Abstract
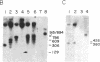
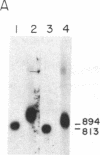
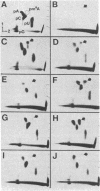
N6-Methyladenosine (m6A) residues, which are found internally in viral and cellular mRNA populations at the sequences Apm6ApC and Gpm6ApC, have been proposed to play a role in mRNA processing and transport. We have developed a sensitive approach to analyze the level and location of m6A in specific purified cellular mRNAs in an attempt to correlate m6A location with function. Polyadenylylated mRNA is hybridized to cDNA clones representing the full size mRNA under study or fragments of it, and the protected RNA is digested and labeled with polynucleotide kinase in vitro. After enrichment for m6A with anti-m6A antibody, the [32P]-pm6A is separated on TLC plates, and compared with the total amount of radiolabeled nucleotides. Using this combination of in vitro RNA labeling and antibody selection, we were able to detect m6A in purified stable mRNAs that cannot be readily labeled in cells with greater sensitivity than was possible by previous techniques. We applied this technique to bovine prolactin mRNA and showed that this mRNA contains m6A. Moreover, all of the m6A residues in this message are found within the 3' two-thirds of the molecule and are highly concentrated (61%) within a sequence of 108 nucleotides at the 3' noncoding region of the message. The nonrandom distribution of m6A in a specific cellular mRNA, as demonstrated for bovine prolactin, will have to be taken into account when designing a model for m6A function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Khoury G. Methylation of nuclear simian virus 40 RNAs. J Virol. 1979 Oct;32(1):52–60. doi: 10.1128/jvi.32.1.52-60.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977 Jun 15;113(1):165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Albers R. J., Coward J. K., Rottman F. M. Effect of undermethylation on mRNA cytoplasmic appearance and half-life. Mol Cell Biol. 1984 Mar;4(3):538–543. doi: 10.1128/mcb.4.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979 Jun 25;6(8):2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Nevins J. R., Darnell J. E., Jr N-6-methyl-adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol. 1979 Dec 15;135(3):733–752. doi: 10.1016/0022-2836(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Cory S., Genin C., Adams J. M. Modified nucleosides and 5'-end groups in purified mouse immunoglobulin light chain mRNA and rabbit globin mRNA detected by borohydride labelling. Biochim Biophys Acta. 1976 Dec 1;454(2):248–262. doi: 10.1016/0005-2787(76)90228-8. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Taylor R. H. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975 Oct;2(10):1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D., Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology. 1983 Dec;131(2):409–425. doi: 10.1016/0042-6822(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Cline M. G. Post-transcriptional modifications of oat coleoptile ribonucleic acids. 5'-Terminal capping and methylation of internal nucleosides in poly(A)-rich RNA. Eur J Biochem. 1980 Feb;104(1):271–277. doi: 10.1111/j.1432-1033.1980.tb04425.x. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Dubin D. T. Methylated constituents of Aedes albopictus poly (A)-containing messenger RNA. Nucleic Acids Res. 1977 Aug;4(8):2671–2682. doi: 10.1093/nar/4.8.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T. D., Lane B. G. Wheat embryo ribonucleates. XIII. Methyl-substituted nucleoside constituents and 5'-terminal dinucleotide sequences in bulk poly(AR)-rich RNA from imbibing wheat embryos. Can J Biochem. 1979 Jun;57(6):927–931. doi: 10.1139/o79-112. [DOI] [PubMed] [Google Scholar]
- Lavi S., Shatkin A. J. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi U., Fernandez-Muñoz R., Darnell J. E., Jr Content of N-6 methyl adenylic acid in heterogeneous nuclear and messenger RNA of HeLa cells. Nucleic Acids Res. 1977 Jan;4(1):63–69. doi: 10.1093/nar/4.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Stringer J. R., Holland L. E., Wagner E. K. 5'-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J Virol. 1977 Aug;23(2):234–239. doi: 10.1128/jvi.23.2.234-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Gershowitz A., Weber L. A., Baglioni C. Histone mRNAs contain blocked and methylated 5' terminal sequences but lack methylated nucleosides at internal positions. Cell. 1977 Jan;10(1):113–120. doi: 10.1016/0092-8674(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Munns T. W., Liszewski M. K., Sims H. F. Characterization of antibodies specific for N6-methyladenosine and for 7-methylguanosine. Biochemistry. 1977 May 17;16(10):2163–2168. doi: 10.1021/bi00629a019. [DOI] [PubMed] [Google Scholar]
- Nilson J. H., Convey E. M., Rottman F. M. Purification of pre-prolactin mRNA from bovine anterior pituitary glands. J Biol Chem. 1979 Mar 10;254(5):1516–1520. [PubMed] [Google Scholar]
- Nilson J. H., Thomason A. R., Horowitz S., Sasavage N. L., Blenis J., Albers R., Salser W., Rottman F. M. Construction and characterization of a cDNA clone containing a portion of the bovine prolactin sequence. Nucleic Acids Res. 1980 Apr 11;8(7):1561–1573. doi: 10.1093/nar/8.7.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell. 1975 Apr;4(4):387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Scherrer K. The methylated constituents of globin mRNA. FEBS Lett. 1975 Sep 1;57(1):73–78. doi: 10.1016/0014-5793(75)80155-4. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Sasavage N. L., Nilson J. H., Horowitz S., Rottman F. M. Nucleotide sequence of bovine prolactin messenger RNA. Evidence for sequence polymorphism. J Biol Chem. 1982 Jan 25;257(2):678–681. [PubMed] [Google Scholar]
- Schibler U., Kelley D. E., Perry R. P. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol. 1977 Oct 5;115(4):695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- Sommer S., Lavi U., Darnell J. E., Jr The absolute frequency of labeled N-6-methyladenosine in HeLa cell messenger RNA decreases with label time. J Mol Biol. 1978 Sep 25;124(3):487–499. doi: 10.1016/0022-2836(78)90183-3. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Dane R. W. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982 Jun;42(3):918–931. doi: 10.1128/jvi.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey S., Nemer M. Methylated blocked 5' terminal sequences of sea urchin embryo messenger RNA classes containing and lacking poly(A). Cell. 1976 Dec;9(4 Pt 1):589–595. doi: 10.1016/0092-8674(76)90041-6. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry. 1977 Apr 19;16(8):1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Camper S. A., Lyons R. H., Horowitz S., Goodwin E. C., Rottman F. M. Cloning and nucleotide sequencing of the bovine growth hormone gene. Nucleic Acids Res. 1982 Nov 25;10(22):7197–7210. doi: 10.1093/nar/10.22.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N. S., Manning R. F., Gage L. P. The blocked and methylated 5' terminal sequence of a specific cellular messenger: the mRNA for silk fibroin of Bombyx mori. Cell. 1976 Mar;7(3):339–347. doi: 10.1016/0092-8674(76)90163-x. [DOI] [PubMed] [Google Scholar]