Abstract
Squamous cell carcinoma of the head and neck (HNSCC) is the sixth most common type of cancer, affecting ~500,000 individuals worldwide annually. Collagen is the major constituent of the extracellular matrix component in tumors and plays a crucial role in tumor development. The aim of this study was to determine the mRNA expression of type XXI, XXII, XXIII and XXIV α1 collagen (COL21A1, COL22A1, COL23A1 and COL24A1, respectively) in head and neck squamous cell carcinoma (HNSCC) and investigate its correlation with disease progression. This study investigated the mRNA expression levels of COL21A1, COL22A1, COL23A1 and COL24A1 in 70 HNSCC primary samples and 44 matched pairs of tumor and adjacent normal mucosal tissues using quantitative polymerase chain reaction (qPCR). Expression data were compared to the clinicopathological variables in order to determine the correlation between expression and disease progression. Our results demonstrated that the mRNA levels of COL22A1 and COL24A1 were significantly higher in HNSCC tissues compared to those in the corresponding normal tissues from the same individuals (n=44; P<0.001 and P=0.019, respectively). The COL22A1 mRNA levels were found to be significantly associated with lymph node metastasis (P=0.018) and pathological stage (P=0.024), whereas the COL24A1 mRNA levels were significantly associated with tumor size (P=0.045). The high expression levels of COL22A1 and COL24A1 mRNA were statistically correlated with a decrease in disease-free survival (DFS) (log-rank test, P<0.001). The results of the multivariate logistic regression analysis revealed that high expression levels of the COL22A1 and COL24A1 gene pair were associated with a high odds ratio for recurrence of 14.62 (95% confidence interval: 2.77–77.26; P=0.002). Therefore, the upregulation of COL22A1 and COL24A1 mRNA may play a critical role in the progression of HNSCC and provide useful information as a prognostic predictor for HNSCC patients.
Keywords: type XXII α1 collagen, type XXIV α1 collagen, quantitative polymerase chain reaction, mRNA expression, head and neck squamous cell carcinoma
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) is the sixth most common type of cancer, affecting ~500,000 individuals annually worldwide (1). Various risk factors are associated with the development of HNSCC, including gender, tobacco smoking and alcohol consumption (2). The prognosis for HNSCC patients remains poor, despite the significant technical advances in surgical treatment, radiotherapy and chemotherapy (3).
Collagens are a large family of at least 28 extracellular matrix proteins that play vital structural and physiological roles in maintaining the integrity and contributing to the homeostasis of the human body (4). Collagen is the major constituent of the extracellular matrix component in tumors and several types of collagen have been identified in cancer tissues (5). Recently, numerous studies investigated the role of collagens that are secreted into the tumor extracellular matrix; however, the number of available studies on transmembrane collagens, including type X, XIII, XVII and XVIII collagen, and their role in promoting tumor growth, invasion and metastasis, is limited (6). Despite the accumulating evidence demonstrating the role of major collagen genes in cancer, the physiological effects of minor collagen genes, such as COL21A1, COL22A1, COL23A1 and COL24A1, have not been fully elucidated.
The COL21A1 gene is localized to 6p11–12 and COL21A1 mRNA is present in a number of tissues, including the heart, stomach, kidney, skeletal muscle and placenta, whereas type XXI collagen is an extracellular matrix component of blood vessel walls (7). The COL21A1 gene encodes the α chain of collagen XXI, which is a member of the fibril-associated collagens with interrupted triple helices (FACIT) collagen family. Similar to other members of the FACIT collagen family, collagen XXI, which localizes to tissues containing type I collagen, may play a role in maintaining the integrity of the extracellular matrix (8).
The COL22A1 gene positioned on human chromosome 8q24.2 encodes a collagen that structurally belongs to the FACIT protein family. Collagen XXII is a novel gene product, which is a specific extracellular matrix protein present only at the tissue junctions of muscles, tendons, the heart, articular cartilage and skin. Collagen XXII is deposited in the basement membrane zone of the myotendinous junction (9).
