Abstract
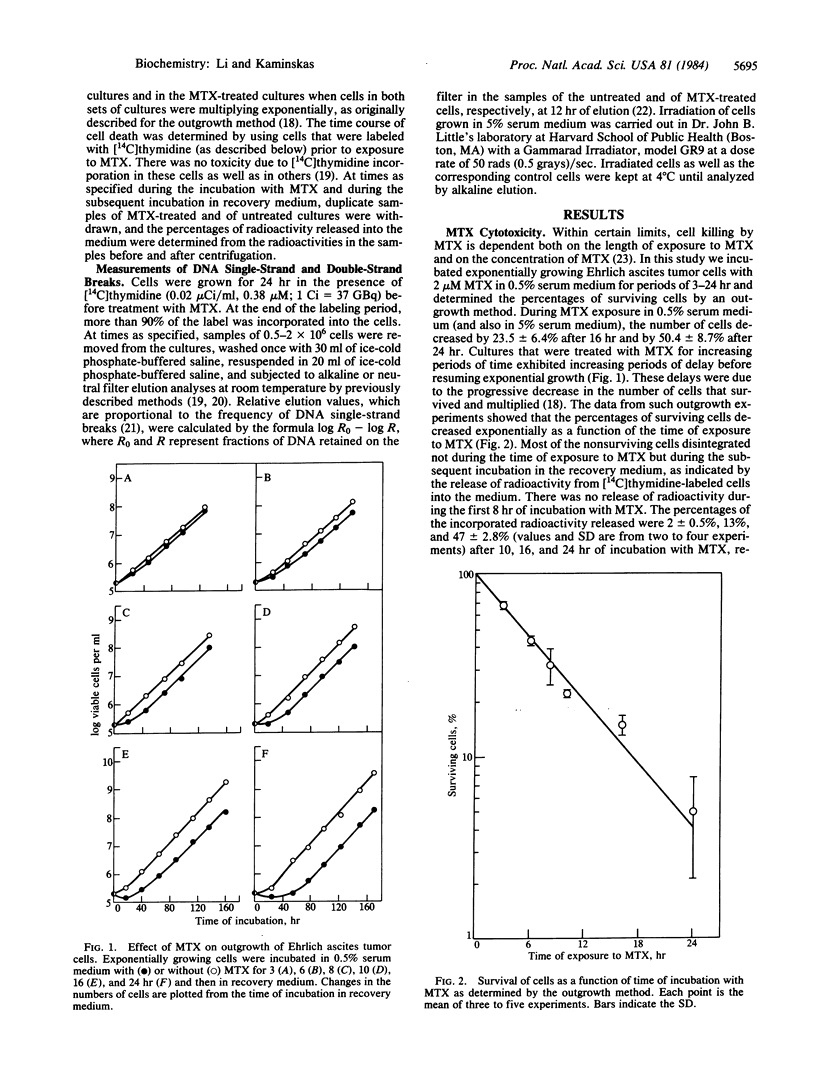
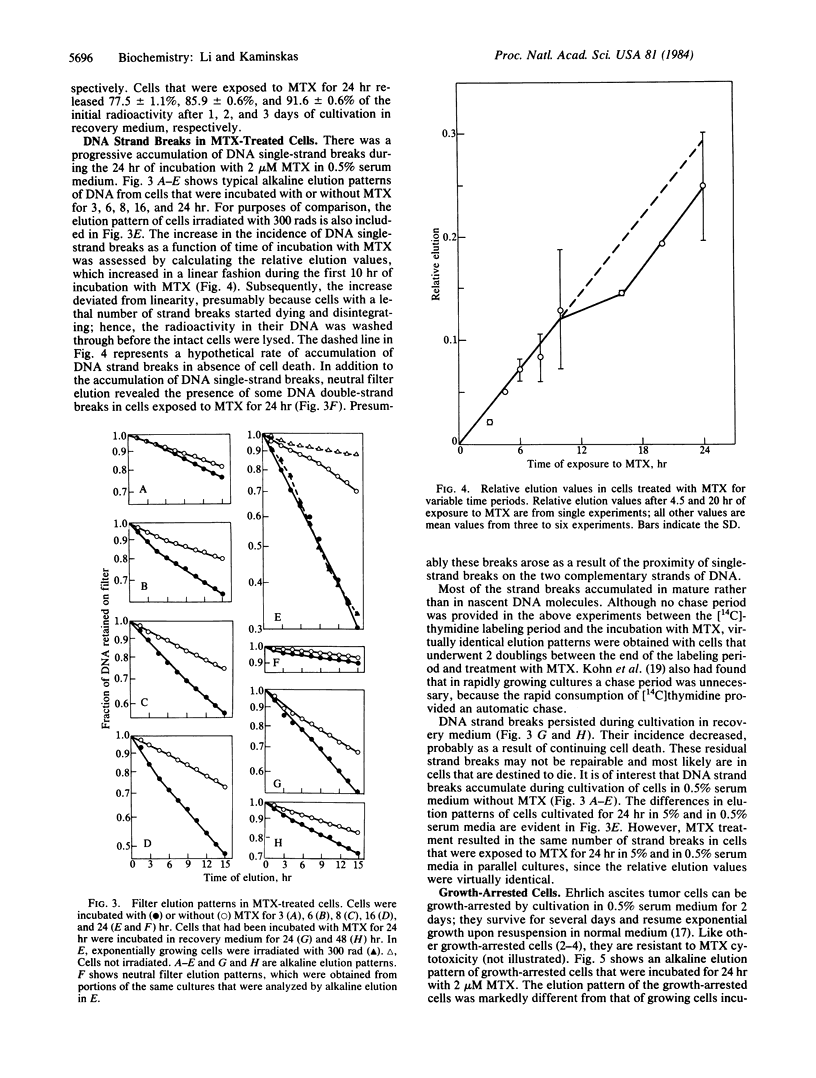
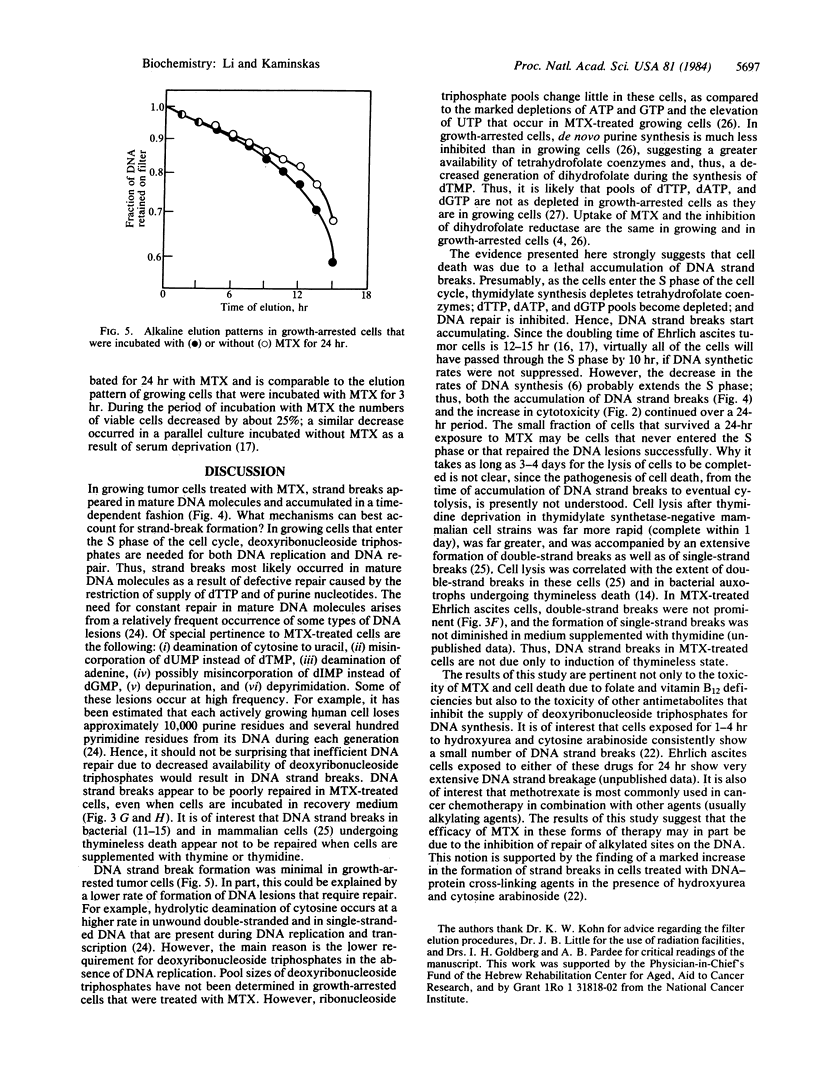
There was a progressive formation of strand breaks in mature DNA of Ehrlich ascites tumor cells that were treated with methotrexate. Cells were labeled with [14C]thymidine before incubation with methotrexate, and DNA strand breaks were measured by alkaline and by neutral filter elution methods. DNA single-strand breaks accumulated in a linear fashion as a function of time during the first 10 hr of incubation with 2 microM methotrexate. Thereafter, the accumulation of DNA strand breaks deviated from linearity because of progressive cell death. The extent of DNA strand breakage in cells that had been incubated with methotrexate for 24 hr was as high as in cells that had been irradiated with 300 rads. DNA strand breaks persisted in cells that were incubated, after exposure to methotrexate, in medium containing thymidine, hypoxanthine, and nonessential amino acids, indicating that these strand breaks were poorly repaired. Cell death commenced after 10 hr of incubation with methotrexate and continued during the following 3-4 days. These findings suggest that cell death was due to a lethal accumulation of DNA strand breaks. The formation of DNA strand breaks is probably due to inefficient DNA repair, resulting from the inhibition of syntheses of thymidylate and of purine nucleotides. The accumulation of DNA strand breaks was minimal in growth-arrested cells, which are resistant to methotrexate toxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Accumulation of DNA strand breaks during thymineless death in thymidylate synthase-negative mutants of mouse FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12448–12454. [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Cell killing studies on the mode of action of methotrexate on L-cells in vitro. Cancer Res. 1969 Apr;29(4):737–744. [PubMed] [Google Scholar]
