Abstract
Peptidyl-prolylcis-trans isomerase NIMA-interacting 1 (PIN1) is a critical catalyst involved in multiple oncogenic signaling pathways. The PIN1 promoter −667T>C (rs2233679) polymorphism plays a role in cancer risk. The association between PIN1 (−667T>C) polymorphism and cancer risk has been previously investigated. However, the available results are inconclusive. To derive a more precise estimation, a meta-analysis of seven published case-control studies including 4,524 cases with different tumor types and 4,561 controls was performed. Published literature from PubMed and EMBASE was retrieved. Crude odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to evaluate the strength of the association. Overall, the results did not suggest any associations between the PIN1 promoter (−667T>C) polymorphism and cancer susceptibility (OR=1.04, 95% CI: 0.91–1.18 for CC vs. TT; OR=0.98, 95% CI: 0.89–1.09 for TC vs. TT; OR=1.00, 95% CI: 0.91–1.10 for TC/CC vs. TT; OR=1.07, 95% CI: 0.97–1.18 for CC vs. TC/TT). Further stratified analysis by cancer type, ethnicity and sample size did not reveal any significant associations in the genetic models. The results of the present study demonstrate that the PIN1 promoter (−667T>C; rs2233679) polymorphism is not associated with cancer susceptibility.
Keywords: peptidyl-prolylcis-trans isomerase NIMA-interacting 1, polymorphism, cancer, meta-analysis
Introduction
Pro-directed phosphorylation, also known as phosphorylation of proteins on serine or threonine residues, is a pivotal intracellular signaling mechanism in regulating diverse cellular processes, such as cell cycle progression, transcriptional regulation, RNA processing and cell proliferation and differentiation (1,2). It has been demonstrated that the deregulation of Pro-directed phosphorylation is a prevalent and specific event in various types of cancer, resulting in cell transformation and oncogenesis (1).
Peptidyl-prolylcis-trans isomerase NIMA-interacting 1 (PIN1) specifically isomerizes the conformation of Pro-directed phosphorylation sites, revealing a novel post-phosphorylation regulatory mechanism (3,4). PIN1 has a high specificity to substrate with Ser/Thr-Pro (Proline) motifs and changes the conformation of phosphoproteins by recognizing and binding to specific phospho-Ser/Thr-Pro motifs (5). PIN1 substrates containing phosphorylated Ser/Thr-Pro motifs include many important cell cycle regulators as well as oncogenic and tumor suppressor proteins, such as cyclin D1 (6), p53 (7), Cdc25 (8), myc (9), c-Jun (7), β-catenin (10), GSK-3 β (9) and Bcl-2 (11). Therefore, by targeting these important substrates which contain phosphorylated Ser/Thr-Pro motifs, PIN1-induced conformational changes may function as a critical catalyst that potentiates multiple oncogenic signaling pathways during cancer development (12). It has been reported that PIN1 is aberrantly overexpressed in many types of cancer, including prostate cancer (13), lung cancer (13), esophageal squamous cell carcinoma (6) and breast cancer (7,10). By contrast, inhibition of PIN1 in cancer cells triggers apoptosis or suppresses the transformed phenotype (14,15). These results indicated that the PIN1 gene may play an oncogenic role in tumorigenesis.
Human PIN1 gene, located at chromosome 19p13, contains 4 exons within a 14-kb region, encodes a 163-amino acid protein and has a promoter region of 1.5 kb (16). Several putative functional single-nucleotide polymorphisms (SNPs) have been identified in the coding and promoter regions of PIN1, the most common one being rs2233679T>C: c.−667T>C in the promoter. The association between PIN1 promoter (−667T>C) polymorphism and risk of cancer of various organs, including liver cancer (17), lung cancer (16), breast cancer (18,19), squamous cell carcinoma of the head and neck (20), nasopharyngeal cancer (21) and esophageal cancer (22) has been recently investigated. However, the results of those studies remain controversial. In consideration of the extensive role of PIN1 in the carcinogenic process, we carried out a meta-analysis on all eligible case-control studies to estimate the overall cancer risk associated with PIN1 promoter (−667T>C) polymorphism and to quantify the potential between-study heterogeneity.
