Abstract
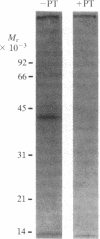
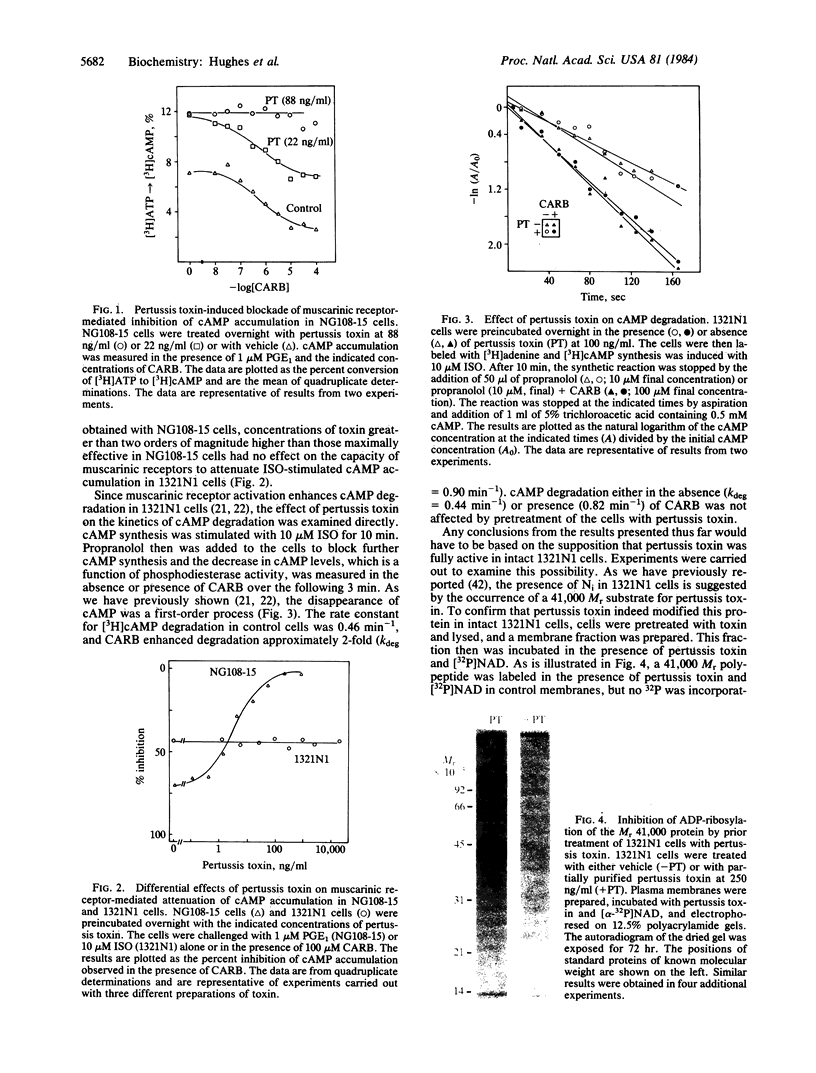
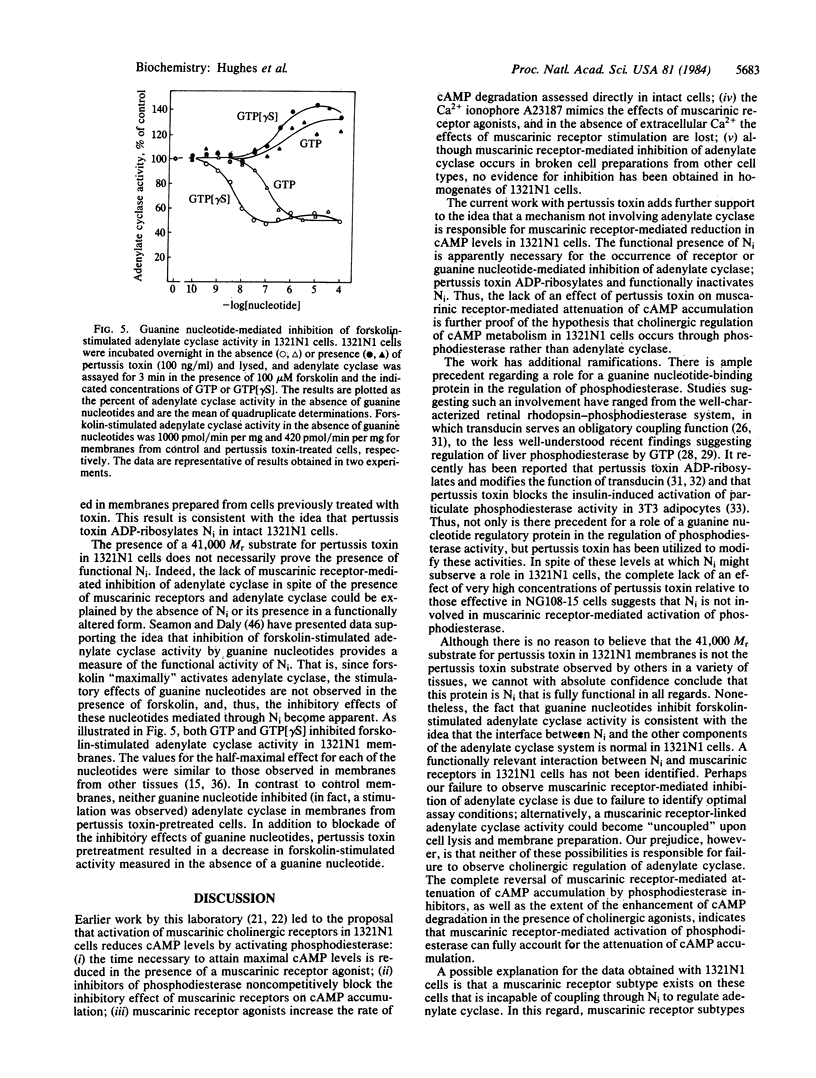
It has been proposed elsewhere [Meeker, R.B. & Harden, T. K. (1982) Mol. Pharmacol. 22, 310-319] that muscarinic cholinergic receptor-mediated attenuation of cAMP accumulation occurs through activation of phosphodiesterase in 1321N1 human astrocytoma cells. Pertussis toxin, which ADP-ribosylates the guanine nucleotide regulatory protein involved in receptor-mediated inhibition of adenylate cyclase (Ni), has been utilized to further differentiate between the mechanism of cholinergic regulation of cAMP metabolism in 1321N1 cells and the mechanism involving inhibition of adenylate cyclase in other tissues. Muscarinic receptor-mediated regulation of cAMP accumulation in NG108-15 neuroblastoma-glioma cells occurs through inhibition of adenylate cyclase. Pretreatment of these cells with pertussis toxin completely blocked the capacity of carbachol to attenuate cAMP accumulation. In contrast, concentrations of pertussis toxin two to three orders of magnitude higher than those effective in NG108-15 cells had no effect on muscarinic receptor-mediated attentuation of cAMP accumulation in 1321N1 cells. In addition, no effect of pertussis toxin was observed either on the control rate or the carbachol-stimulated rate of cAMP degradation measured directly in intact 1321N1 cells. A 41,000 Mr protein previously proposed to be the alpha subunit of Ni was labeled during incubation of a plasma membrane fraction from 1321N1 cells with [32P]NAD and pertussis toxin. Pertussis toxin is apparently active in 1321N1 cells, since this protein substrate was not labeled in plasma membrane preparations from cells previously incubated with toxin. Functional activity of Ni was demonstrated by the observation that guanosine 5'-[gamma-thio]triphosphate- and GTP-mediated inhibition of forskolin-stimulated adenylate cyclase activity occurred in cell-free preparations from 1321N1 cells. The inhibitory activity of these guanine nucleotides was lost in membrane preparations from pertussis toxin-treated cells. The data suggest that adenylate cyclase is not involved in cholinergic action in 1321N1 cells and, furthermore, Ni is not involved in muscarinic receptor-mediated activation of phosphodiesterase in these cells. Thus, pertussis toxin can be used to differentiate between two mechanisms of cholinergic regulation of cAMP metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Schultz G., Jakobs K. H. Cholera toxin does not impair hormonal inhibition of adenylate cyclase and concomitant stimulation of a GTPase in adipocyte membranes. Biochim Biophys Acta. 1982 Oct 28;719(1):58–64. doi: 10.1016/0304-4165(82)90307-5. [DOI] [PubMed] [Google Scholar]
