Abstract
Background
Vitamin D deficiency has been associated with a number of diseases, including influenza. Whether or not this reflects a causal relationship is unknown. We therefore wanted to examine if supplementation with vitamin D would affect the incidence and severity of influenza-like disease.
Methods
Questionnaires on influenza were sent to subjects participating in ongoing placebo-controlled intervention studies with vitamin D supplementation, up until the end of April 2010.
Results
Five hundred and sixty-nine subjects from 10 different clinical trials were included in the study, of whom 289 were randomized to receive vitamin D (1111–6800 IU/day) and 280 to receive placebo. Influenza-like disease during the previous fall/winter was reported in 38 subjects in the vitamin D group and 42 in the placebo group (non-significant), of whom 25 and 26 subjects, respectively, fulfilled our clinical criteria for influenza. In these latter subjects, the duration of illness was significantly longer among those in the vitamin D group than among those in the placebo group (median 7 (range 2–60) days vs median 4 (range 2–18) days; p = 0.007). However, this difference was not statistically significant if all 38 (vitamin D) and 42 (placebo) subjects who reported symptoms were included.
Conclusion
Our results do not support the hypothesis that high doses of vitamin D supplementation will have a pronounced effect on influenza-like disease in populations not targeted for high influenza risk.
Keywords: H1N1, influenza, randomized clinical trial, vitamin D
Introduction
The presence of the vitamin D receptor (VDR) and of the enzymes necessary to hydroxylate 25-hydroxyvitamin D (25(OH)D) to its active form 1,25-dihydroxyvitamin D (1,25(OH)2D) throughout the body [1,2], makes it evident that vitamin D is important for more than skeletal health. Thus, low circulating levels of 25(OH)D have been associated with a number of diseases, such as coronary heart disease [3], peripheral artery disease [4], stroke [5], diabetes [6], hypertension [7], and cancer, and also immunological and infectious diseases including upper respiratory infections and influenza [8].
For influenza, as for practically all of the other diseases mentioned above, the association with vitamin D is based on observational studies. Thus, in temperate climates, non-pandemic influenza occurs mostly in the winter season when the serum 25(OH) D levels are low [9], influenza pandemics are associated with solar activity cycles [10], and even the mortality rates during influenza pandemics may be related to the level of solar ultraviolet B radiation [11]. In addition, vitamin D appears to stimulate the innate immune response and increase the expression of antimicrobial peptides in human monocytes and neutrophils [12,13]. Accordingly, it has been hypothesized that low levels of vitamin D may be the stimulus for the seasonality of epidemic influenza [14,15]. However, so far there is conflicting evidence from interventional studies that supplementation with vitamin D will prevent the spread of influenza or upper respiratory tract infections [16–20].
In March 2009 several cases of swine influenza, a porcine respiratory disease that rarely infects humans, were reported from Mexico [21]. The virus appeared to be a novel reassortant, containing genetic elements of influenza viruses found in swine, birds, and human beings, and was renamed influenza H1N1 [22]. The virus was expected to spread quickly around the world [21,22]. At this time, in Tromsø, Northern Norway, we had more than 400 subjects randomized to vitamin D supplementation versus placebo in ongoing intervention studies. We therefore had the opportunity to examine if vitamin D had a protective effect on influenza H1N1 or not. In order to increase the number of subjects, we also invited other research groups to participate in the study.
Methods
The study aimed to send questionnaires to subjects who had participated in vitamin D intervention studies during the influenza epidemic to examine if those randomized to vitamin D were less affected by influenza-like symptoms than those given placebo.
Recruitment of study centres and inclusion criteria
From the databases http://www.controlled-trials.com/mrct/active, http://www.who.int/ictrp/en/, and http://www.clinicaltrials.gov, 104 vitamin D researchers with ongoing or planned vitamin D intervention studies were contacted by e-mail and invited to participate if their study was randomized, placebo-controlled and double-blind, approved by the local medical research ethics committee, the vitamin D intervention dose was > 800 IU per day, and if data regarding influenza (including obtaining randomization status of the participants) would be available by August 2010.
