and 56.2% of those in cluster 2.
DISCUSSION
EPM1 was the most frequently occurring form of PME in our series, accounting for more than one-third of the patients. Most came from southern Italy, but there was also a cluster from northwestern Italy, which may indicate a separate wave of migration from northern Europe. However, even in the southern Italian population (more than 15 million inhabitants), the prevalence of EPM1 was lower than that observed in Finland, where there are about 200 EPM1 patients among a resident population of slightly more than 5 million inhabitants.10
In patients with EPM2 (the second most frequently occurring form of PME in our series), NHLRC1 mutations were more prevalent than other mutations (found in 70% of the subjects), similar to previous reports.22 Follow-up showed that a few patients had a relatively long life expectancy despite the typically severe prognosis of EPM2.
PMEs due to other identified causes were rarer and accounted for 15.2% of the study population as a whole. The next most frequently occurring cause was NCL (5.9% of the series); most NCL patients carried CLN6 mutations, which have recently been described as a major cause of the PME phenotype associated with NCL in adults and teenagers23,24; true PME phenotypes were rarely associated with other mutations in this patient series, and, if they were, the patients always presented with other symptoms at disease onset and only later presented with a PME phenotype. Other identified cause of PMEs were SCARB2 mutations, which caused PME with or without renal failure.3,18
A large proportion of our patients had PMEs of undetermined cause (27.9%), including heterogeneous disorders characterized by variable clinical, neurophysiologic, and neuroimaging findings. We found during cluster analysis that cluster 1 consisted mainly of patients with an earlier onset of disease and myoclonus, psychomotor delay before PME presentation, more severe epilepsy, PSW elicited by IPS, and more common MRI findings of cerebral or cerebellar atrophy. Furthermore, comparison of findings at the time of the last observation showed that cluster 1 included subjects with more severe myoclonus. Cluster 2 mainly included patients with a later onset and signs other than those of the cardinal definition of a PME phenotype. The type of associated signs and the pattern of the brain atrophy, as revealed by MRI (e.g., prominent cerebral atrophy vs cerebellar atrophy), were not different in the 2 clusters.
Taken together, these findings suggest that the PME phenotype of patients in cluster 1 represents an unusual evolution of an early neurodegenerative disorder with “age-related” appearance of cortical myoclonus, which more often appears in late childhood and adolescence, in the same age range as the myoclonic presentation of more frequent PME phenotypes.5,6
The patients in cluster 2 more often presented with severe myoclonus at onset, whereas epilepsy was less severe. A frequent positive family history in this group also supports the possibility that PME is a recessive trait in these patients, perhaps including a myoclonic variant of progressive cerebellar disease previously observed in a few families and individual subjects,25–27 or a late phenotype of yet unknown genetic disorders.
Specific molecular tests for rare or recently described PMEs, such as SCARB2, PRICKLE1, or GOSR2, were undertaken on selected cases based on clinical phenotype. In some subjects, the skin or muscle biopsy revealed abnormal but nonspecific features suggesting that unidentified glycogen or lipid storage diseases may be the cause. Although most patients underwent an extensive series of genetic, biochemical, and morphologic examinations, we cannot exclude the possibility that some patients were inadequately investigated for rare or nonobvious diagnoses.
We aimed to identify important components of a “rational” diagnostic workup in patients with PMEs. Our findings indicate that in patients with early and typical PME presentations, CSTB and EPM2A and EPM2B gene investigations should be a priority.10 However, the occurrence of severe illness in patients with variants of EPM112,28 suggests molecular analysis of CSTB gene studies should also be conducted in patients with early onset and polymorphic seizures. Similarly, the SCARB2 gene should be investigated in patients with phenotypic features resembling EPM1 but showing severe cortical myoclonus and preserved cognitive function.18
MERRF syndrome is relatively rare in our series, despite implementation of a diagnostic workflow specifically investigating mitochondrial dysfunction.19 This finding suggests that a strict PME phenotype accounts for a minority of patients with mitochondrial disorders. Four of 5 patients with MERRF had a maternal inheritance, as previously described in patients with MERFF29; given the extreme variability of this disorder in terms of associated signs and age at onset, the families of such patients should also be carefully screened. The polymerase gamma gene (POLG), which is reported to be occasionally associated with a MERRF-like phenotype,27,29 was investigated in several patients but the results were also negative.