- Barber R., Ray K. P., Butcher R. W. Turnover of adenosine 3',5'-monophosphate in WI-38 cultured fibroblasts. Biochemistry. 1980 Jun 10;19(12):2560–2567. doi: 10.1021/bi00553a004. [DOI] [PubMed] [Google Scholar]
- Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Guanine nucleotides modulate muscarinic receptor binding in the heart. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1000–1005. doi: 10.1016/s0006-291x(79)80006-6. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Brown J. H. Cholinergic inhibition of catecholamine-stimulable cyclic AMP accumulation in murine atria. J Cyclic Nucleotide Res. 1979 Dec;5(6):423–433. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- Elks M. L., Watkins P. A., Manganiello V. C., Moss J., Hewlett E., Vaughan M. Selective regulation by pertussis toxin of insulin-induced activation of particulate cAMP phosphodiesterase activity in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):593–598. doi: 10.1016/0006-291x(83)90565-x. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Meeker R. B., Martin M. W. Interaction of a radiolabeled agonist with cardiac muscarinic cholinergic receptors. J Pharmacol Exp Ther. 1983 Dec;227(3):570–577. [PubMed] [Google Scholar]
- Harden T. K., Scheer A. G., Smith M. M. Differential modification of the interaction of cardiac muscarinic cholinergic and beta-adrenergic receptors with a guanine nucleotide binding component(s). Mol Pharmacol. 1982 May;21(3):570–580. [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Heyworth C. M., Rawal S., Houslay M. D. Guanine nucleotides can activate the insulin-stimulated phosphodiesterase in liver plasma membranes. FEBS Lett. 1983 Apr 5;154(1):87–91. doi: 10.1016/0014-5793(83)80880-1. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Hoffman B. B., Yim S., Tsai B. S., Lefkowitz R. J. Preferential uncoupling by manganese of alpha adrenergic receptor mediated inhibition of adenylate cyclase in human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):724–731. doi: 10.1016/s0006-291x(81)80235-5. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Lasch P., Minuth M., Aktories K., Schultz G. Uncoupling of alpha-adrenoceptor-mediated inhibition of human platelet adenylate cyclase by N-ethylmaleimide. J Biol Chem. 1982 Mar 25;257(6):2829–2833. [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Kurose H., Katada T., Amano T., Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Boone G., Blume A. Muscarinic receptor regulation of NG108-15 adenylate cyclase: requirement for Na+ and GTP. J Cyclic Nucleotide Res. 1979 Oct;5(5):367–375. [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- Manning D. R., Fraser B. A., Kahn R. A., Gilman A. G. ADP-ribosylation of transducin by islet-activation protein. Identification of asparagine as the site of ADP-ribosylation. J Biol Chem. 1984 Jan 25;259(2):749–756. [PubMed] [Google Scholar]
