Abstract
N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL), a quorum-sensing signal molecule produced by Pseudomonas aeruginosa (P. aeruginosa), is involved in the expression of bacterial virulence factors and in the modulation of host immune responses by directly disrupting nuclear factor-κB (NF-κB) signaling and inducing cell apoptosis. The unfolded protein response (UPR) triggered by endoplasmic reticulum (ER) stress may suppress inflammatory responses in the later phase by blocking NF-κB activation. It was recently demonstrated that 3-oxo-C12-HSL may induce UPR in human aortic endothelial cells (HAECs). Therefore, 3-oxo-C12-HSL may also inhibit NF-κB activation and suppress inflammatory responses by activating UPR. However, the possible underlying mechanism has not been fully elucidated. Accordingly, we investigated the effects of 3-oxo-C12-HSL on cellular viability, UPR activation, lipopolysaccharide (LPS)-induced NF-κB activation and inflammatory response in the RAW264.7 mouse macrophage cell line. Treatment with 6.25 μM 3-oxo-C12-HSL was not found to affect the viability of RAW264.7 cells. However, pretreating RAW264.7 cells with 6.25 μM 3-oxo-C12-HSL effectively triggered UPR and increased the expression of UPR target genes, such as CCAAT/enhancer-binding protein β (C/EBP β) and CCAAT/enhancer-binding protein-homologous protein (CHOP). The expression of C/EBP β and CHOP was found to be inversely correlated with LPS-induced NF-κB activation. 3-Oxo-C12-HSL pretreatment was also shown to inhibit LPS-stimulated proinflammatory cytokine production. Hence, 3-oxo-C12-HSL may attenuate LPS-induced inflammation via UPR-mediated NF-κB inhibition without affecting cell viability. This may be another mechanism through which P. aeruginosa evades the host immune system and maintains a persistent infection.
Keywords: Pseudomonas aeruginosa, homoserine lactone, unfolded protein response, nuclear factor-κB, inflammation
Introduction
N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL), a quorum-sensing signal molecule produced by the opportunistic pathogen Pseudomonas aeruginosa (P. aeruginosa), plays an important role in the regulation of bacterial virulence genes, as well as in the modulation of inflammatory responses (1–3). 3-Oxo-C12-HSL directly disrupts lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) signaling and represses NF-κB-responsive genes that encode inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-12 in macrophages (2,4). 3-Oxo-C12-HSL also induces cellular apoptosis in macrophages/lymphocytes and alleviates host immune responses (5). These data indicate that 3-oxo-C12-HSL plays a critical role in the inhibition of inflammation.
The unfolded protein response (UPR) is an adaptive cellular response that protects cells against the stress of unfolded proteins in the endoplasmic reticulum (ER) (6). Recent studies reported that UPR triggers NF-κB activation in the early phase (7) and suppresses inflammatory responses to stimuli in the later phase through NF-κB activity inhibition (8–12). CCAAT/enhancer-binding protein (C/EBP) is a family of transcription factors that possess a highly conserved, basic-leucine zipper (bZIP) domain, which is required for DNA binding and dimerization (13). C/EBP β, one of the 6 C/EBP family members, may be triggered by ER stress and interact with transcription factors, including NF-κB (14). Several studies confirmed that C/EBP β induced by UPR depresses NF-κB phosphorylation (12,15,16). CCAAT/enhancer-binding protein-homologous protein (CHOP) is a transcription factor that stablely forms heterodimers with C/EBP family members through their bZIP domain and is upregulated by the activation of UPR (17). Recent studies demonstrated that UPR-mediated induction of CHOP reduces inflammatory cytokine secretion and NF-κB activation (18,19). A recent study also demonstrated that 3-oxo-C12-HSL may induce the expression of UPR genes, such as C/EBP β and CHOP, in human aortic endothelial cells (HAECs) (20). These data strongly indicate that 3-oxo-C12-HSL may inhibit NF-κB activity through the induction of UPR.