Materials and methods
Identification and eligibility of relevant studies
A search was conducted on the PubMed and EMBASE databases using the search terms ‘PIN1’, ‘polymorphism’ and ‘cancer’ (last search update was 8 May 2013) in order to include all the case-control studies concerning the association between PIN1 (−667T>C) polymorphism and cancer risk. The search was limited to English language studies. Additional studies were identified by manual search of the references of original studies. When more than one study of the same population was included in several publications, the most recent studies with the largest sample size were selected. Studies included in our meta-analysis were required to meet the following criteria: i) evaluation of the PIN1 (−667T>C) polymorphism and cancer risk; ii) use of a case-control design; and iii) containing available genotype frequency.
Data extraction
Two authors extracted data independently according to the inclusion criteria and reached a consensus on all the items. The following characteristics were collected from each study: the first author’s last name, year of publication, cancer type, country of origin, ethnicity, source of control groups (population- or hospital-based controls), genotyping method, number of cases and controls and P-value for Hardy-Weinberg equilibrium (HWE) in the controls (Table I). Different ethnic descents were categorized as European and Asian. For studies including subjects of different ethnic groups, data were extracted separately for each ethnic group whenever possible. Additionally, studies investigating more than one sample were considered as individual data sets.
Table I.
Characteristics of studies included in the meta-analysis.
| Authors (year) | Tumor type | Country | Ethnicity | Source of controls | Genotyping method | HWE | Sample size (case/control) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Segat et al (2007) | Liver cancer | Italy | European | Hospital | PCR-RFLP | NA | 228/250 | (17) |
| Lu et al (2011)a | Lung cancer | China | Asian | Hospital | PCR-RFLP | >0.05 | 1,056/1,056 | (16) |
| Lu et al (2011)b | Lung cancer | China | Asian | Hospital | PCR-RFLP | >0.05 | 503/623 | (16) |
| Naidu et al (2011) | Breast cancer | Malaysia | Asian | Hospital | PCR-RFLP | 0.986 | 107/80 | (19) |
| Breast cancer | China | Asian | Hospital | PCR-RFLP | 0.856 | 219/111 | ||
| Breast cancer | India | Asian | Hospital | PCR-RFLP | 0.981 | 61/61 | ||
| Han et al (2010) | Breast cancer | USA | European | Hospital | PCR-RFLP | 0.229 | 467/488 | (18) |
| Lu et al (2009) | SCCHN | USA | European | Hospital | PCR-RFLP | 0.080 | 1,006/1,007 | (20) |
| Lu et al (2013) | Nasopharyngeal cancer | China | Asian | Hospital | PCR-RFLP | 0.056 | 178/156 | (21) |
| You et al (2013) | Esophageal cancer | China | Asian | Hospital | PCR-RFLP | 0.578 | 699/729 | (22) |
These samples were collected between March 2007 and March 2009 in Southern China.
These samples were collected between March 2008 and May 2010 in Eastern China.
HWE, Hardy-Weinberg equilibrium; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; NA, not available; SCCHN, squamous cell carcinoma of the head and neck.
