Abstract
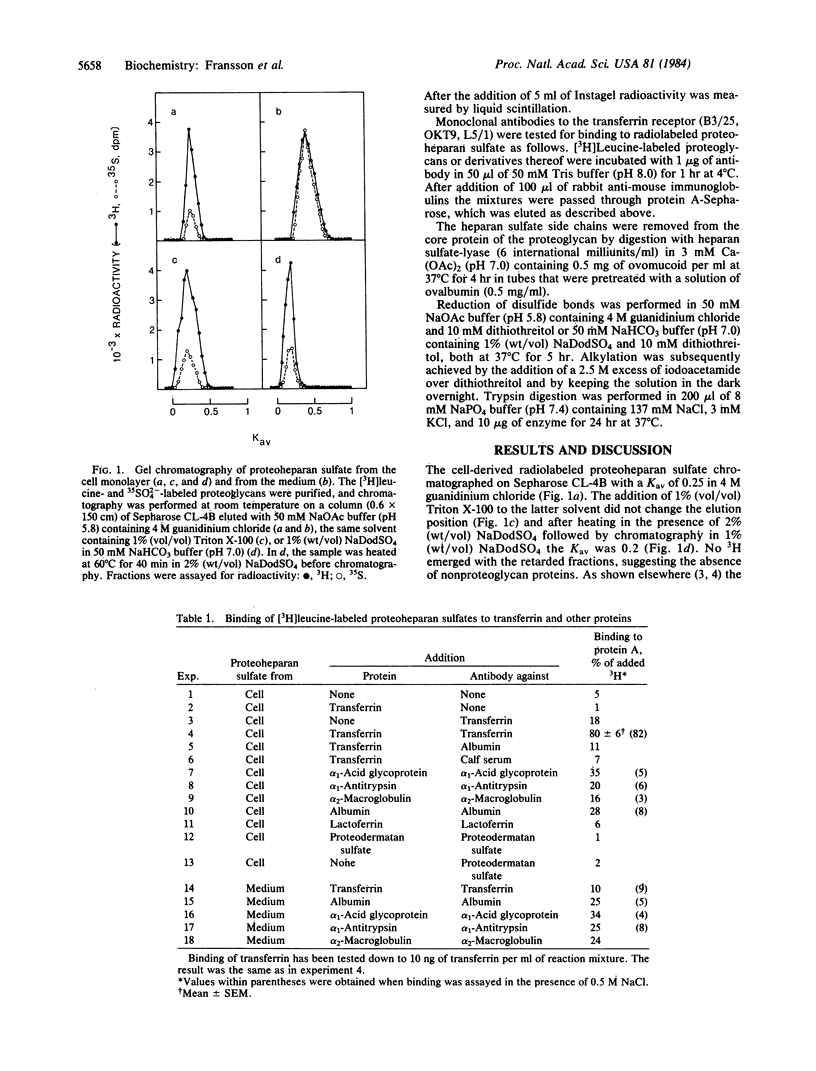
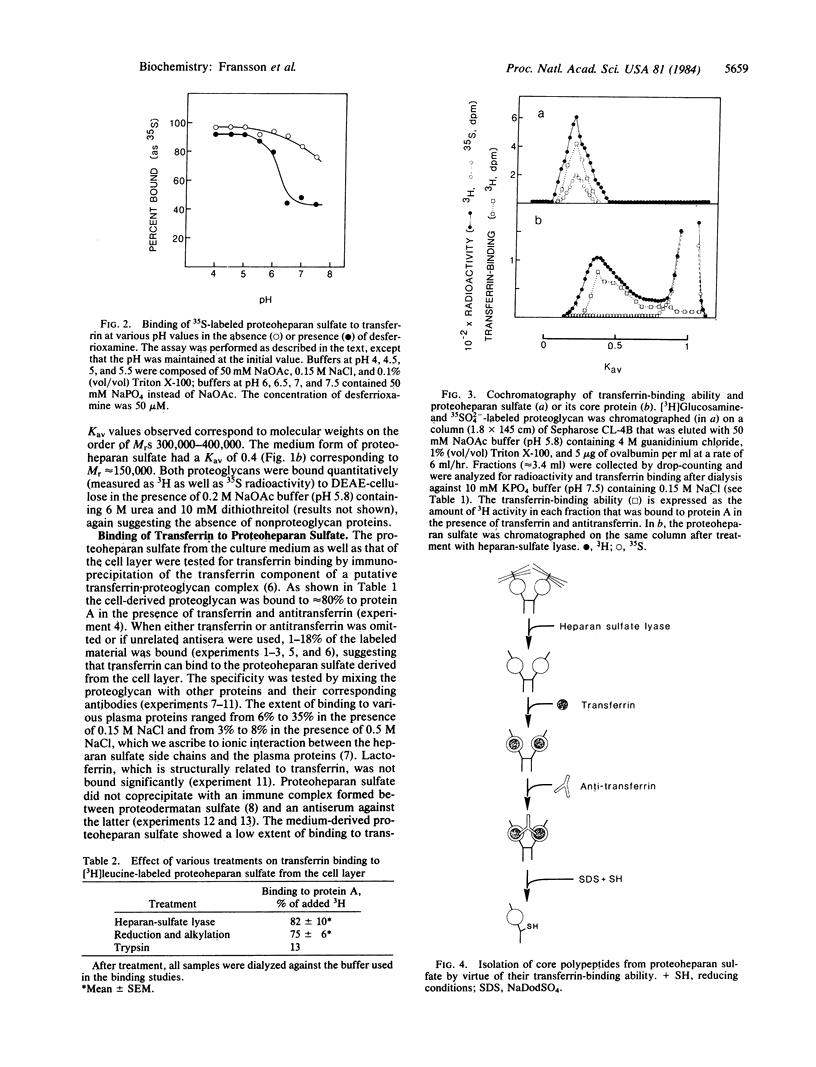
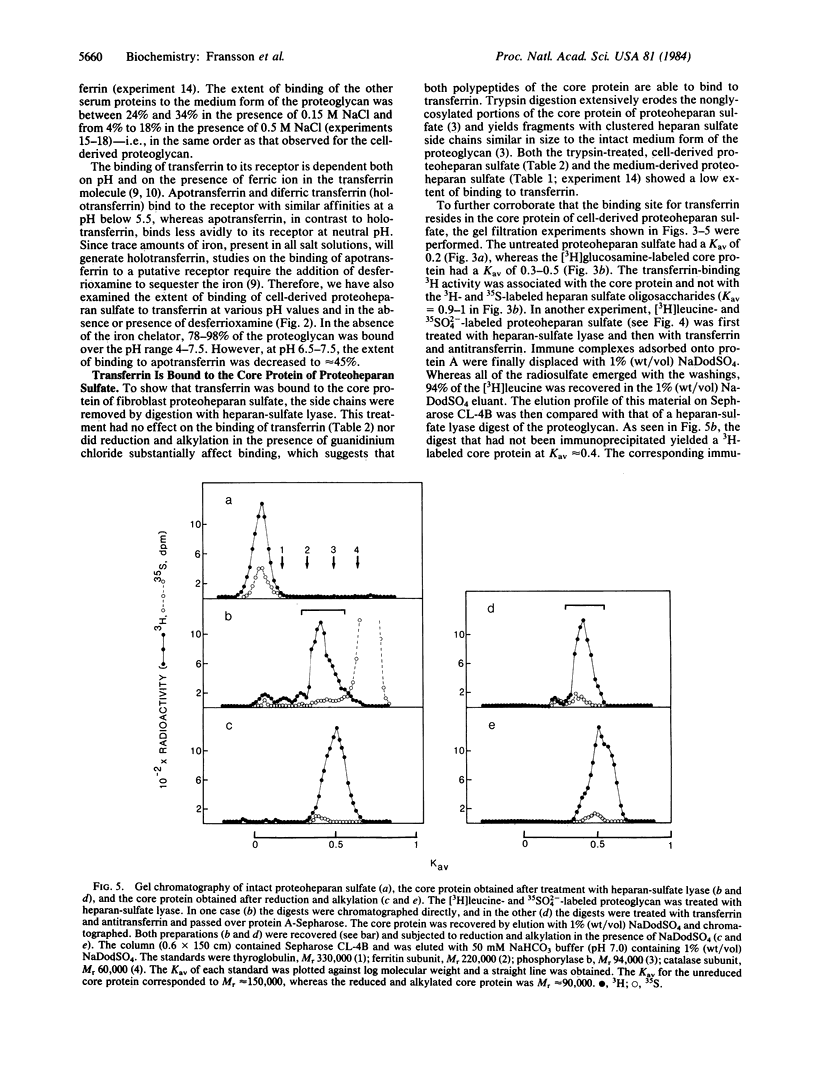
Cell-surface-associated proteoheparan sulfate from confluent human skin fibroblasts appears to consist of two disulfide-bonded polypeptides of Mr approximately equal to 90,000. The transferrin receptor, a ubiquitous cell-surface component of proliferating cells, also consists of two subunits of Mr 90,000 linked by S--S bonds. Radiolabeled proteoheparan sulfate mixed with holotransferrin or apotransferrin at pH 4-5 followed by rabbit anti-human transferrin was adsorbed onto protein A-Sepharose to approximately equal to 80-90%. At pH 7.5 apotransferrin bound approximately equal to 40% of the proteoglycan, whereas approximately equal to 80% was bound to holotransferrin. Trypsin digestion of the proteoglycan markedly lowered its ability to bind transferrin. However, binding was essentially unaffected by heparan-sulfate lyase treatment and after reduction and alkylation. Over 90% of the 3H activity of an L-[3H]leucine-labeled proteoglycan was recovered by immunoprecipitation (transferrin.antitransferrin) of a heparan-sulfate lyase digest of the proteoglycan. The immunoprecipitated core protein had an apparent Mr of 150,000 before reduction and Mr of 90,000 after reduction