3-Oxo-C12-HSL may inhibit the expression levels of TNF-α and monocyte chemoattractant protein-1 (MCP-1) by directly impairing the regulation of NF-κB functions when stimulating bone marrow-derived macrophages and peripheral blood mononuclear cells together with LPS for ≤3 h (2). However, whether pretreatment with 3-oxo-C12-HSL is able to induce UPR and whether this UPR can inhibit LPS-induced inflammation in macrophages has yet to be determined. In the present study, we investigated the effects of 3-oxo-C12-HSL-triggered UPR on LPS-induced NF-κB activation and inflammation in RAW264.7 cells.
Materials and methods
Reagents
P. aeruginosa quorum-sensing molecule 3-oxo-C12-HSL and LPS from Escherichia coli were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
RAW264.7 cells (China Center for Type Culture Collection, Wuhan, China) were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (Hyclone, Logan, UT, USA). The cultured cells were incubated with serum-free basal medium for 12 h and then washed twice prior to the challenge. For the challenge experiments, the cells were exposed to various concentrations of 3-oxo-C12-HSL (6.25–100 μM). At indicated times following incubation, the cultures were centrifuged at 450 × g for 10 min at 4°C. The cultured supernatants and the cells were collected and stored at -80°C for further experiments.
Cell viability assay
The cells (5×103 cells/well) were cultured in a flat-bottom 96-well microtiter plate containing 100 μl of serum-free basal medium with various concentrations of 3-oxo-C12-HSL (21). The cell viability was determined using the WST-1 cell proliferation and cytotoxicity assay kit (Beyotime, Haimen, China) according to the manufacturer’s instructions. Absorbance was measured using a microplate reader (BioTek, Winooski, VT, USA) at 450 nm and the reference wavelength was set at 630 nm. The percentage cytotoxicity was calculated in comparison with that of the untreated control cells.
Apoptosis assay
Cell apoptosis was analyzed by flow cytometry using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit (Keygen Biotech, Nanjing, China). The RAW264.7 cells (1×106 cells/ml) were incubated with different concentrations of 3-oxo-C12-HSL for 9 h. After the challenge, the cells were suspended and labeled with propidium iodide and Annexin V-FITC for 15 min at 25°C in the dark. The cells were observed on FACSAria (BD Biosciences, San Diego, CA, USA) and the data were analyzed using the FACSDiva Cytometer software (BD Biosciences). The cells that stained positive for Annexin V were counted as apoptotic.
RNA extraction, quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
TRIzol reagent (Invitrogen Life Technologies) was used to extract total RNA. The primers used for qPCR are as follows: GAPDH, forward 5′-TGCACC ACCAACTGCTTAG-3′ and reverse 5′-GATGCAGGGATG ATGTTC-3′; C/EBP β, forward 5′-ACCGGGTTTCGGGAC TTGA-3′ and reverse 5′-GTTGCGTAGTCCCGTGTCCA-3′; CHOP, forward 5′-CTGGAAGCCTGGTATGAGGAT-3′ and reverse 5′-CAGGGTCAAGAGTAGTGAAGGT-3′. RT-PCR was performed using the ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) and qPCR analysis was performed with SYBR-Green Realtime PCR master mix-plus (Toyobo) according to the manufacturer’s instructions.
Western blot analysis
Following the challenge and removal of the culture medium, the cells were lysed in radioimmunoprecipitation assay buffer (ProMab, Changsha, China) and then boiled for 5 min. Aliquots of cell extracts were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad, Richmond, CA, USA) and blotted with anti-β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-C/EBP β (BioLegend, San Diego, CA, USA), anti-CHOP (Santa Cruz Biotechnology, Inc.) and anti-phospho-NF-κB p65 pSer536 antibody (Cell Signaling Technology, Inc., Danvers, MA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The cytokine levels of MCP-1 and TNF-α were measured by ELISA using mouse MCP-1 and TNF-α kits (R&D Systems, Minneapolis, MN, USA), as previously described (22). Cell supernatant was added to the wells, which were coated with antibodies against MCP-1 and TNF-α. After washing twice with washing buffer, peroxidase-conjugated avidin, biotinylated antibodies and chromogenic substrate were added to each well. The absorbance was read at 450 nm in an ELISA plate reader.