Statistical analysis
For the control group of each study, the allelic frequency was calculated and HWE was assessed using the Chi-square goodness-of-fit test and P<0.05 was considered representative of a departure from HWE. The strength of the association between the PIN1 (−667T>C) polymorphism and cancer risk was measured by odds ratios (ORs) with 95% confidence intervals (CIs). We first estimated the risks of the TC and CC genotypes on various cancer types, compared with the wild-type TT homozygote and then evaluated the risks of TC/CC vs. TT and CC vs. TC/TT on these cancer types, assuming dominant and recessive effects of the variant C allele, respectively. Stratified analyses were also performed by cancer type (if one cancer type occurred in only one individual study, it was combined into the ‘other cancers’ group), ethnicities and sample size (subjects >500 in both cases and controls). In consideration of the possibility of heterogeneity across the studies, a statistical test for heterogeneity was performed based on the Q statistic (23). If P>0.1 of the Q test, indicating a lack of heterogeneity among studies, the summary estimates of ORs of each study was calculated by the fixed-effects model (the Mantel-Haenszel method) (24). Otherwise, the random-effects model (the DerSimonian and Laird method) (25) was used. Furthermore, the meta-regression model was used to explore the possible source of heterogeneity among different types of studies (26). Sensitivity analyses were performed to assess the stability of the results, i.e., a single study in the meta-analysis was deleted each time to reflect the effect of the individual data set to the pooled OR. Publication bias was evaluated using the Begg’s funnel plot and Egger’s test (27). Analyses were performed with Stata software (version 11.0; StataCorp LP, College Station, TX, USA), and all tests were two-sided.
Results
Characteristics of studies
Seven articles in English on PIN1 (−667T>C) polymorphism and cancer risk were available for this analysis. One article investigated two individual samples collected at different time periods. Another article investigated three individual samples obtained from different countries. Each of these articles was counted as individual studies. Ten case-control studies met our inclusion criteria, including 4,524 cases and 4,561 controls. The characteristics of the selected studies are shown in Table I. The study comprised case-control studies only, including four breast cancer studies, two lung cancer, with the remaining studies being classified as the ‘other cancer’ group. Three of the studies obtained comprised individuals of European descent and seven studies of Asian descent. Cancers were confirmed histologically or pathologically in the majority of studies. Genotyping methods used were polymerase chain reaction-restriction fragment length polymorphism. Genotype distribution in the controls of all the studies was consistent with HWE, with the exception of one study (17) (without data for all three genotypes).
Quantitative synthesis
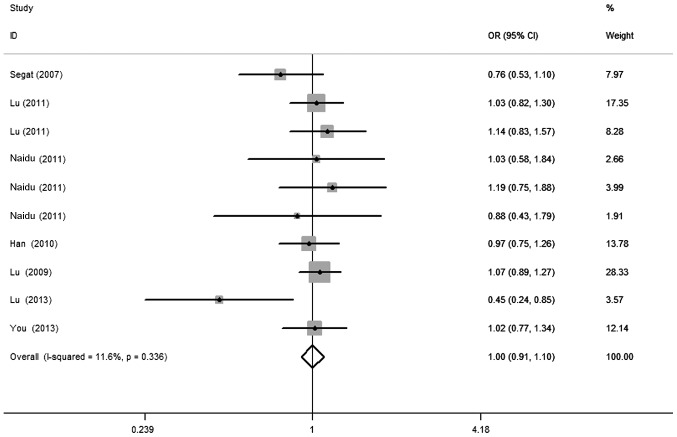
There was a wide variation of the C allele frequencies across different ethnicities. The C allele frequencies in the European and Asian populations were 34.7 and 47.3% on average, respectively (Fig. 1). As shown in Table II, no significant associations between the PIN1 promoter (−667T>C) polymorphism and cancer risk were observed in any of the genetic models [OR=1.04, 95% CI: 0.91–1.18 for CC vs. TT; OR=0.98, 95% CI: 0.89–1.09 for TC vs. TT; OR=1.00, 95% CI: 0.91–1.10 for TC/CC vs. TT (Fig. 2); OR=1.07, 95% CI: 0.97–1.18 for CC vs. TC/TT]. Similarly, in further stratified analysis by cancer type, ethnicity and sample size, no significant associations were observed in any of the genetic models (Table II).
Figure 1.