COL23A1, as a transmembrane collagen, belongs to the subfamily of non-fibrillar collagens that contain a single-pass hydrophobic transmembrane domain. The collagen XXIII protein is detected at very low levels in benign prostatic tissues, whereas significantly increased levels have been detected in prostatic cancer tissues (10). Collagen XXIII is also expressed in a high proportion of tissue and urine samples from patients with non-small-cell lung cancer (11). A correlation between the upregulation of COL23A1 and tumor progression was previously described for several types of cancer (10,11).
COL24A1, which is predominantly expressed in bone tissue, is a poorly characterized member of the fibril-forming family of collagen molecules (12). This fibrillar collagen may be involved in the regulation of vital physiological processes in bone and cartilage. The expression of collagen XXIV is detected at lower levels in non-skeletal tissues, such as the brain and the eye, suggesting a potentially broader role in organogenesis (13).
In this study, we aimed to investigate the mRNA expression levels of COL21A1 and COL24A1 in HNSCC tissues (typical SCC specimens) and normal mucosal tissues from the same individuals, in order to determine the correlation between their expression and disease progression.
Materials and methods
Tumor specimens and patients
Patients diagnosed with HNSCC (n=70) who were treated at the Department of Otolaryngology/Head and Neck Surgery, Hamamatsu University School of Medicine (Shizuoka, China), were included in this study. HNSCC tumor specimens were obtained from the 70 patients during surgery. Clinical information, including age, gender, tumor site, smoking status, alcohol consumption, tumor size, lymph node status and tumor stage were obtained from the clinical records. The mean patient age was 65.0 years (range, 37–85 years) and the male:female ratio was 55:15. The primary tumors were located in the oral cavity (n=24), pharynx (n=19), larynx (n=15) and paranasal sinuses (n=12). Matched pairs of head and neck tumors and adjacent normal mucosal tissues were obtained from the surgical specimens of 44 patients for initial expression screening. All patients provided written informed consent under a protocol approved by the Institutional Review Board of the Hamamatsu University School of Medicine.
RNA extraction and quantitative polymerase chain reaction (qPCR)
Frozen tissue specimens were stored at −80°C until RNA extraction. Total RNA was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany) and treated with RNase-Free DNase (Qiagen). cDNA was generated from DNase-treated total RNA using random primers (Invitrogen Life Technologies, Carlsbad, CA, USA) with Superscript II reverse transcriptase (Invitrogen Life Technologies). The primer sequences and PCR conditions are provided in Table I. All the qPCR reactions were performed with the Thermal Cycler Dice™ Real Time System TP800 (Takara, Tokyo, Japan). For each PCR evaluation, 2 μl of diluted cDNA, 12.5 μl of SYBR® Premix Ex Taq™ Perfect Real Time (Takara) and 0.5 μl of the primers were added to a final volume of 25 μl. The thermal cycler conditions were as follows: an initial denaturation step at 95°C for 10 sec, followed by 45 cycles of denaturation at 95°C for 5 sec and annealing/extension at 60°C for 30 sec (two-step reaction). Analysis was performed with Thermal Cycler Dice Real Time System TP800 software, version 1.03A (Takara) according to the manufacturer’s instructions. For comparisons between samples, the mRNA expression of the target genes was normalized to GAPDH mRNA expression.
Table I.