Statistical analysis
Representative data from triple independent experiments are expressed as means ± SD. One-way ANOVA was performed to determine statistical significance among multiple groups and the Student’s t-test was used to compare differences between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Determination of a proper concentration of 3-oxo-C12-HSL in RAW264.7 cells
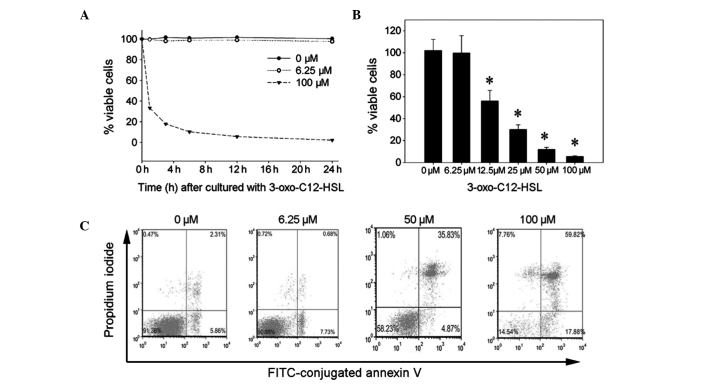
At a proper concentration, 3-oxo-C12-HSL may activate UPR without affecting cell viability, as previously described (11). 3-Oxo-C12-HSL was shown to induce cell apoptosis and death in macrophage/lymphocytes (5). Thus, the cytotoxic effects of different concentrations of 3-oxo-C12-HSL were investigated in order to determine a proper concentration that elicits an appropriate UPR. The RAW264.7 cells exhibited a significantly reduced viability following treatment with 100 μM 3-oxo-C12-HSL (Fig. 1A). Cell viability decreased from 33.4% at 1 h to 2.25% at 24 h following 3-oxo-C12-HSL addition (Fig. 1A). By contrast, 6.25 μM 3-oxo-C12-HSL did not affect cell viability in this assay at 24 h. After 12 h of incubation with 12.5–100 μM 3-oxo-C12-HSL, cell viability was significantly decreased (Fig. 1B). These data indicated that 3-oxo-C12-HSL reduces RAW264.7 cell viability in a concentration-dependent manner and that 6.25 μM is the proper concentration for RAW264.7 cells. To further verify these findings, the effects of different concentrations of 3-oxo-C12-HSL on cell apoptosis/necrosis were also determined with the Annexin V-FITC apoptosis assay. Following incubation with 0, 6.25, 50, or 100 μM 3-oxo-C12-HSL for 9 h, ~40 and 80% of apoptotic cells were observed in response to 50 and 100 μM 3-oxo-C12-HSL, respectively (Fig. 1C). However, there were no significant apoptotic effects in the 6.25-μM group. These data confirmed that treatment with 6.25 μM 3-oxo-C12-HSL for 1–24 h does not affect RAW264.7 cell viability.
Figure 1.
Effects of N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL) on RAW264.7 cell viability. (A) RAW264.7 cells were treated with 6.25 or 100 μM 3-oxo-C12-HSL for 1–24 h. Cell viability was measured with the WST-1 assay. (B) Following incubation with increasing concentrations of 3-oxo-C12-HSL for 12 h, cell viability was measured with the WST-1 assay (*P<0.05 compared to the 0 and 6.25 μM 3-oxo-C12-HSL-treated groups). (C) The RAW264.7 cells were treated with 0, 6.25, 50 or 100 μM 3-oxo-C12-HSL for 9 h. The cells were stained with propidium iodide and fluorescein isothiocyanate (FITC)-conjugated Annexin V and the percentage of Annexin V-positive cells was measured by flow cytometry.