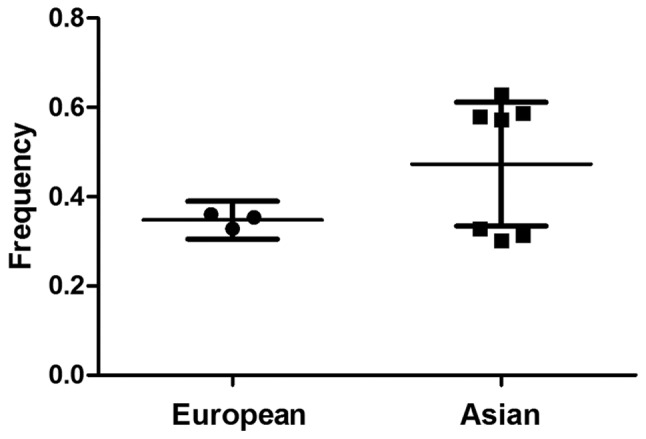
Peptidyl-prolylcis-trans isomerase NIMA-interacting 1 C allele frequency among controls stratified by ethnicity.
Table II.
Summary OR of the PIN1 (−667T>C) polymorphism on cancer risk.
| Variables | na | Cases/controls | CC vs. TT OR (95% CI)b |
Pc | TC vs. TT OR (95% CI)b |
Pc | TC/CC vs. TT (dominant) OR (95% CI)b |
Pc | CC vs. TC/TT (recessive) OR (95% CI)b |
Pc |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 10 | 4,524/4,561 | 1.04 (0.91–1.18) | 0.484 | 0.98 (0.89–1.09) | 0.404 | 1.00 (0.91–1.10) | 0.336 | 1.07 (0.97–1.18) | 0.938 |
| Cancer types | ||||||||||
| Breast cancer | 4 | 854/740 | 0.97 (0.70–1.35) | 0.995 | 1.02 (0.82–1.26) | 0.783 | 1.01 (0.82–1.23) | 0.865 | 0.97 (0.71–1.32) | 0.979 |
| Lung cancer | 2 | 1,559/1,679 | 1.11 (0.91–1.37) | 0.608 | 1.04 (0.85–1.26) | 0.701 | 1.07 (0.89–1.29) | 0.631 | 1.09 (0.94–1.25) | 0.734 |
| Other cancers | 4 | 2,111/2,142 | 0.99 (0.82–1.21) | 0.066 | 0.95 (0.82–1.09) | 0.057 | 0.97 (0.84–1.10) | 0.037 | 1.08 (0.92–1.26) | 0.428 |
| Ethnicities | ||||||||||
| Europen | 3 | 1,701/1,745 | 1.00 (0.80–1.26) | 0.273 | 0.99 (0.86–1.14) | 0.428 | 0.99 (0.87–1.13) | 0.263 | 1.01 (0.82–1.26) | 0.445 |
| Asian | 7 | 2,822/2,816 | 1.05 (0.90–1.23) | 0.446 | 0.90 (0.76–1.08) | 0.181 | 0.89 (0.75–1.06) | 0.197 | 0.91 (0.72–1.15) | 0.969 |
| Sample sized | ||||||||||
| ≤500 | 6 | 1,260/1,146 | 0.81 (0.62–1.05) | 0.571 | 0.90 (0.76–1.08) | 0.181 | 0.89 (0.75–1.06) | 0.197 | 0.91 (0.72–1.15) | 0.969 |
| >500 | 4 | 3,264/3,415 | 1.12 (0.97–1.30) | 0.965 | 1.03 (0.91–1.16) | 0.915 | 1.06 (0.94–1.19) | 0.955 | 1.11 (0.99–1.24) | 0.933 |
Number of comparisons.
Random-effects model was used when P=0.05 for heterogeneity test; otherwise, fixed-effects model was used.
P-value of Q-test for heterogeneity test.
Both cases and controls.
OR, odds ratio; PIN1, peptidyl-prolylcis-trans isomerase NIMA-interacting 1; CI, confidence interval.
Figure 2.
Forest plot of cancer risk associated with the peptidyl-prolylcis-trans isomerase NIMA-interacting 1 promoter (−667T>C) polymorphism for TC/CC vs. TT. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the study-specific weight (inverse of the variance). The diamond represents the summary OR and 95% CI. OR, odds ratio; CI, confidence interval; I2, measure to quantify the degree of heterogeneity in the meta-analysis.