- Manning D. R., Gilman A. G. The regulatory components of adenylate cyclase and transducin. A family of structurally homologous guanine nucleotide-binding proteins. J Biol Chem. 1983 Jun 10;258(11):7059–7063. [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated activation of phosphodiesterase. Mol Pharmacol. 1982 Sep;22(2):310–319. [PubMed] [Google Scholar]
- Meeker R. B., Harden T. K. Muscarinic cholinergic receptor-mediated control of cyclic AMP metabolism. Agonist-induced changes in nucleotide synthesis and degradation. Mol Pharmacol. 1983 Mar;23(2):384–392. [PubMed] [Google Scholar]
- Motulsky H. J., Hughes R. J., Brickman A. S., Farfel Z., Bourne H. R., Insel P. A. Platelets of pseudohypoparathyroid patients: evidence that distinct receptor-cyclase coupling proteins mediate stimulation and inhibition of adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4193–4197. doi: 10.1073/pnas.79.13.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Guanosine 5'-(beta, gamma-imido)triphosphate inhibition of forskolin-activated adenylate cyclase is mediated by the putative inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1982 Oct 10;257(19):11591–11596. [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Smith M. M., Harden T. K. Modification of receptor-mediated inhibition of adenylate cyclase in NG108-15 neuroblastoma X glioma cells by n-ethylmaleimide. J Pharmacol Exp Ther. 1984 Feb;228(2):425–433. [PubMed] [Google Scholar]
- Spina A., Chiosi E., Paolisso G., Laurenza A., Illiano G. A stimulating effect of guanyl nucleotides on the rat-liver soluble cyclic GMP high-affinity phosphodiesterase activity. FEBS Lett. 1983 Jul 4;157(2):351–355. doi: 10.1016/0014-5793(83)80574-2. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Van Sande J., Erneux C., Dumont J. E. Negative control of TSH action by iodide and acetylcholine: mechanism of action in intact thyroid cells. J Cyclic Nucleotide Res. 1977 Oct;3(5):335–345. [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Watanabe A. M., McConnaughey M. M., Strawbridge R. A., Fleming J. W., Jones L. R., Besch H. R., Jr Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem. 1978 Jul 25;253(14):4833–4836. [PubMed] [Google Scholar]
- Yajima M., Hosoda K., Kanbayashi Y., Nakamura T., Nogimori K., Mizushima Y., Nakase Y., Ui M. Islets-activating protein (IAP) in Bordetella pertussis that potentiates insulin secretory responses of rats. Purification and characterization. J Biochem. 1978 Jan;83(1):295–303. doi: 10.1093/oxfordjournals.jbchem.a131904. [DOI] [PubMed] [Google Scholar]
- de Mazancourt P., Giudicelli Y. Guanine nucleotide- and GTP-dependent N6-phenylisopropyladenosine stimulation of the membrane-bound cyclic AMP high affinity phosphodiesterase in rat brain. FEBS Lett. 1984 Feb 13;167(1):142–146. doi: 10.1016/0014-5793(84)80849-2. [DOI] [PubMed] [Google Scholar]