Male and female subjects over the age of 18 y could be included, and the subjects had to have received the study drug for at least 12 weeks during the anticipated influenza period (from 1 September 2009 to 30 April 2010). Inclusion in the original study based on a specific disease state (such as osteoporosis, kidney failure, etc.) did not exclude participation in the present study.
If a study was not completed by August 2010, and accordingly the randomization code not yet broken, a procedure for obtaining the randomization status without de-blinding the researchers had to be available. The procedure to be used in Tromsø and recommended to the other centres was as follows: “When all questionnaires are collected, a file will be made with the patient’s name, study identification number, age, gender, and answers to the questionnaire. This file will be sent to the university hospital’s research department where the randomization code is kept. The research department will add the randomization status to the file, and return the file without the patient’s name and identification number. With this procedure, no one taking part in the study will be un-blinded.”
Questionnaire
The subjects were contacted by mail (or at one of the regular study visits), despatched by the end of April 2010, and were asked to fill in a simple questionnaire as shown in Table I.
Table I.
Questionnaire sent to subjects participating in studies with vitamin D supplementation versus placebo.
|
To be answered by all: Did you have influenza this fall/winter? (Answer ‘yes’ if diagnosed by a doctor or if you had serious symptoms that you considered caused by the flu) |
| Did you receive an influenza vaccine this fall/winter? If so, was the vaccine against common influenza or H1N1 (‘Swine flu’) When did you receive the vaccine(s)? |
|
To be answered only by those who had influenza this fall/winter: If you had influenza this fall/winter approximately when was it? How many days were you ill? When you had the influenza, did you have fever, cough, sore throat, runny nose, limb or joint pain, headache, vomiting or diarrhoea? |
| Did you see a doctor because of the influenza? Was the diagnosis of influenza confirmed with laboratory tests? If so, was it found to be common influenza or H1N1 (‘Swine flu’)? |
| Were you treated with Tamiflu because of the influenza? Were you admitted to hospital because of the influenza? If so, how many days were you admitted? |
Statistics
Subjects who answered ‘yes’ to the question on influenza and in addition reported fever and at least 2 additional symptoms (cough, sore throat, limb or joint pain, runny nose, headache, vomiting or diarrhoea) were considered to have had influenza, which was the primary endpoint in the study. The severity of disease (number of days ill) was the secondary endpoint.
In the power calculation we anticipated that 10% of the population would fall ill with influenza and that the vitamin D supplementation would reduce the incidence by 50%. If so, with 1000 subjects randomized 1:1 to vitamin D or placebo, we would have a 90% chance of detecting a difference between the groups at a p-level of 0.05.
The vitamin D group and the placebo group were compared with the Chi-square test for the dichotomous variables and with the Mann–Whitney U-test for the duration of disease. A p-value of < 0.05 was considered statistically significant. Duration data are presented as the median and range.
Results
Ten intervention studies being carried out at 6 centres were included in the analyses (Table II). There were 569 subjects in total (242 females and 327 males), and their median age was 63 (range 32–84) y. Two hundred and eighty-nine subjects were given vitamin D3 (cholecalciferol) in doses ranging from 1111 to 6800 IU per day and 280 were given placebo (Table III).
Table II.
Details of intervention studies included.