The mRNA expression of molecular chaperones in the UPR pathway
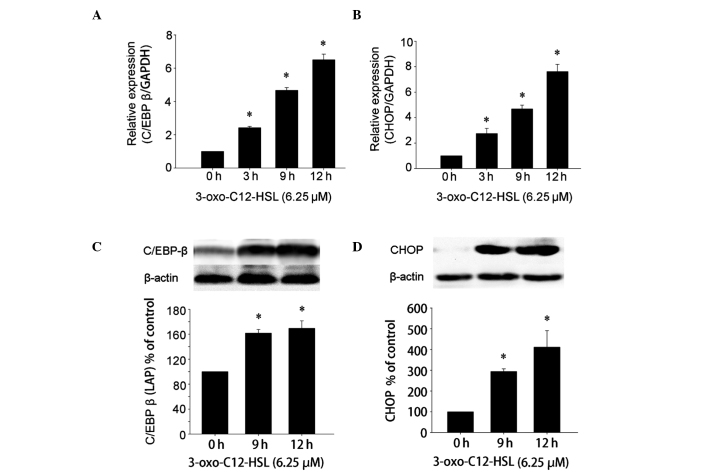
Kim et al (20) confirmed that 50 μM 3-oxo-C12-HSL effectively induces UPR and activates UPR target genes, such as C/EBP β and CHOP in HAECs. Therefore, the mRNA expression of representative target genes of UPR activation, such as C/EBP β and CHOP, was analyzed using qRT-PCR to determine whether 6.25 μM 3-oxo-C12-HSL was able to efficiently activate these genes (Fig. 2A and B). C/EBP β mRNA was found to be increased at 3 h and reached a peak at 12 h (Fig. 2A). Similarly, CHOP mRNA expression was also increased after 3-oxo-C12-HSL stimulation at time points between 3 and 12 h (Fig. 2B). These results suggested that 6.25 μM 3-oxo-C12-HSL may effectively stimulate the expression of UPR target genes in RAW264.7 cells. The C/EBP β [liver-enriched transcriptional activator protein (LAP) isoform] and CHOP proteins are activated by UPR and may suppress the activation of NF-κB in the later phase (15,18). Thus, we also investigated the protein expression pattern of UPR following incubation with 3-oxo-C12-HSL. Enhanced expression of the C/EBP β (LAP isoform) and CHOP proteins was observed following stimulation with 3-oxo-C12-HSL. The protein level of C/EBP β (LAP isoform) increased from 9 to 12 h (Fig. 2C). The level of CHOP was also significantly increased after 9 h, reaching a peak at 12 h (Fig. 2D). These results indicated that 3-oxo-C12-HSL pretreatment may effectively activate UPR-mediated C/EBP β and CHOP expression. Although the levels of C/EBP β (LAP isoform) and CHOP at 12 h were marginally higher compared to those at 9 h, no statistically significant difference was observed (P>0.05). Therefore, the time point for analyzing the abrogation of LPS-induced inflammation in RAW264.7 cells would be the point at which the UPR target genes were effectively activated following pretreatment with 3-oxo-C12-HSL. In the present study, 9 h was selected as the appropriate time point based on the aforementioned results.
Figure 2.
Expression of molecular chaperones in the unfolded protein response pathway. RAW264.7 cells were treated with 6.25 μM N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL) for 0, 3, 9 and 12 h prior to gene expression analysis. The relative quantitative expression of (A) CCAAT/enhancer-binding protein (C/EBP β) and (B) CCAAT/enhancer-binding protein-homologous protein (CHOP) at different time points were determined using quantitative reverse transcription-polymerase chain reaction analysis (*P<0.05 compared to untreated controls). Subsequently, the RAW264.7 cells were incubated with 6.25 μM 3-oxo-C12-HSL for the indicated times and the level of (C) C/EBP β [liver-enriched activator protein (LAP)] and (D) CHOP were determined by western blot analysis and semi-quantified by densitometry (*P<0.05 compared to untreated controls; P>0.05 compared to each other).