- Bradley M. O., Dysart G., Fitzsimmons K., Harbach P., Lewin J., Wolf G. Measurements by filter elution of DNA single- and double-strand breaks in rat hepatocytes: effects of nitrosamines and gamma-irradiation. Cancer Res. 1982 Jul;42(7):2592–2597. [PubMed] [Google Scholar]
- Chu M. Y., Fischer G. A. The incorporation of 3H-cytosine arabinoside and its effect on murine leukemic cells (L5178Y). Biochem Pharmacol. 1968 May;17(5):753–767. doi: 10.1016/0006-2952(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Detection of DNA single-strand breaks produced during the repair of damage by DNA-protein cross-linking agents. Cancer Res. 1982 Jan;42(1):145–149. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Little J. B. DNA crosslinking induced by x-rays and chemical agents. Biochim Biophys Acta. 1977 Aug 16;477(4):343–355. doi: 10.1016/0005-2787(77)90253-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Single-strand breaks in bacterial DNA associated with thymine starvation. J Mol Biol. 1969 Oct 14;45(1):1–7. doi: 10.1016/0022-2836(69)90205-8. [DOI] [PubMed] [Google Scholar]
- Fridland A., Brent T. P. DNA replication in methotrexate-treated human lymphoblasts. Eur J Biochem. 1975 Sep 15;57(2):379–385. doi: 10.1111/j.1432-1033.1975.tb02311.x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniuk W. M., Fischer G. A., Bertino J. R. S-phase cells of rapidly growing and resting populations. Differences in response to methotrexate. Mol Pharmacol. 1969 Nov;5(6):557–564. [PubMed] [Google Scholar]
- Hryniuk W. M. Purineless death as a link between growth rate and cytotoxicity by methotrexate. Cancer Res. 1972 Jul;32(7):1506–1511. [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Abelson H. T. Resistance of resting 3T6 mouse fibroblasts to methotrexate cytotoxicity. Cancer Res. 1978 Aug;38(8):2408–2412. [PubMed] [Google Scholar]
- Kaminskas E. Effects of methotrexate on ribonucleotide pools in growing and in growth-arrested tumor cells and antagonism by RNA synthesis inhibitors. J Biol Chem. 1982 Apr 25;257(8):4279–4284. [PubMed] [Google Scholar]