| Location | Subjects | Main outcome measure | Intervention |
|---|---|---|---|
| Tromsø 1, Norway | Subjects with reduced glucose tolerance | Development of type 2 diabetes | 20,000 IU vitamin D3 per week or placebo |
| Tromsø 2, Norway | Subjects with serum 25(OH)D < 50 nmol/l | Blood pressure, lipids, depression | 40,000 IU vitamin D3 per week or placebo |
| Tromsø 3, Norway | Subjects with serum 25(OH)D < 50 nmol/l | Glucose sensitivity | 40,000 IU vitamin D3 per week or placebo |
| Vienna, Austria | Kidney transplant recipients with 25(OH)D < 50 nmol/l | Graft function | 6800 IU vitamin D3 per day or placebo |
| Seattle, USA | Type 2 diabetes with 25(OH) D < 75 nmol/l and urine albumin excretion ≥ 30 mg/day | Urine albumin excretion | 2000 IU vitamin D3 per day or placebo |
| Dundee 1, Scotland | Age > 70 y, isolated systolic hypertension and 25(OH)D < 75 nmol/l | Blood pressure | 100,000 units vitamin D3 or placebo, taken at 0, 3, 6, and 9 months |
| Dundee 2, Scotland | Adults with a past history of myocardial infarction | Change in endothelial function | 100,000 units vitamin D3 or placebo, taken at 0, 2, and 4 months |
| Dundee 3, Scotland | Adults with resistant hypertension and 25(OH)D level < 75 nmol/l | Blood pressure | 100,000 units vitamin D3 or placebo, taken at 0, 2, and 4 months |
| Aarhus, Denmark | Subjects with serum 25(OH) D < 80 nmol/l | PTH and calcium metabolism | 2800 IU vitamin D3 per day or placebo |
| Leuven, Belgium | Patients with moderate to very severe COPD | COPD exacerbations | 100,000 units vitamin D3 or placebo every 4 weeks during 1 y |
25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; COPD, chronic obstructive pulmonary disease.
Table III.
Gender, age, and questionnaire answers in the vitamin D and placebo groups in relation to vitamin D study.
| Study | Females/males | Age, median (range) y | Vaccine yes/no a | Vitamin D group
|
Placebo group
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Answered ‘yes’ to influenza
|
Answered ‘no’ | Answered ‘yes’ to influenza
|
Answered ‘no’ | ||||||
| Classified as influenza b | Influenza unlikely | Classified as influenza b | Influenza unlikely | ||||||
| Tromsø 1 | 150/202 | 65 (40–82) | 190/162 | 18 | 8 | 148 | 23 | 9 | 146 |
| Tromsø 2 | 45/33 | 55 (34–73) | 38/40 | 3 | 2 | 37 | 1 | 3 | 32 |
| Tromsø 3 | 8/9 | 56 (38–72) | 11/6 | 1 | 1 | 9 | 0 | 0 | 6 |
| Vienna | 7/13 | 61 (32–72) | 0/8 | 1 | 1 | 8 | 0 | 4 | 6 |
| Seattle | 1/6 | 60 (46–76) | 4/3 | 0 | 0 | 3 | 1 | 0 | 3 |
| Dundee 1 | 10/12 | 77 (70–84) | 17/5 | 0 | 0 | 12 | 0 | 0 | 10 |
| Dundee 2 | 6/25 | 69 (50–82) | 26/5 | 0 | 0 | 15 | 0 | 0 | 16 |
| Dundee 3 | 1/4 | 69 (58–74) | 5/0 | 0 | 0 | 4 | 0 | 0 | 1 |
| Aarhus | 13/3 | 65 (33–77) | 4/12 | 1 | 1 | 6 | 1 | 0 | 7 |
| Leuven | 1/20 | 72 (56–82) | 19/2 | 1 | 0 | 9 | 0 | 0 | 11 |
| Total | 242/327 | 63 (32–84) | 314/243 | 25 | 13 | 251 | 26 | 16 | 238 |
Not all subjects answered this question.
Answered ‘yes’ to the question on influenza and in addition had fever plus 2 of the following 6 symptoms: cough, sore throat, limb or joint pain, runny nose, headache, and vomiting or diarrhoea.
Eighty-five subjects answered ‘yes’ to the question on influenza. Three of them had the influenza prior to the start of the vitamin D or placebo intervention and 2 reported the disease after 1 May 2010 and were considered as not having been ill in the period under study. Among the remaining 80 subjects (38 in the vitamin D group and 42 in the placebo group), 29 (13 in the vitamin D group and 16 in the placebo group) did not fulfil the clinical criteria for influenza (fever plus 2 additional symptoms). Thus, 25 subjects in the vitamin D group and 26 in the placebo group were considered to have had influenza (Table II). Among these 51 subjects, 10 subjects had 5 symptoms in addition to fever, 23 subjects had 4 additional symptoms, 10 subjects had 3 additional symptoms, and 8 subjects had 2 additional symptoms.
