Abstract
It is well-established that triple-negative breast cancer (TNBC) is a subtype of breast cancer, characterized by a poor prognosis and aggressive biological behavior. However, the available relevant data on TNBC in non-Western populations are limited. In order to analyze the clinicopathological and molecular biological characteristics and observe survival and prognostic factors, 972 breast cancer patients (156 of whom had TNBC) who received treatment at the First Affiliated Hospital of Medical School of Xi'an Jiaotong University and the First Hospital of China Medical University, between January, 2004 and January, 2007 were retrospectively evaluated. In the univariate analysis, tumor size, TNM stage, axillary lymph node status and recurrence or metastasis were identified as prognostic factors for 7-year disease-free survival (DFS) and overall survival (OS). Our multivariate Cox's regression analysis demonstrated that tumor size and axillary lymph node status were significant prognostic factors for 7-year DFS and OS. Notably, tumor subgroup (TNBC vs. non-TNBC) was a significant prognostic factor associated with 7-year DFS and OS in breast cancer. It was suggested that TNBC exhibited a worse 7-year survival compared with that in non-TNBC patients, most likely due to its more aggressive behavior and insensitivity to specific therapy.
Keywords: clinicopathological characteristics, prognostic analysis, survival analysis, triple-negative breast cancer
Introduction
Breast cancer is the most common malignancy among females (1) and the second most common cause of cancer-related mortality behind lung cancer (2). Due to changes in lifestyle, the incidence of breast cancer, which is currently on the increase in developing countries, including China, has increased significantly. Based on DNA microarray techniques, breast cancer is classified into five subtypes: luminal A, luminal B, normal breast-like, human epidermal growth factor receptor 2 (HER2/neu)-overexpressing and basal-like (3). The basal-like and normal breast-like subtypes, which are immunohistochemically characterized by the lack of expression of the estrogen receptor (ER), progesterone receptor (PgR) and HER2, are defined as triple-negative breast cancer (TNBC) (4).
TNBC is a distinct breast cancer subtype, which accounts for ~10–17% of all breast carcinomas (5). TNBC, usually occurring in young females, is generally considered to exhibit an aggressive clinical behavior and poor prognosis, due to the fact that it is insensitive to endocrine and targeted therapy (6). Furthermore, the TNBC subgroup is associated with a higher risk of distant recurrence and mortality compared to its non-triple-negative counterparts, particularly during the first 3–5 years of follow-up (6). However, few studies have been conducted among non-Western populations (7) and the information on the Asian TNBC subtype remains confusing and limited (8). Kurebayashi et al (9) reported that Japanese patients with TNBC are mostly superimposable for disease-free survival (DFS) and overall survival (OS). Lin et al (10) indicated that Taiwanese TNBC patients exhibited a better 5-year OS compared with HER2-positive patients. Yin et al (7) revealed that recurrence-free survival in Chinese TNBC patients was superior to that of HER2-positive patients. In order to elucidate whether there are regional differences among patients in different Chinese cities and whether they differ from Western populations, it is critical to further delve into the clinical characteristics and prognosis of TNBC in mainland Chinese patients.
In the present study, a retrospective analysis was perfomed on the clinicopathological characteristics of TNBC patients who received conventional treatment at the Department of Oncology, First Affiliated Hospital of Medical School of Xi'an Jiaotong University and the Department of Breast Surgery, General Surgery, First Hospital of China Medical University. In this study, the aim was to determine the clinicopathological characteristics of this breast cancer subtype, evaluate the survival of patients treated by the currently available conventional methods and analyze the prognostic factors. The internal information on clinical TNBC cases may elucidate the implications of the underlying distinction in tumor biology from other breast cancer subgroups.
Materials and methods
Patient characteristics
Between January 1, 2004 and December 31, 2007, a total of 972 female patients with breast cancer confirmed by surgery and pathological examination in the Department of Oncology, First Affiliated Hospital of Medical School of Xi'an Jiaotong University and the Department of Breast Surgery, General Surgery, First Hospital of China Medical University, were retrospectively investigated. The study protocol was approved by the Institutional Review Board of the two participating hospitals. The baseline data included age, tumor characteristics (including, tumor size, lymph node metastasis, distant metastasis, tumor grade, pathological stage, ER/PgR/HER2 expression and histological type) and treatment modalities. The quality of the cancer registry database was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Medical School of Xi'an Jiaotong University.