The patients with NCL had the most polymorphic clinical pictures, ranging from childhood onset with polymorphic seizures and mental impairment (with a delayed onset of cortical myoclonus) to a severe and almost pure myoclonic phenotype in adults. The recent identification of CLN6 mutations in patients who develop Kufs disease at various ages suggests that this genetic test should now be considered an initial diagnostic step before invasive and not always fruitful tissue examination.30 The presence of strong and persistent photosensitivity23,30,31 in all of our patients with CLN6 mutations, regardless of the age at PME onset (adolescent or adult), may indicate utility as a meaningful marker. Nevertheless, because of the genetic and phenotypical heterogeneity of the “PME” forms of NCL, additional CLN genes may be considered in patients with cortical myoclonus.21,31
Genomic analyses represent promising tools to dissect the complex genetics of PMEs of undetermined cause and to efficiently exploit extensive genetic testing in this group of heterogeneous disorders. However, the proper interpretation and use of growing amount of genetic data in a clinical setting will require increasing recognition of these disorders and an accurate phenotypic characterization. The definition of new forms of PME will have an impact on their clinical management by limiting the need for extensive and invasive procedures and providing essential information for appropriate genetic counseling.
Supplementary Material
GLOSSARY
- IPS
intermittent photic stimulation
- LICE
Italian League against Epilepsy
- MERRF
myoclonic epilepsy with ragged-red fibers
- NCL
neuronal ceroid lipofuscinosis
- PME
progressive myoclonic epilepsy
- PSW
polyspikes and waves
- TC
tonic-clonic
Footnotes
Editorial, page 378
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Franceschetti conceived the study, clinically evaluated the patients, discussed the results, and drafted and revised the manuscript. Dr. Michelucci conceived the study, clinically evaluated the patients, and discussed the results. Dr. Canafoglia clinically evaluated the patients, discussed the results, and drafted and revised the manuscript. Dr. Striano clinically evaluated the patients, discussed the results, and revised the manuscript. Dr. Gambardella clinically evaluated the patients, discussed the results, and revised the manuscript. Dr. Magaudda clinically evaluated the patients and discussed the results. Dr. Tinuper clinically evaluated the patients and discussed the results. Dr. La Neve clinically evaluated the patients and discussed the results. Dr. Ferlazzo clinically evaluated the patients and discussed the results. Dr. Gobbi clinically evaluated the patients and discussed the results. Dr. Giallonardo clinically evaluated the patients and discussed the results. Dr. Capovilla clinically evaluated the patients and discussed the results. Dr. Visani carried out the statistical analyses and discussed the results. Dr. Panzica revised the statistical analyses and discussed the results. Dr. Avanzini clinically evaluated the patients and discussed the results. Dr. Tassinari clinically evaluated the patients and discussed the results. Dr. Bianchi clinically evaluated the patients and discussed the results. Dr. Zara genetically analyzed the patients, discussed the results, and revised the manuscript. The Collaborative LICE study group on PMEs clinically investigated the patients and reported the data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.de Siqueira LF. Progressive myoclonic epilepsies: review of clinical, molecular and therapeutic aspects. J Neurol 2010;257:1612–1619 [DOI] [PubMed] [Google Scholar]
- 2.Bassuk AG, Wallace RH, Buhr A, et al. A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet 2008;83:572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibbens LM, Michelucci R, Gambardella A, et al. SCARB2 mutations in progressive myoclonus epilepsy (PME) without renal failure. Ann Neurol 2009;66:532–536 [DOI] [PubMed] [Google Scholar]
- 4.Corbett MA, Schwake M, Bahlo M, et al. A mutation in the Golgi Qb-SNARE gene GOSR2 causes progressive myoclonus epilepsy with early ataxia. Am J Hum Genet 2011;88:657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkovic SF, Andermann F, Carpenter S, Wolfe LS. Progressive myoclonus epilepsies: specific causes and diagnosis. N Engl J Med 1986;315:296–305 [DOI] [PubMed] [Google Scholar]
- 6.Marseille Consensus Group Classification of progressive myoclonus epilepsies and related disorders. Ann Neurol 1990;28:113–116 [DOI] [PubMed] [Google Scholar]