An influenza vaccine had been given to 314 subjects (29 against ordinary influenza, 193 against swine influenza, and 91 a combination of both; 1 subject did not state the type of vaccine) – 157 in the vitamin D group and 157 in the placebo group. Of the 25 subjects in the vitamin D group considered to have had influenza, 12 had received a vaccine, and among the 26 in the placebo group, 18 had been vaccinated (non-significant).
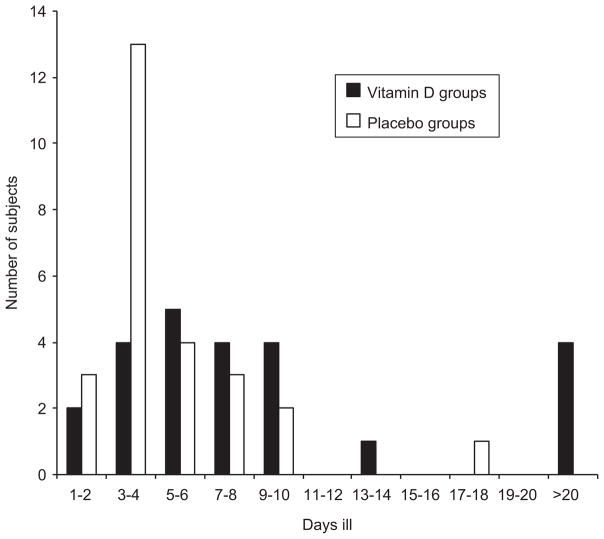
One of the subjects in the vitamin D group with influenza did not state the number of days ill. For the remaining 24 subjects in the vitamin D group the median duration of the illness was 7 (range 2–60) days and in the 26 subjects in the placebo group it was 4 (range 2–18) days (p = 0.007) (Figure 1). If examining all subjects who had answered ‘yes’ to the question on influenza together and not excluding those without significant clinical signs (fever and 2 additional symptoms), then the difference in duration of illness between the vitamin D group and the placebo group was not statistically significant.
Figure 1.
Number of days ill in the 24 subjects randomized to vitamin D and in the 26 subjects randomized to placebo.
Ten of the 25 subjects in the vitamin D group and 4 of the 26 in the placebo group consulted a doctor because of the influenza (p = 0.064, non-significant). The diagnosis was confirmed by laboratory testing in 3 subjects in the vitamin D group (2 with ordinary influenza, 1 with H1N1) and in 1 subject in the placebo group (H1N1). One subject in the vitamin D group but none in the placebo group were admitted to hospital because of the influenza.
Discussion
In the present study we did not find supplementation with vitamin D to protect against ‘influenza’. Furthermore, the subjects in the vitamin D group who fell ill with ‘influenza’ had a significantly more prolonged disease than those in the placebo group.
Our study was based on what the subjects themselves considered as ‘influenza’, provided they also had subjective fever and 2 of the following additional clinical symptoms: cough, sore throat, limb or joint pain, runny nose, headache, and vomiting or diarrhoea. These symptoms were chosen based on symptoms reported to predict seasonal influenza [23], and also those reported for the H1N1 influenza, for which vomiting and diarrhoea were frequently reported [24]. However, among the 8 subjects classified as having had ‘influenza’ based on only 2 additional symptoms, none of them had gastrointestinal complaints. Accordingly, inclusion of the gastrointestinal symptoms as a diagnostic criterion did not have any effects on our results.
The questionnaire used by us has not been formally validated in a population at low risk for developing influenza. However, in the retrospective study by Monto et al. [23] on 3744 subjects with influenza-like symptoms who later underwent laboratory testing, the positive predictive value of fever and cough combined with any one of nasal congestion, myalgia, sore throat, or headache, was close to 80%. Furthermore, we do not have any data on how other respiratory viral or bacterial infections would present with these combinations of symptoms.