Test of heterogeneity, publication bias and sensitivity analyses
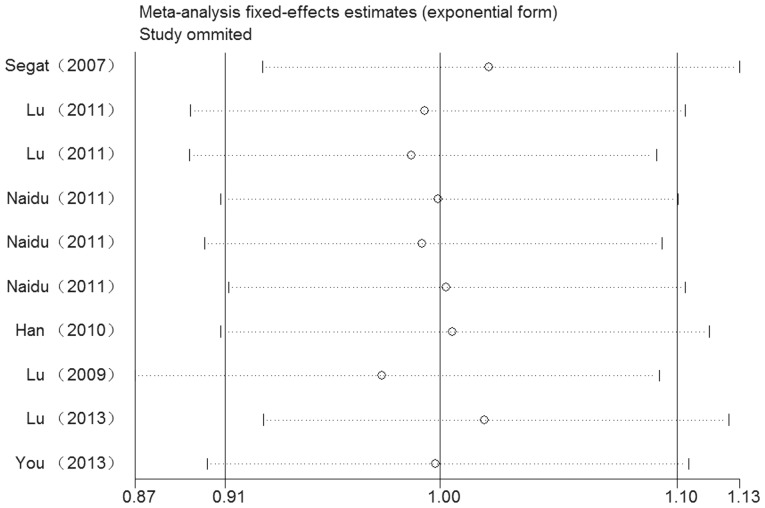
No significant heterogeneity between the studies was observed in the overall comparisons (Table II). Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the studies. The shapes of the funnel plots did not show any evidence of obvious asymmetry in any of the compared models. The Egger’s test was used to provide statistical evidence of funnel plot symmetry. As expected, the results did not reveal any evidence of publication bias (t=−1.57, P=0.154 for TC/CC vs. TT; Fig. 3). The effect of each study on the pooled OR was examined by repeating the leave-one-out sensitivity analysis and the sensitivity analysis proved that our results were reliable and robust (Fig. 4). Furthermore, following exclusion of the study by Segat et al (17) for which data on HWE were not available, no change in the estimated pool OR was evident.
Figure 3.
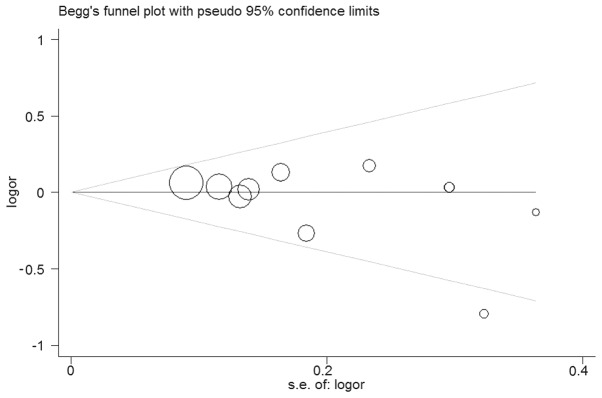
Begg’s funnel plot of publication bias test for TC/CC vs. TT. Each point represents a separate study for the indicated association. Log(OR), natural logarithm of odds ratio; horizontal line, mean effect size.
Figure 4.
Sensitivity analysis of cancer risk associated with the peptidyl-prolylcis-trans isomerase NIMA-interacting 1 promoter (−667T>C) polymorphism for TC/CC vs. TT. The figure shows the influence of individual studies on the summary odds ratio.