- Kaminskas E. Inactivation of protein synthesis stimulating activity in serum by cells. J Cell Physiol. 1973 Dec;82(3):475–488. doi: 10.1002/jcp.1040820316. [DOI] [PubMed] [Google Scholar]
- Kaminskas E., Nussey A. C. Effects of methotrexate and of environmental factors on glycolysis and metabolic energy state in cultured Ehrlich ascites carcinoma cells. Cancer Res. 1978 Sep;38(9):2989–2996. [PubMed] [Google Scholar]
- Keefe D. A., Capizzi R. L., Rudnick S. A. Methotrexate cytotoxicity for L5178Y/Asn- lymphoblasts: relationship of dose and duration of exposure to tumor cell viability. Cancer Res. 1982 May;42(5):1641–1645. [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- Makino F., Munakata N. Deoxyuridine residues in DNA of thymine-requiring Bacillus subtilis strains with defective N-glycosidase activity for uracil-containing DNA. J Bacteriol. 1978 Apr;134(1):24–29. doi: 10.1128/jb.134.1.24-29.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hanawalt P. Sedimentation analysis of deoxyribonucleic acid from thymine-starved Escherichia coli. J Bacteriol. 1975 Feb;121(2):537–547. doi: 10.1128/jb.121.2.537-547.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Kutler M., Brown O. E. Antifolate-induced misincorporation of deoxyuridine monophosphate into DNA: inhibition of high molecular weight DNA synthesis in human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):917–921. doi: 10.1073/pnas.78.2.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. W., Slowiaczek P., Francis P. R., Tattersall M. H. Purine modulation of methotrexate cytotoxicity in mammalian cell lines. Cancer Res. 1982 Dec;42(12):5159–5164. [PubMed] [Google Scholar]
- Walker J. R. Thymine Starvation and Single-Strand Breaks in Chromosomal Deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1391–1392. doi: 10.1128/jb.104.3.1391-1392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K. Double-strand scission of DNA involved in thymineless death of Escherichia coli 15 TAU. Biochim Biophys Acta. 1973 Jan 19;294(1):204–213. [PubMed] [Google Scholar]