Primers for quantitative polymerase chain reaction.
| Primer name | Sequence | Base pairs | Temperature (°C) | Cycles | |
|---|---|---|---|---|---|
| COL21A1 | sense | 5′-GGATTAATGGGTAGTCCCGGTTTC-3′ | 149 | 60 | 45 |
| antisense | 5′-TGTCCTGGAGGCCCAATTTC-3′ | ||||
| COL22A1 | sense | 5′-GTGATTGGCAAGCGCCTCTAC-3′ | 98 | 60 | 45 |
| antisense | 5′-CAAGTCTCCAATTCTGCGTGTCTC-3′ | ||||
| COL23A1 | sense | 5′-AAGCTCCATCCGAATGTGTCTG-3′ | 108 | 60 | 45 |
| antisense | 5′-GGTAGCCATCTCGTCCTGATTG-3′ | ||||
| COL24A1 | sense | 5′-CCCAGCACGAATCTGCAAAG-3′ | 135 | 60 | 45 |
| antisense | 5′-GTCTGGCCACCAGCACTGAA-3′ | ||||
| GAPDH | sense | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 138 | 60 | 40 |
| antisense | 5′-TGGTGAAGACGCCAGTGGA-3′ | ||||
Statistical analysis
The differences in the expression levels of COL21A1, COL22A1, COL23A1 and COL24A1 mRNA between normal and malignant tissues were assessed with the Wilcoxon signed-rank test. Statistical analyses of the associations between variables were performed by the Mann-Whitney U test. The disease-free survival (DFS) was measured from the date of treatment initiation to the date of diagnosis of locoregional recurrence or distant metastasis. DFS probabilities were estimated by the Kaplan-Meier method and the log-rank test was applied to assess the significance of the differences among actuarial survival curves. A multivariate logistic regression analysis was used to identify the predictive value of prognostic factors, including age, gender, smoking status, alcohol intake, tumor stage and the expression of each gene (14,15). P<0.05 was considered to indicate a statistically significant difference. Data are expressed as the means ± SD. Statistical analyses were performed with the StatMate IV software package (ATMS Co., Ltd., Tokyo, Japan).
Results
mRNA expression levels in matched pairs of HNSCC and adjacent normal mucosal tissues
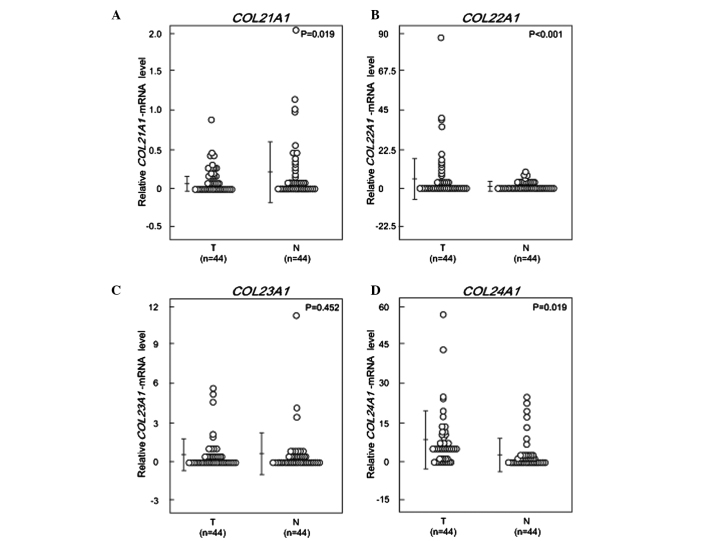
The expression levels of COL21A1, COL22A1, COL23A1 and COL24A1 mRNA were determined in 44 HNSCC and 44 paired normal mucosal tissues using qPCR. The mRNA levels were normalized to GAPDH and are shown in Fig. 1. The COL21A1 mRNA level in HNSCC tissues was found to be 4-fold lower compared to that in the paired non-cancerous mucosa (P=0.019, Wilcoxon signed-rank test) (Fig. 1A). There was a significant 15-fold increase in COL22A1 mRNA expression in HNSCC tissues compared to that in normal tissues (P<0.001) (Fig. 1B). There were no significant differences in the COL23A1 mRNA levels between cancerous and normal tissues (Fig. 1C). The levels of COL24A1 mRNA were increased by 2.5-fold in HNSCC compared to those in normal tissues (P=0.019) (Fig. 1D).
Figure 1.