Discussion
PIN1 is not an oncogene itself, but it can serve as an indispensable translator and amplifier of oncogenic signal transduction. PIN1 specifically recognizes phospho-Ser/Thr-Pro motifs and regulates the conformation of the Pro-directed phosphorylation site which potentates multiple oncogenic signaling pathways during carcinogenesis (12). PIN1 overexpression is a prevalent and specific event in human cancers (28). Consequently, results of previous studies have demonstrated that a high expression of PIN1 is correlated with poor prognosis in patients with different cancer types, such as prostate cancer (29) and esophageal squamous cell carcinoma (30). Thus, it is biologically reasonable that the functional polymorphisms of PIN1 may play an important role in the aetiology of cancer. Thus, since the identification of a functional polymorphism known as −667T>C (rs2233679) in PIN1 promoter, accumulating studies have evaluated the association between the PIN1 (−667T>C) polymorphism and cancer risk. Findings of an Italian study showed that the −667T>C SNP over-represented the T allele in the hepatocellular carcinoma cases (17). Recently, Lu et al (21) showed that the PIN1 promoter (−667T>C) polymorphism was associated with a decreased risk of nasopharyngeal carcinoma (NPC) in Chinese populations. However, it has been indicated that the variant −667C allele was not associated with risk of cancer (16,18–20,22). To resolve this conflict, we performed a meta-analysis of seven published case-control studies including 4,524 cases with different tumor types and 4,561 controls to derive a more precise estimation of the association. To the best of our knowledge, this is the first meta-analysis focusing on a comprehensive assessment of the relationship between the PIN1 polymorphism (−667T>C) and cancer risk. Results of this study suggest that the PIN1 −667T>C (rs2233679) polymorphism is not associated with risk of cancer in all the studied populations.
In the stratification analysis of cancer type, no significant associations were observed in breast, lung and other cancers in any of the genetic models, suggesting that tumor origin did not play a clear role in the PIN1 promoter (−667T>C) polymorphism associated with risk of cancer. A similar result was observed in the subgroup analysis by sample size. Ethnicity was an important influence factor for the development of cancer. As shown in Fig. 1, the frequency of −667C allele in the Asian controls, was significantly higher than that in the European controls (P<0.05). Beyond our expectation, in this study, there were no significant associations found in Europeans and Asians in any of the genetic models, suggesting that ethnic differences in genetic backgrounds and/or environmental and social factors did not affect the association between PIN1 promoter (−667T>C) polymorphism and cancer risk. These insignificant results may be due to the limited number of studies with available data on these characteristics, which had insufficient statistical power to detect a slight effect or may have generated a fluctuated risk estimate.
In interpreting the current results, some limitations of the meta-analysis should be considered. First, our result was based on unadjusted estimates, while a more precise analysis should be conducted if more detailed individual data were available, which would allow for an adjusted estimate by other factors such as age and gender. Lack of information relevant to the data analysis may cause serious confounding bias. Second, the lack of original data of the reviewed studies limited our evaluation of potential interactions, as the interactions among gene-gene, gene-environment and even different polymorphic loci of the same gene may modulate cancer risk. Third, in this meta-analysis, the studies were all based on hospital and only comprised European and Asian populations. Thus, validations with larger population-based studies in different ethnic groups are necessary. Fourth, the number of published studies was not sufficiently large for a comprehensive analysis, particularly for the single type of cancer. However, there were benefits to our meta-analysis that should be considered. First, a substantial number of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis. Second, the quality of case-control studies included in current meta-analysis was satisfactory and did not detect any publication bias suggesting that the whole pooled result should be unbiased.
In conclusion, our meta-analysis suggests that the PIN1 promoter (−667T>C; rs2233679) polymorphism is not associated with risk of cancer, indicating that this polymorphism is not a biomarker for susceptibility to cancer. However, large-scale studies in different ethnic groups using standardized unbiased methods, enrolling precisely defined cancer patients and well-matched controls, with more detailed individual data are needed to validate our findings. Investigations of the gene-environmental interaction may lead to an improved, more comprehensive understanding of the roles of PIN1 polymorphisms in the aetiology of cancer.
Acknowledgements
This study was supported by grants from the Anhui Provincial Health Administration (no. 09A070).