During an influenza epidemic the presence of these symptoms makes it more likely that the subject actually is ill with influenza, however the likelihood is much less when there is not an ongoing severe epidemic, as was the case in the winter of 2010. Most of the subjects did not consult a doctor because of the illness, and only a few had the diagnosis of ordinary influenza or H1N1 confirmed by laboratory testing. Also, many of those who reported illness had received an influenza vaccine, which makes it less likely that the illness was actually caused by an influenza virus. Accordingly, what we have actually registered should more properly be termed ‘influenza-like symptoms’ or ‘upper respiratory tract infection’ than true influenza.
The finding that those randomized to vitamin D had a more prolonged disease than those given placebo was surprising and hard to explain regarding the similar number of subjects who fell ill. Furthermore, the difference was not significant when including all subjects who answered ‘yes’ to influenza, and should therefore be viewed with caution. However, there are indications that the effect curve of vitamin D has a U-shaped form, at least regarding mortality [25], and the doses given to most of the subjects were more than 3000 IU per day, which is fairly high. On the other hand, much higher doses have been well tolerated in other studies without any reports of increased incidence of infections [26].
Low serum levels of 25(OH)D have consistently been associated with a number of disease states, including respiratory tract infection [8]. Thus, in the Third National Health and Nutrition Examination Survey in the USA including 18,883 subjects, those with serum 25(OH)D levels in the range 25–75 nmol/l had a 24% higher chance of having had a recent respiratory tract infection compared to those with levels >75 nmol/l [27]. Similarly, in a study from Finland including 800 men at a military base, those with serum 25(OH)D levels< 40 nmol/l had almost twice as many days of absence from duty due to respiratory infection than did the control subjects [28]. Furthermore, in a longitudinal study by Sabetta et al. who followed 198 healthy adults over the fall and winter 2009–2010, those with serum 25(OH)D > 95 nmol/l had a 2-fold reduction in the risk of developing an acute respiratory tract infection compared to the rest of the cohort [29].
However, interventional studies have been less conclusive [16]. Thus, Laaksi et al. included 164 young Finnish men and randomized the subjects to 400 IU per day versus placebo, but no significant effect on the main outcome variable, number of days absent from duty due to respiratory tract infection, was found [17]. Similarly, Li-Ng et al. randomized 162 adults to 2000 IU daily versus placebo and found no benefit of the supplementation regarding the incidence or severity of symptomatic upper respiratory infections during the winter [18].
On the other hand, Aloia and Li-Ng reported in a letter to the editor a post-hoc analysis showing that among 208 post-menopausal women randomized to 2000 IU vitamin D versus placebo, the incidence of cold and influenza symptoms was 3 times higher in the placebo group than in the vitamin D group [19]. Furthermore, in a recent study from Japan specifically designed for testing the effect of vitamin D supplementation to prevent influenza, 167 children were randomized to 1200 IU vitamin D and 167 to placebo. Influenza was diagnosed with antigen testing with a nasopharyngeal swab specimen, and influenza type A was diagnosed in only 18 children in the vitamin D group versus 31 in the placebo group (p = 0.04) [20]. Reasons for the different outcomes when comparing ‘positive’ studies to ‘negative’ studies, including ours, are not clear, but may relate to the different influenza risk of the different study populations (e.g. higher in schoolchildren during the 2009 influenza season), differences in case ascertainment, or chance variation if the true effect of vitamin D is small or null. Accordingly, the effects of vitamin D on respiratory virus infections remain to be determined.