3-oxo-C12-HSL pretreatment suppresses LPS-induced NF-κB activation and inflammatory cytokine production
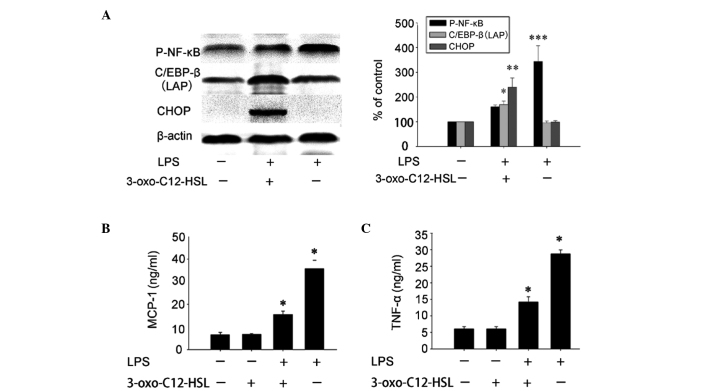
To further verify whether 3-oxo-C12-HSL pretreatment suppresses LPS-induced NF-κB activation, we observed the LPS-induced NF-κB phosphorylation in cells pretreated with 3-oxo-C12-HSL and untreated cells. Following stimulation with LPS for 12 h, NF-κB phosphorylation was significantly decreased in the group pretreated with 3-oxo-C12-HSL for 9 h. Furthermore, the expression of C/EBP β (LAP isoform) and CHOP was significantly negatively correlated with NF-κB phosphorylation (Fig. 3A).
Figure 3.
The activation of the unfolded protein response suppressed the lipopolysaccharide (LPS)-induced activation of NF-κB and inflammatory responses in RAW264.7 cells. The RAW264.7 cells were preincubated with 6.25 μM N-3-oxododecanoyl homoserine lactone (3-oxo-C12-HSL) for 9 h and then stimulated with 10 ng/ml LPS for 12 h. (A) Phospho-NF-κB, CCAAT/enhancer-binding protein (C/EBP β) [liver-enriched transcriptional activator protein (LAP)] and CCAAT/enhancer-binding protein-homologous protein (CHOP) were determined by western blot analysis and semi-quantified by densitometry (***P<0.05 compared to the untreated and LPS-treated groups; ***P<0.05 compared to the untreated and 3-oxo-C12-HSL-stimulated groups). (B and C) The culture supernatants were collected and the concentrations of monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α) were determined by ELISA (*P<0.05 compared to each other; P<0.05 compared to the control and the 3-oxo-C12-HSL-treated group).
Since LPS-induced MCP-1 and TNF-α production involves NF-κB phosphorylation (23,24), the effects of 3-oxo-C12-HSL on LPS-induced cytokine production were then investigated in RAW264.7 cells. LPS-induced MCP-1 and TNF-α production was significantly decreased in cells pretreated with 3-oxo-C12-HSL compared to that in untreated cells (Fig. 3B and C). These results indicated that pretreatment with 6.25 μM 3-oxo-C12-HSL significantly inhibits LPS-induced inflammatory responses in RAW264.7 cells.
Therefore, 3-oxo-C12-HSL may inhibit NF-κB phosphorylation and cytokine production in RAW264.7 cells through the activation of UPR, particularly the expression of UPR target genes, such as C/EBP β and CHOP.
Discussion
P. aeruginosa is an opportunistic pathogen that causes persistent infection in patients with cystic fibrosis, mechanical ventilation, HIV infection and various malignancies (25). It was previously confirmed that macrophage dysfunction contributes to the susceptibility to infection and the persistence of P. aeruginosa in experimental models (1,3). 3-Oxo-C12-HSL, a vital quorum-sensing signal molecule produced by P. aeruginosa, regulates the expression of P. aeruginosa extracellular virulence factors and induces the incompetence of macrophages by impairing the regulation of NF-κB function and accelerating macrophage apoptosis (2,5,26,27). Incubation with 12–50 μM 3-oxo-C12-HSL was shown to directly induce the apoptosis of bone marrow-derived macrophages (5). Incubation with 25 μM 3-oxo-C12-HSL may also directly disrupt NF-κB activation (2). Those results indicated that 3-oxo-C12-HSL at concentrations >12 μM may directly inhibit macrophage activation.