COL21A1, COL22A1, COL23A1 and COL24A1 mRNA patterns in matched pairs of head and neck tumors and adjacent normal mucosal tissues. The relative expression levels of (A) COL21A1, (B) COL22A1, (C) COL23A1 and (D) COL24A1 were analyzed in the clinical samples. The changes between cancerous and normal mucosal tissues were considered to be significant, as determined by the Student’s t-test and the Wilcoxon signed-rank test. The P-values were calculated by the Wilcoxon signed-rank test. T, tumor; N, normal mucosal tissues.
COL21A1, COL22A1, COL23A1 and COL24A1 mRNA expression in 70 HNSCC primary samples
Clinical data, including age, gender, smoking status, alcohol exposure, tumor stage and survival were recorded for 70 patients. Samples from previously untreated primary tumors were tested with the same primers (Table I). COL21A1 and COL23A1 mRNA expression levels were not found to be associated with any of the clinicopathological characteristics. The expression levels of COL22A1 mRNA were significantly increased in cases with lymph node metastasis (P=0.018) and stage IV tumor (P=0.024), but did not differ significantly among tumors of varying sizes (P=0.473). Notably, the COL24A1 mRNA expression levels were significantly higher in T1–2 tumors compared with those in T3–4 tumors (P=0.045), suggesting that an increase in mRNA expression may occur early during the course of carcinogenesis (Table II and III).
Table II.
mRNA expression levels of COL21A1 and COL22A1 in HNSCC samples (n=70).
| Patient and tumor characteristics (n=70) | COL21A1 expression levels | P-valueb | COL22A1 expression levels | P-valueb |
|---|---|---|---|---|
| Age (years) | ||||
| <70 (50) | 0.12±0.20 | 0.511 | 3.17±8.73 | 0.107 |
| ≥70 (20) | 0.24±0.40 | 5.69±10.72 | ||
| Gender | ||||
| Male (55) | 0.15±0.26 | 0.511 | 3.64±8.63 | 0.492 |
| Female (15) | 0.19±0.33 | 4.79±11.83 | ||
| Smoking status | ||||
| Non-smoker (22) | 0.12±0.19 | 0.586 | 4.22±5.78 | 0.407 |
| Smoker (48) | 0.17±0.31 | 3.73±10.6 | ||
| Alcohol exposure | ||||
| No (29) | 0.17±0.32 | 0.642 | 4.11±11.06 | 0.482 |
| Yes (41) | 0.15±0.25 | 3.73±8.02 | ||
| Tumor size | ||||
| T1–2 (21) | 0.19±0.33 | 0.995 | 2.55±3.40 | 0.473 |
| T3–4 (49) | 0.14±0.25 | 4.46±10.89 | ||
| Lymph-node status | ||||
| N0 (36) | 0.16±0.29 | 0.967 | 1.61±2.84 | 0.018a |
| N+ (34) | 0.16±0.27 | 6.30±12.80 | ||
| Tumor stage | ||||
| I, II, III (35) | 0.10±0.18 | 0.760 | 1.41±2.47 | 0.024a |
| IV (35) | 0.22±0.34 | 6.36±12.61 | ||
Data are expressed as means ± standard deviation.
P<0.05.
Mann-Whitney U test.
HNSCC, head and neck squamous cell carcinoma. COL21A1, collagen type XXI α1 mRNA; COL22A1, collagen type XXII α1 mRNA.