of disulfide bonds. The core protein of the proteoglycan was recognized by the monoclonal antibody B3/25, which is known to be receptor specific. The present findings suggest that the core polypeptides of proteoheparan sulfate and the transferrin receptor may be identical or closely similar.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlstedt I., Cöster L., Malmström A., Fransson L. A. Proteoheparan sulfate from human skin fibroblasts. Isolation and structural characterization. J Biol Chem. 1983 Oct 10;258(19):11629–11635. [PubMed] [Google Scholar]
- Carlstedt I., Cöster L., Malmström A. Isolation and characterization of dermatan sulphate and heparan sulphate proteoglycans from fibroblast culture. Biochem J. 1981 Jul 1;197(1):217–225. doi: 10.1042/bj1970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Malström A., Carlstedt I., Fransson L. A. The core protein of fibroblast proteoheparan sulphate consists of disulphide-bonded subunits. Biochem J. 1983 Nov 1;215(2):417–419. doi: 10.1042/bj2150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M., Tobey R. A. Cell-cycle dependent desquamation of heparan sulfate from the cell surface. J Cell Biol. 1972 Dec;55(3):713–717. doi: 10.1083/jcb.55.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman D., Trucco M., Bottero L., Lange B., Pessano S., Rovera G. A monoclonal antibody that detects expression of transferrin receptor in human erythroid precursor cells. Blood. 1982 Mar;59(3):671–678. [PubMed] [Google Scholar]