UPR induced by a subcytotoxic dose of subtilase cytotoxin was shown to prevent LPS-associated inflammatory responses through a UPR-dependent inhibition of NF-κB activation, without affecting RAW264.7 cell viability (11). A recent investigation also reported that 3-oxo-C12-HSL effectively activated UPR in HAECs (20). Therefore, it is important to determine whether pre-incubation with a subcytotoxic dose of 3-oxo-C12-HSL may induce UPR and inhibit NF-κB activation in RAW264.7 cells, as this may be another mechanism through which 3-oxo-C12-HSL inhibits the function of macrophages and maintains the persistent infection of P. aeruginosa. The present data demonstrated that 6.25 μM 3-oxo-C12-HSL may effectively induce the expression of UPR-responsive genes and proteins without affecting cell viability. UPR induced by 3-oxo-C12-HSL was significantly inversely correlated with the LPS-induced inflammatory responses in RAW264.7 cells.
3-Oxo-C12-HSL at a concentration range of 10–100 μM was shown to induce cell death in bone marrow-derived macrophage, breast cancer, mast and epithelial cell lines (5,21,28). A concentration of 50 μM 3-oxo-C12-HSL was also found to induce the expression of C/EBP β and CHOP through UPR (20). In the present study, we observed that incubation with 12.5–100 μM 3-oxo-C12-HSL induced apoptosis and death in RAW264.7 cells. Moreover, 6.25 μM 3-oxo-C12-HSL did not affect cell viability but effectively activated UPR gene expression, increasing the mRNA expression levels of C/EBP β and CHOP. Thus, we selected 6.25 μM as the proper concentration to stimulate RAW264.7 cells. C/EBP β and CHOP protein levels were significantly elevated at 9 h following pretreatment with 3-oxo-C12-HSL. The levels of these proteins were higher at the time point of 12 h compared to those at 9 h. However, there was no significant difference between the two groups. Therefore, 9 h was selected as the appropriate pretreatment time in this experiment.
UPR was first confirmed to abrogate the induction of MCP-1 and inducible nitric oxide synthase by TNF-α (8). Other studies reported that UPR activation also suppresses cytokine or LPS-triggered activation of NF-κB and the subsequent production of proinflammatory cytokines (9–12). In a previous study, it was proven that the C/EBP β gene has an unfolded protein response element (UPRE) at its 3′-end. This UPRE was shown to be responsible for the upregulation of C/EBP β by ER stress (29). Another study confirmed that C/EBPs interact with the Rel domain of NF-κB via its bZIP domain (30). Prosch et al (31) further indicated that C/EBP α/β interacts with the NF-κB p65 subunit and inhibits the activation of NF-κB. The upregulated C/EBP β induced by UPR was confirmed to inhibit NF-κB phosphorylation (12,15). Although the exact underlying mechanism remains unclear, it is hypothesized that CHOP expression is upregulated by UPR and consequently reduces the phosphorylation of NF-κB and inflammatory cytokine production (16,18). In the present study, we demonstrated that the expression of C/EBP β and CHOP is significantly inversely correlated with the phosphorylation of NF-κB in RAW264.7 cells. In addition, pretreatment with 3-oxo-C12-HSL represses NF-κB-mediated MCP-1 and TNF-α production in these cells. All these findings indicated that C/EBP β and CHOP, which are upregulated by 3-oxo-C12-HSL-mediated UPR, inhibit LPS-induced NF-κB activation and inflammatory cytokine production.