Table III.
mRNA expression levels of COL23A1 and COL24A1 in HNSCC samples (n=70).
| Patient and tumor characteristics (n=70) | COL23A1 expression levels | P-valueb | COL24A1 expression levels | P-valueb |
|---|---|---|---|---|
| Age (years) | ||||
| <70 (50) | 1.84±3.17 | 0.200 | 22.85±29.60 | 0.104 |
| ≥70 (20) | 0.95±1.77 | 39.28±62.86 | ||
| Gender | ||||
| Male (55) | 1.63±2.32 | 0.694 | 31.98±27.12 | 0.062 |
| Female (15) | 1.58±3.01 | 26.33±45.17 | ||
| Smoking status | ||||
| Non-smoker (22) | 1.78±4.11 | 0.939 | 20.67±23.39 | 0.257 |
| Smoker (48) | 1.50±2.11 | 30.69±47.91 | ||
| Alcohol exposure | ||||
| No (29) | 1.33±2.01 | 0.716 | 25.53±33.38 | 0.703 |
| Yes (41) | 1.77±3.33 | 28.97±47.25 | ||
| Tumor size | ||||
| T1–2 (21) | 0.71±0.92 | 0.238 | 39.66±60.84 | 0.045a |
| T3–4 (49) | 1.96±3.30 | 22.35±29.75 | ||
| Lymph-node status | ||||
| N0 (36) | 1.29±1.83 | 0.577 | 23.94±23.19 | 0.930 |
| N+ (34) | 1.90±3.65 | 31.36±55.31 | ||
| Tumor stage | ||||
| I, II, III (35) | 1.38±1.80 | 0.819 | 25.12±32.42 | 0.438 |
| IV (35) | 1.79±3.64 | 29.97±49.86 | ||
Data are expressed as means ± standard deviation.
P<0.05.
Mann-Whitney U test.
HNSCC, head and neck squamous cell carcinoma. COL21A1, collagen type XXI α1 mRNA; COL23A1, collagen type XXIII α1 mRNA.
DFS
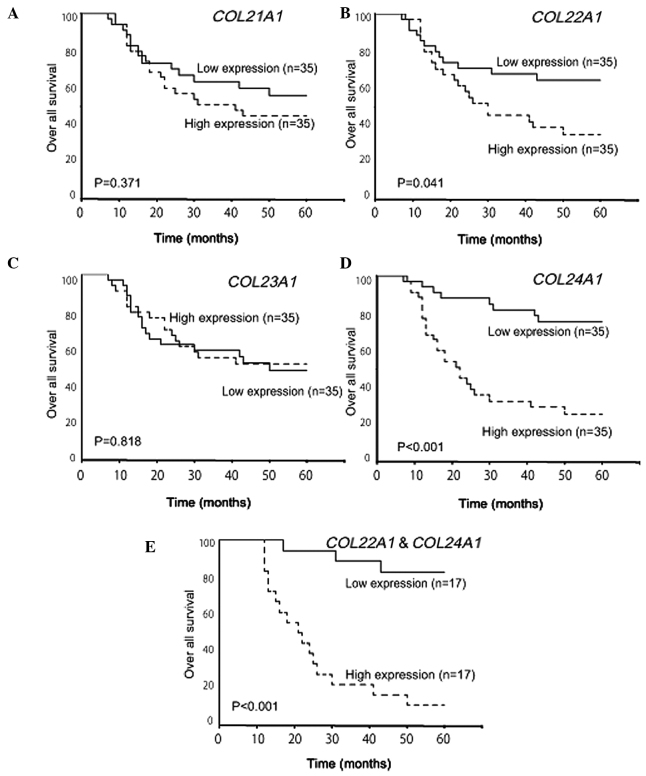
The correlation of COL21A1, COL22A1, COL23A1 and COL24A1 mRNA expression status with patient DFS was assessed with the Kaplan-Meier method. At each cut-off value (median), the data was divided into low- and high-mRNA expression groups. COL21A1 and COL23A1 mRNA expression was not found to be associated with any difference in DFS (Fig. 2A and C), whereas high COL22A1 mRNA expression levels were significantly associated with earlier disease recurrence (P=0.041, log-rank test) (Fig. 2B). Furthermore, high COL24A1 mRNA expression was associated with a statistically significant decrease in DFS (P<0.001, log-rank test) (Fig. 2D) and high COL22A1 and COL24A1 mRNA expression was associated with a DFS rate of 11.1% compared with 83.0% in the low-expression group (P<0.001, log-rank test) (Fig. 2E).