- 7.Bhatia KP, Brown P, Gregory R, et al. Progressive myoclonic ataxia associated with coeliac disease: the myoclonus is of cortical origin, but the pathology is in the cerebellum. Brain 1995;118:1087–1093 [DOI] [PubMed] [Google Scholar]
- 8.Testa D, Ambrosoni E, Franceschetti S, Salmaggi A, Soliveri P, Girotti F. Progressive myoclonic ataxia with intrathecal immune activation in six patients. Neurol Sci 2007;28:199–204 [DOI] [PubMed] [Google Scholar]
- 9.Magaudda A, Ferlazzo E, Nguyen VH, Genton P. Unverricht-Lundborg disease, a condition with self-limited progression: long-term follow-up of 20 patients. Epilepsia 2006;47:860–866 [DOI] [PubMed] [Google Scholar]
- 10.Kälviäinen R, Khyuppenen J, Koskenkorva P, Eriksson K, Vanninen R, Mervaala E. Clinical picture of EPM1-Unverricht-Lundborg disease. Epilepsia 2008;49:549–556 [DOI] [PubMed] [Google Scholar]
- 11.Genton P. Unverricht-Lundborg disease (EPM1). Epilepsia 2010;51(suppl 1):37–39 [DOI] [PubMed] [Google Scholar]
- 12.Canafoglia L, Gennaro E, Capovilla G, et al. Electroclinical presentation and genotype-phenotype relationships in patients with Unverricht-Lundborg disease carrying compound heterozygous CSTB point and indel mutations. Epilepsia 2012;53:2120–2127 [DOI] [PubMed] [Google Scholar]
- 13.Van Heycoptenhamm, De Jager H. Progressive myoclonus epilepsy with Lafora bodies: clinical-pathological features. Epilepsia 1963;4:95–119 [DOI] [PubMed] [Google Scholar]
- 14.Minassian BA. Lafora's disease: towards a clinical, pathologic, and molecular synthesis. Pediatr Neurol 2001;25:21–29 [DOI] [PubMed] [Google Scholar]
- 15.Rossi Sebastiano D, Soliveri P, Panzica F, et al. Cortical myoclonus in childhood and juvenile onset Huntington's disease. Parkinsonism Relat Disord 2012;18:794–797 [DOI] [PubMed] [Google Scholar]
- 16.Canafoglia L, Bugiani M, Uziel G, et al. Rhythmic cortical myoclonus in Niemann-Pick disease type C. Mov Disord 2006;21:1453–1456 [DOI] [PubMed] [Google Scholar]
- 17.Canafoglia L, Franceschetti S, Uziel G, et al. Characterization of severe action myoclonus in sialidoses. Epilepsy Res 2011;94:86–93 [DOI] [PubMed] [Google Scholar]
- 18.Rubboli G, Franceschetti S, Berkovic SF, et al. Clinical and neurophysiologic features of progressive myoclonus epilepsy without renal failure caused by SCARB2 mutations. Epilepsia 2011;52:2356–2363 [DOI] [PubMed] [Google Scholar]
- 19.Canafoglia L, Franceschetti S, Antozzi C, et al. Epileptic phenotypes associated with mitochondrial disorders. Neurology 2001;56:1340–1346 [DOI] [PubMed] [Google Scholar]
- 20.Binelli S, Canafoglia L, Panzica F, Pozzi A, Franceschetti S. Electroencephalographic features in a series of patients with neuronal ceroid lipofuscinoses. Neurol Sci 2000;21(3 suppl):S83–S87 [DOI] [PubMed] [Google Scholar]
- 21.Cannelli N, Nardocci N, Cassandrini D, et al. Revelation of a novel CLN5 mutation in early juvenile neuronal ceroid lipofuscinosis. Neuropediatrics 2007;38:46–49 [DOI] [PubMed] [Google Scholar]
- 22.Franceschetti S, Gambardella A, Canafoglia L, et al. Clinical and genetic findings in 26 Italian patients with Lafora disease. Epilepsia 2006;47:640–643 [DOI] [PubMed] [Google Scholar]
- 23.Arsov T, Smith KR, Damiano J, et al. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet 2011;88:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade DM, Paton T, Turnbull J, Marshall CR, Scherer SW, Minassian BA. Mutation of the CLN6 gene in teenage-onset progressive myoclonus epilepsy. Pediatr Neurol 2012;47:205–208 [DOI] [PubMed] [Google Scholar]
- 25.Coppola G, Criscuolo C, De Michele G, et al. Autosomal recessive progressive myoclonus epilepsy with ataxia and mental retardation. J Neurol 2005;252:897–900 [DOI] [PubMed] [Google Scholar]
- 26.Gribaa M, Salih M, Anheim M, et al. A new form of childhood onset, autosomal recessive spinocerebellar ataxia and epilepsy is localized at 16q21-q23. Brain 2007;130:1921–1928 [DOI] [PubMed] [Google Scholar]
- 27.Synofzik M, Srulijes K, Godau J, Berg D, Schöls L. Characterizing POLG ataxia: clinics, electrophysiology and imaging. Cerebellum 2012;11:1002–1011 [DOI] [PubMed] [Google Scholar]
- 28.Koskenkorva P, Hyppönen J, Aikiä M, et al. Severer phenotype in Unverricht-Lundborg disease (EPM1) patients compound heterozygous for the dodecamer repeat expansion and the c.202C>T mutation in the CSTB gene. Neurodegener Dis 2011;8:515–522 [DOI] [PubMed] [Google Scholar]
- 29.Finsterer J, Zarrouk Mahjoub S. Epilepsy in mitochondrial disorders. Seizure 2012;21:316–321 [DOI] [PubMed] [Google Scholar]
- 30.Berkovic SF, Carpenter S, Andermann F, Andermann E, Wolfe LS. Berkovic Kufs' disease: a critical reappraisal. Brain 1988;111:27–62 [DOI] [PubMed] [Google Scholar]
- 31.Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 2012;79:183–191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.