Three major branches of UPR were generally triggered by ER stress to cause downstream transcriptional events: the double-stranded RNA-activated protein kinase-like ER kinase pathway, the inositol-requiring enzyme 1 (IRE1) pathway and the activating transcription factor 6 pathway (6). Although the role of stress response pathways in 3-oxo-C12-HSL-mediated cytotoxicity has not been fully elucidated, it was recently reported that 3-oxo-C12-HSL does not activate the splicing X-box binding protein 1 (XBP-1) function of IRE1 (32). However, another study indicated that the splicing XBP-1 function of IRE1 is not associated with the induction of C/EBP β (12). Thus, 3-oxo-C12-HSL may activate the UPR of ER stress through other branches in order to inhibit NF-κB activation.
All three branches of UPR are involved in the regulation of inflammatroy responses and different branches of UPR play major roles in different cell types (8–11). Therefore, the exact role of each UPR branch on 3-oxo-C12-HSL-induced UPR-mediated inhibition of NF-κB requires further investigation.
In summary, this preliminary study indicated that 3-oxo-C12-HSL-mediated UPR in macrophages may inhibit LPS-induced inflammatory responses by disrupting NF-κB activation. Although 3-oxo-C12-HSL was originally identified as a bacterial autoinducer for controlling virulence gene expression in P. aeruginosa infection (27), it may also prevent LPS-mediated inflammatory responses by UPR, a mechanism possibly used by P. aeruginosa to evade the host immune system and maintain persistent infection. Further investigations are required to determine the exact role of C/EBP β and CHOP or other branches of UPR in 3-oxo-C12-HSL-mediated NF-κB inhibition through loss-of-function studies.
Acknowledgements
This study was partly supported by the Clinical Medicine Science Foundation of Health and Family Planning Commission of Wuhan, China (grant no. WX13B14).
References
- 1.Glasser SW, Senft AP, Whitsett JA, et al. Macrophage dysfunction and susceptibility to pulmonary Pseudomonas aeruginosa infection in surfactant protein C-deficient mice. J Immunol. 2008;181:621–628. doi: 10.4049/jimmunol.181.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kravchenko VV, Kaufmann GF, Mathison JC, et al. Modulation of gene expression via disruption of NF-κB signaling by a bacterial small molecule. Science. 2008;321:259–263. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- 3.McClure CD, Schiller NL. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr Microbiol. 1996;33:109–117. doi: 10.1007/s002849900084. [DOI] [PubMed] [Google Scholar]
- 4.Telford G, Wheeler D, Williams P, et al. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateda K, Ishii Y, Horikawa M, et al. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 7.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano Y, Hiramatsu N, Okamura M, et al. Suppression of cytokine response by GATA inhibitor K-7174 via unfolded protein response. Biochem Biophys Res Commun. 2007;360:470–475. doi: 10.1016/j.bbrc.2007.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa K, Hiramatsu N, Okamura M, et al. Blunted activation of NF-κB and NF-κB-dependent gene expression by geranylgeranylacetone: involvement of unfolded protein response. Biochem Biophys Res Commun. 2008;365:47–53. doi: 10.1016/j.bbrc.2007.10.115. [DOI] [PubMed] [Google Scholar]
- 10.Okamura M, Takano Y, Hiramatsu N, et al. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am J Physiol Renal Physiol. 2008;295:F1495–F1503. doi: 10.1152/ajprenal.00602.2007. [DOI] [PubMed] [Google Scholar]