Figure 2.
Kaplan-Meier estimates of disease-free survival (DFS) of 70 patients based on collagen gene expression status. Patient DFS by (A) COL21A1, (B) COL22A1, (C) COL23A1 and (D) COL24A1 expression status. A total of 35 patients with head and neck squamous cell carcinoma (HNSCC) were classified as the high-expression (dotted line) and 35 patients as the low-expression group (solid line). The cut-off was determined by the median in 70 patients. DFS in patients by (E) COL22A1 and COL24A1 expression status: 17 HNSCC patients exhibited a high expression of the two genes (solid line), whereas 17 HNSCC patients exhibited a low expression of the two genes (dotted line); these differences were statistically significant (P<0.001, log-rank test).
Multivariate logistic regression analysis
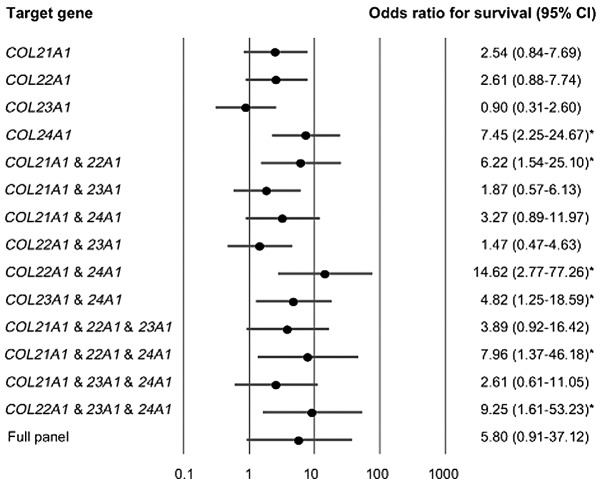
The multivariate logistic regression analysis revealed that there was an association between the estimated odds-of-recurrence and methylation of the COL21A1, COL22A1, COL23A1 and COL24A1 genes. The association between the expression of COL21A1, COL22A1 and COL23A1 in the primary tumor and the increase in the odds-of-recurrence was not considered to be significant. When COL24A1 was highly expressed in the primary tumor, the adjusted odds ratio (OR) for recurrence was 7.45 [95% confidence interval: 2.25–24.67; P=0.001]. Patients with high expression levels of COL22A1 and COL24A1 mRNA exhibited a higher OR for recurrence (OR=14.62, 95% confidence interval: 2.77–77.26; P=0.002) compared with those without high expression of this gene pair (Fig. 3).
Figure 3.
Odds ratio for overall survival determined by the multivariate logistic regression model adjusted for age (≥70 vs. <70 years), gender, smoking status, alcohol exposure and tumor stage (I, II, III vs. IV). The multivariate logistic regression analysis revealed the estimated odds of recurrence associated with the expression of COL21A1, COL22A1, COL23A1 and COL24A1 genes. The COL22A1 and COL24A1 gene pair in the primary tumor was found to be associated with the most significant odds ratios for recurrence. *P<0.05.
Discussion
To the best of our knowledge, this study is the first to quantitatively assess the changes in the expression levels of COL21A1, COL22A1, COL23A1 and COL24A1 mRNA in head and neck cancerous and normal mucosal tissues from the same individual. Collagen is a major component of the extracellular matrix and the basement membrane and provides a protective barrier that must be compromised in order for tumor cells to metastasize to distant sites (16,17). The role of the tumor microenvironment, along with extracellular matrix elements, such as laminin, fibronectin and a variety of collagens, are critical in promoting tumor growth, survival and dissemination (18,19). Several studies have suggested that the serum levels of type I and III collagen degradation products are associated with survival in patients with HNSCC (20). Endostatin, a collagen XVIII fragment, was shown to inhibit tumor progression by directly affecting the tumor cells, rather than only acting via the endothelial cells to block angiogenesis (21). Furthermore, the hypermethylation of COL1A2 was found to be an independent predictor of survival in patients with head and neck cancer (22).