- McKay E. J., Laurell C. B. The interaction of heparin with plasma proteins. Demonstration of different binding sites for antithrombin III complexes and antithrombin III. J Lab Clin Med. 1980 Jan;95(1):69–80. [PubMed] [Google Scholar]
- Oldberg A., Heldin C. H., Wasteson A., Busch C., Hök M. Characterization of a platelet endoglycosidase degrading heparin-like polysaccharides. Biochemistry. 1980 Dec 9;19(25):5755–5762. doi: 10.1021/bi00566a014. [DOI] [PubMed] [Google Scholar]
- Oosta G. M., Favreau L. V., Beeler D. L., Rosenberg R. D. Purification and properties of human platelet heparitinase. J Biol Chem. 1982 Oct 10;257(19):11249–11255. [PubMed] [Google Scholar]
- Schneider C., Asser U., Sutherland D. R., Greaves M. F. In vitro biosynthesis of the human cell surface receptor for transferrin. FEBS Lett. 1983 Jul 25;158(2):259–264. doi: 10.1016/0014-5793(83)80591-2. [DOI] [PubMed] [Google Scholar]
- Schneider C., Kurkinen M., Greaves M. Isolation of cDNA clones for the human transferrin receptor. EMBO J. 1983;2(12):2259–2263. doi: 10.1002/j.1460-2075.1983.tb01732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]