References
- 1.Lu KP. Pinning down cell signaling, cancer and Alzheimer’s disease. Trends Biochem Sci. 2004;29:200–209. doi: 10.1016/j.tibs.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lu KP. Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell. 2003;4:175–180. doi: 10.1016/s1535-6108(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 3.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 4.Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 5.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita H, Mori S, Motegi K, Fukumoto M, Uchida T. Pin1 is overexpressed in oral squamous cell carcinoma and its levels correlate with cyclin D1 overexpression. Oncol Rep. 2003;10:455–461. [PubMed] [Google Scholar]
- 7.Wulf GM, Ryo A, Wulf GG, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 2001;20:3459–3472. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou XZ, Kops O, Werner A, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- 9.Arnold HK, Zhang X, Daniel CJ, et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28:500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- 11.Pathan N, Aime-Sempe C, Kitada S, Basu A, Haldar S, Reed JC. Microtubule-targeting drugs induce bcl-2 phosphorylation and association with Pin1. Neoplasia. 2001;3:550–559. doi: 10.1038/sj.neo.7900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryo A, Liou YC, Lu KP, Wulf G. Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J Cell Sci. 2003;116:773–783. doi: 10.1242/jcs.00276. [DOI] [PubMed] [Google Scholar]
- 13.Ayala G, Wang D, Wulf G, et al. The prolyl isomerase Pin1 is a novel prognostic marker in human prostate cancer. Cancer Res. 2003;63:6244–6251. [PubMed] [Google Scholar]
- 14.Ryo A, Liou YC, Wulf G, Nakamura M, Lee SW, Lu KP. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol Cell Biol. 2002;22:5281–5295. doi: 10.1128/MCB.22.15.5281-5295.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryo A, Uemura H, Ishiguro H, et al. Stable suppression of tumorigenicity by Pin1-targeted RNA interference in prostate cancer. Clin Cancer Res. 2005;11:7523–7531. doi: 10.1158/1078-0432.CCR-05-0457. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Yang L, Zhao H, et al. The polymorphism and haplotypes of PIN1 gene are associated with the risk of lung cancer in Southern and Eastern Chinese populations. Hum Mutat. 2011;32:1299–1308. doi: 10.1002/humu.21574. [DOI] [PubMed] [Google Scholar]
- 17.Segat L, Milanese M, Crovella S. Pin1 promoter polymorphisms in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2618–2620. doi: 10.1053/j.gastro.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Han CH, Lu J, Wei Q, et al. The functional promoter polymorphism (−842G>C) in the PIN1 gene is associated with decreased risk of breast cancer in non-Hispanic white women 55 years and younger. Breast Cancer Res Treat. 2010;122:243–249. doi: 10.1007/s10549-009-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidu R, Har YC, Taib NA. Analysis of peptidyl-propyl-cis/trans isomerase 1 (PIN1) gene −842(G > C) and −667(T > C) polymorphic variants in relation to breast cancer risk and clinico-pathological parameters. Scand J Clin Lab Invest. 2011;71:500–506. doi: 10.3109/00365513.2011.590223. [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Hu Z, Wei S, et al. A novel functional variant (−842G>C) in the PIN1 promoter contributes to decreased risk of squamous cell carcinoma of the head and neck by diminishing the promoter activity. Carcinogenesis. 2009;30:1717–1721. doi: 10.1093/carcin/bgp171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Huang GL, Pu XX, et al. Association between PIN1 promoter polymorphisms and risk of nasopharyngeal carcinoma. Mol Biol Rep. 2013;40:3777–3782. doi: 10.1007/s11033-012-2454-6. [DOI] [PubMed] [Google Scholar]
- 22.You Y, Deng J, Zheng J, et al. Functional polymorphisms in PIN1 promoter and esophageal carcinoma susceptibility in Chinese population. Mol Biol Rep. 2013;40:829–838. doi: 10.1007/s11033-012-2122-x. [DOI] [PubMed] [Google Scholar]
- 23.Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil. 2006;87:875. doi: 10.1016/j.apmr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol. 2004;164:1727–1737. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arboleda MJ, Lyons JF, Kabbinavar FF, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 30.Fukuchi M, Fukai Y, Kimura H, et al. Prolyl isomerase Pin1 expression predicts prognosis in patients with esophageal squamous cell carcinoma and correlates with cyclinD1 expression. Int J Oncol. 2006;29:329–334. [PubMed] [Google Scholar]