Type XXI and XXII collagens are members of the FACIT collagen family, which also includes type IX, XII, XIV and XIX collagens. These collagens may serve as molecular bridges that enable the organization and stability of the extracellular matrix (3,9). Our results revealed reduced expression levels of COL21A1 mRNA in HNSCC tissues compared to those in normal control tissues from the same patient. However, when COL21A1 mRNA expression was compared according to DFS, no significant differences were observed (P=0.371; Fig. 3A). It is hypothesized that the downregulation of COL21A1 mRNA is a necessary, but insufficient event in the process of carcinogenesis.
However, COL22A1 mRNA expression was significantly upregulated in HNSCC tissues compared with that in normal tissues from the same patient. Collagen XXII is known to act as a cell adhesion ligand for skin epithelial cells and fibroblasts (9). Recently, single-nucleotide polymorphisms in the COL22A1 gene were found to be associated with serum creatinine levels and may represent potential candidates for further functional analysis (23). In this study, we observed that the upregulation of COL22A1 mRNA was associated with a statistically significant decrease in DFS. However, the results of the multivariate Cox proportional hazards regression analysis indicated that the upregulation of COL22A1 mRNA was not statistically significantly associated with DFS.
With regard to other types of cancer, a number of studies compared COL23A1 expression between malignant and normal tissues. Those studies reported that COL23A1 is upregulated in prostate and non-small-cell lung cancer (10,11,24). In prostate cancer, an increased collagen XXIII level was found to be a significant independent predictor of disease recurrence (10), whereas, in non-small-cell lung cancer, collagen XXIII was found to be expressed in ~80% of patient tissues and urine samples and was correlated with a shorter recurrence-free survival (11). In our study, COL23A1 mRNA expression was not detected in the majority of HNSCC patients, which may be explained by the differences in cell origin and molecular pathogenesis between different types of cancer.
Our data demonstrated that the expression level of COL24A1 mRNA was higher in patients with a T1–T2 tumors, compared to that in patients with T3–T4 tumors. Additionally, we observed that high levels of COL24A1 mRNA was predictive of patient DFS, suggesting that this gene may be specifically associated with recurrence and distant metastasis. The results of the multivariate logistic regression analyses demonstrated that a high expression of the COL24A1 gene was associated with a significant OR for recurrence. Patients with high expression levels of COL22A1 and COL24A1 exhibited a significantly higher OR for recurrence compared to those with a low expression of this gene pair.
Although the examination of tissue biomarkers may initially assist with HNSCC diagnosis and guide treatment decisions, non-invasive procedures are essential for screening and post-treatment monitoring. Body fluids may carry whole cells, as well as protein, DNA and RNA species that allow for the detection of cell alterations associated with cancer. As with all screening and detection modalities, an optimum combination of genes for qPCR-based HNSCC detection in salivary rinses or urine samples requires further validation in an independent cohort.
In conclusion, we demonstrated that the COL22A1 and COL24A1 mRNA expression profiles may be valuable biomarkers for the prognosis of HNSCC. Our findings may be used to identify patients with high-risk HNSCC who may benefit from adjuvant therapy and cautious observation following resection of the primary tumor. Further analyses of the collagen genes COL22A1 and COL24A1 may help elucidate their biological roles in head and neck carcinogenesis and their value as biomarkers for the early detection and prognostication of HNSCC.