- 11.Harama D, Koyama K, Mukai M, et al. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J Immunol. 2009;183:1368–1374. doi: 10.4049/jimmunol.0804066. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K, Nakajima S, Hiramatsu N, et al. ER stress depresses NF-κB activation in mesangial cells through preferential induction of C/EBP β. J Am Soc Nephrol. 2010;21:73–81. doi: 10.1681/ASN.2009040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 14.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima S, Kato H, Takahashi S, Johno H, Kitamura M. Inhibition of NF-κB by MG132 through ER stress-mediated induction of LAP and LIP. FEBS Lett. 2011;585:2249–2254. doi: 10.1016/j.febslet.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima S, Hiramatsu N, Hayakawa K, et al. Selective abrogation of BiP/GRP78 blunts activation of NF-κB through the ATF6 branch of the UPR: involvement of C/EBPβ and mTOR-dependent dephosphorylation of Akt. Mol Cell Biol. 2011;31:1710–1718. doi: 10.1128/MCB.00939-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 18.Jeong M, Cho J, Cho WS, Shin GC, Lee K. The glucosamine-mediated induction of CHOP reduces the expression of inflammatory cytokines by modulating JNK and NF-κB in LPS-stimulated RAW264.7 cells. Genes & Genomics. 2009;31:251–260. [Google Scholar]
- 19.Du S, Hiramatsu N, Hayakawa K, et al. Suppression of NF-κB by cyclosporin A and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J Immunol. 2009;182:7201–7211. doi: 10.4049/jimmunol.0801772. [DOI] [PubMed] [Google Scholar]
- 20.Kim JB, Xia YR, Romanoski CE, et al. Paraoxonase-2 modulates stress response of endothelial cells to oxidized phospholipids and a bacterial quorum-sensing molecule. Arterioscler Thromb Vasc Biol. 2011;31:2624–2633. doi: 10.1161/ATVBAHA.111.232827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Wang L, Ye L, et al. Influence of Pseudomonas aeruginosa quorum sensing signal molecule N-(3-oxododecanoyl) homoserine lactone on mast cells. Med Microbiol Immunol. 2009;198:113–121. doi: 10.1007/s00430-009-0111-z. [DOI] [PubMed] [Google Scholar]
- 22.Gong F, Zhan W, Wang L, Song Y, Xing M, Song J. Role of MexA-MexB-OprM efflux pump system in chronic Pseudomonas Aeruginosa pulmonary infection in mice. J Huazhong Univ Sci Technolog Med Sci. 2012;32:546–551. doi: 10.1007/s11596-012-0094-7. [DOI] [PubMed] [Google Scholar]
- 23.Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor α signaling in septic shock. Shock. 2005;24:206–209. doi: 10.1097/01.shk.0000180074.69143.77. [DOI] [PubMed] [Google Scholar]
- 24.Ueda A, Okuda K, Ohno S, et al. NF-κB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 25.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson JP, Gray KM, Passador L, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzer C, Fu Z, Patanwala M, et al. Pseudomonas aeruginosa biofilm-associated homoserine lactone C12 rapidly activates apoptosis in airway epithelia. Cell Microbiol. 2012;14:698–709. doi: 10.1111/j.1462-5822.2012.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Dudenhausen EE, Pan YX, Zhong C, Kilberg MS. Human CCAAT/enhancer-binding protein β gene expression is activated by endoplasmic reticulum stress through an unfolded protein response element downstream of the protein coding sequence. J Biol Chem. 2004;279:27948–27956. doi: 10.1074/jbc.M313920200. [DOI] [PubMed] [Google Scholar]
- 30.Stein B, Cogswell PC, Baldwin AS., Jr Functional and physical associations between NF-κB and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993;13:3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prosch S, Heine AK, Volk HD, Kruger DH. CCAAT/enhancer-binding proteins α and β negatively influence the capacity of tumor necrosis factor α to up-regulate the human cytomegalovirus IE1/2 enhancer/promoter by nuclear factor κB during monocyte differentiation. J Biol Chem. 2001;276:40712–40720. doi: 10.1074/jbc.M009815200. [DOI] [PubMed] [Google Scholar]
- 32.Valentine CD, Anderson MO, Papa FR, Haggie PM. X-box binding protein 1 (XBP1s) is a critical determinant of Pseudomonas aeruginosa homoserine lactone-mediated apoptosis. PLoS Pathog. 2013;9:e1003576. doi: 10.1371/journal.ppat.1003576. [DOI] [PMC free article] [PubMed] [Google Scholar]