It must be emphasized that our study has a number of weaknesses. The study was retrospective and relied on self-reported symptoms, which obviously is not an accurate method for collecting this type of data. Therefore, we did not have a definite diagnosis of influenza. We did not analyze the 25(OH)D levels during the study, and the number of subjects included was lower than that needed according to the power calculation. Thus, with 569 subjects included, the study would only have a power of 0.65 in detecting a 50% reduction in the rate of ‘influenza’ seen in the present cohort. Only a few of our subjects were completely healthy; a large group had glucose intolerance, and we also included subjects with co-morbidities like type 2 diabetes, hypertension, chronic obstructive pulmonary disease, and subjects with a past history of myocardial infarction and kidney transplant recipients. Our results may therefore not apply to a more healthy population. None of the trials included were specifically designed for studying influenza susceptibility, and accordingly, the present report is a secondary analysis. Our results therefore do not exclude an effect of vitamin D supplementation on influenza or upper respiratory tract infections.
There are also important strengths of this study: the multi-centre, international design lends substantial external validity; all 10 studies included were placebo-controlled and double-blind, all studies used high doses of vitamin D that are likely to achieve substantial separation in vitamin D status by treatment assignment, and data were analyzed on the participant level (not meta-analyzed), yielding the (to our knowledge) largest study published to date assessing the effect of vitamin D supplementation on influenza-like symptoms.
In conclusion, and in line with other aspects of vitamin D and health, one should not rely on observational data regarding vitamin D and infections [30], or on underpowered intervention studies with influenza as the secondary endpoint, as the present one. To our knowledge, there has been only one properly performed randomized clinical trial on influenza prevention published to date [20]. Until further similar studies are performed, preferably in populations at risk of the disease and/or with insufficient vitamin D status, the role of vitamin D for the prevention of influenza and upper respiratory infections remains unresolved.
Acknowledgments
The superb assistance of the staff of the Clinical Research Unit, University Hospital of North Norway is gratefully acknowledged.
Footnotes
Declaration of interest: The present study received funding from The Northern Norway Regional Health Authority, The Norwegian Research Foundation, The Chief Scientist Office, Scottish Government, Chest Heart and Stroke Scotland, The National Institutes of Health grants 5KL2RR025015, P30 DK017047, and UL1 RR025014. None of the authors have any financial involvement or affiliation with any organization whose financial interests may be affected by material in the manuscript, or which may potentially bias it.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Maalouf NM. The noncalciotropic actions of vitamin D: recent clinical developments. Curr Opin Nephrol Hypertens. 2008;17:408–15. doi: 10.1097/MNH.0b013e3283040c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease. Results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–85. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low vitamin D levels predicts stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–3. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- 6.Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jenssen T, Njølstad I, et al. Serum 25-hydroxyvitamin D and incident type 2 diabetes mellitus—eleven years follow-up from the Tromsø Study 1994–95. Diabetic Med. 2010;27:1107–15. doi: 10.1111/j.1464-5491.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 7.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25- hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55:792–8. doi: 10.1161/HYPERTENSIONAHA.109.143990. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 9.Juzeniene A, Ma LW, Kwitniewski M, Polev GA, Lagunova Z, Dahlback A, et al. The seasonality of pandemic and non-pandemic influenzas: the roles of solar radiation and vitamin D. Int J Infect Dis. 2010;14:1099–105. doi: 10.1016/j.ijid.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Hayes DP. Influenza pandemics, solar activity cycles, and vitamin D. Med Hypotheses. 2010;74:831–4. doi: 10.1016/j.mehy.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215–9. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 13.White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–8. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol J. 2008;5:29. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–49. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamäki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;202:809–14. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 18.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 19.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 21.Editorial. Swine influenza: how much of a global threat? Lancet. 2009;373:1495. doi: 10.1016/S0140-6736(09)60826-6. [DOI] [PubMed] [Google Scholar]
- 22.Gallaher WR. Towards a sane and rational approach to management of influenza H1N1 2009. Virol J. 2009;6:51. doi: 10.1186/1743-422X-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 24.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 25.Michaëlsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundström J, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92:841–8. doi: 10.3945/ajcn.2010.29749. [DOI] [PubMed] [Google Scholar]
- 26.Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008;159:675–84. doi: 10.1530/EJE-08-0339. [DOI] [PubMed] [Google Scholar]
- 27.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, et al. An association of serum vitamin D concentrations < 40 nmol/l with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 29.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]