Acknowledgements
The authors would like to thank Yuko Mohri for her excellent technical support. This study was supported by a Grant-in-Aid for Scientific Research (nos. 23592524, 24592594 and 25861485) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 4.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Yin Y, Zhao Y, Li AQ, Si JM. Collagen: a possible prediction mark for gastric cancer. Med Hypotheses. 2009;72:163–165. doi: 10.1016/j.mehy.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Spivey KA, Chung I, Banyard J, Adini I, Feldman HA, Zetter BR. A role for collagen XXIII in cancer cell adhesion, anchorage-independence and metastasis. Oncogene. 2012;31:2362–2372. doi: 10.1038/onc.2011.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald J, Bateman JF. A new FACIT of the collagen family: COL21A1. FEBS Lett. 2001;505:275–280. doi: 10.1016/s0014-5793(01)02754-5. [DOI] [PubMed] [Google Scholar]
- 8.Chou MY, Li HC. Genomic organization and characterization of the human type XXI collagen (COL21A1) gene. Genomics. 2002;79:395–401. doi: 10.1006/geno.2002.6712. [DOI] [PubMed] [Google Scholar]
- 9.Koch M, Schulze J, Hansen U, et al. A novel marker of tissue junctions, collagen XXII. J Biol Chem. 2004;279:22514–22521. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banyard J, Bao L, Hofer MD, et al. Collagen XXIII expression is associated with prostate cancer recurrence and distant metastases. Clin Cancer Res. 2007;13:2634–2642. doi: 10.1158/1078-0432.CCR-06-2163. [DOI] [PubMed] [Google Scholar]
- 11.Spivey KA, Banyard J, Solis LM, et al. Collagen XXIII: a potential biomarker for the detection of primary and recurrent non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:1362–1372. doi: 10.1158/1055-9965.EPI-09-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo N, Tanaka S, Gordon MK, Koch M, Yoshioka H, Ramirez F. CREB-AP1 protein complexes regulate transcription of the collagen XXIV gene (Col24a1) in osteoblasts. J Biol Chem. 2006;281:5445–5452. doi: 10.1074/jbc.M509923200. [DOI] [PubMed] [Google Scholar]
- 13.Koch M, Laub F, Zhou P, et al. Collagen XXIV, a vertebrate fibrillar collagen with structural features of invertebrate collagens: selective expression in developing cornea and bone. J Biol Chem. 2003;278:43236–43244. doi: 10.1074/jbc.M302112200. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–4236. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- 15.Katz MH. Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- 16.Nerenberg PS, Salsas-Escat R, Stultz CM. Collagen - a necessary accomplice in the metastatic process. Cancer Genomics Proteomics. 2007;4:319–328. [PubMed] [Google Scholar]
- 17.Kalluri R. Basement membranes: structure, assembly and role in tumor angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 18.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurmenniemi S, Koivula MK, Nyberg P, et al. Type I and III collagen degradation products in serum predict patient survival in head and neck squamous cell carcinoma. Oral Oncol. 2012;48:136–140. doi: 10.1016/j.oraloncology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Nyberg P, Heikkila P, Sorsa T, et al. Endostatin inhibits human tongue carcinoma cell invasion and intravasation and blocks the activation of matrix metalloprotease-2, −9, and −13. J Biol Chem. 2003;278:22404–22411. doi: 10.1074/jbc.M210325200. [DOI] [PubMed] [Google Scholar]
- 22.Misawa K, Kanazawa T, Misawa Y, et al. Hypermethylation of collagen α2 (I) gene (COL1A2) is an independent predictor of survival in head and neck cancer. Cancer Biomark. 2011;10:135–144. doi: 10.3233/CBM-2012-0242. [DOI] [PubMed] [Google Scholar]
- 23.Pattaro C, De Grandi A, Vitart V, et al. A meta-analysis of genome-wide data from five European isolates reveals an association of COL22A1, SYT1, and GABRR2 with serum creatinine level. BMC Med Genet. 2010;11:41. doi: 10.1186/1471-2350-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banyard J, Bao L, Zetter BR. Type XXIII collagen, a new transmembrane collagen identified in metastatic tumor cells. J Biol Chem. 2003;278:20989–20994. doi: 10.1074/jbc.M210616200. [DOI] [PubMed] [Google Scholar]