Tumor characteristics
The pathological diagnosis was in accordance with the histological classification of tumors developed by the World Health Organization and clinical staging was based on the TNM staging of breast cancer developed by the American Joint Committee on Cancer. The immunohistochemical expression of ER, PgR and HER2 was detected with the streptavidin peroxidase conjugated method (SP method) using 5-μm serial sections. The primary antibosdies used were as follows: rabbit monoclonal anti-human ER antibody, rabbit monoclonal anti-human PgR antibody (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) and rabbit anti-human HER2 antibody (Roche Diagnostics, Shanghai, China). The secondary antibody used was HRP-ploymer anti mouse/rabbit IgG (Fuzhou Maixin Biotechnology Development Co., Ltd.). Cells accounting for ≥10% were considered as positive expression. The size of the primary breast tumor was determined by using dual-track measurement.
Treatment
Routine preoperative chemotherapy was administered to 116 out of the 972 cases (11.9%). All patients underwent surgical treatment of breast cancer. The postoperative adjuvant therapy was administered based on the recommendations of the National Comprehensive Cancer Network guidelines. In our study, the majority of the patients received the CMF chemotherapy regimen: cyclophosphamide, 600 mg/m2; methotrexate, 40 mg/m2; and 5-FU, 500 mg/m2. In certain high-risk patients, taxanes (paclitaxel, docetaxel) were added to 5-FU, epirubicin and cyclophosphamide (FEC regimen).
Follow-up
The patients were followed-up from the first day following surgery to tumor recrudescence, metastasis or death from any cause. Our final follow-up deadline was December 31, 2011. The required information on therapeutic effect and prognosis was collected mainly through letters, telephonical communication or outpatient review. Local recurrence was defined as clinical or histological recurrence in the ipsilateral breast, chest wall or axillary lymph nodes. Distant metastasis referred to the clinical and imaging identification of distant metastatic lesions. DFS was defined as the time period from the first day after surgery to the first local recurrence or distant metastasis. OS was measured from the first day of follow-up.
Statistical analysis
The statistical software SPSS 17.0 (SPSS Inc., Chicafo, IL, USA) was used to analyze the collected data. Data were expressed as means ± SD for continuous variables. An independent t-test was used for the comparison of continuous variables. Categorical variables were assessed using the Chi-square test when appropriate. P<0.05 was considered to indicate a statistically significant difference. A cumulative survival analysis of breast cancer patients was performed with the Kaplan-Meier method and the log-rank test was used for single-factor analysis. The multivariate analysis was performed using the Cox proportional hazards regression model.
Results
Clinical characteristics
All included patients were followed up for 6–84 months, without any losses. The patients in the TNBC group accounted for 16.05% (156/972). The average age of the 156 TNBC patients was 51.7 years, with a median age of 52.5 years. The patients with TNBC had a similar age at diagnosis with non-TNBC patients (P=0.943). The TNBC patients prior to menopause accounted for 43.59% (68/156), which was not different from the non-TNBC group.
In the TNBC group, the major pathological type was infiltrative ductal carcinoma (82.05%), followed by infiltrative lobular (5.77%) and medullary carcinoma (5.13%), which was a distribution similar to that of the non-TNBC group (P=0.995). The tumor diameter in TNBC patients was commonly 2–5 cm, accounting for 60.26% (94/156), with an average tumor diameter of 3.1 cm. The percentage of tumor grade III was 51.92% in TNBC, which was significantly higher compared with that of the non-TNBC group (P=0.002). The cases with tumor stage ≥II accounted for 85.90% (134/156). The percentage of axillary lymph node-positive cases was lower in the TNBC compared to that in the non-TNBC group (P=0.009). The number of positive lymph nodes was most commonly 1–3, accounting for 45.45% (25/156), with 4–9 accounting for 34.55% (19/156) and ≥10 accounting for 20% of the cases (11/156). Clinicopathological data are summarized in Table I. The Chi-square test revealed that there was no correlation between axillary lymph node metastasis and the tumor size in the TNBC group (P=0.536, Table II).
Table I.
Clinicopathological characteristics of patients with breast cancer according to tumor subgroup.
| Characteristics | Total (n=972) | Subgroup, n (%) | P-value | |
|---|---|---|---|---|
|
| ||||
| TNBC (n=156) | Non-TNBC (n=816) | |||
| Mean age at diagnosis (years) | 51.7 | 51.5 | 0.943 | |
| Menopausal status (%) | 0.887 | |||
| Prior to menopause | 416 | 68 (43.59) | 348 (42.65) | |
| Following menopause | 556 | 88 (56.41) | 468 (57.35) | |
| Pathological type | 0.995 | |||
| Invasive ductal carcinoma | 790 | 128 (82.05) | 662 (81.13) | |
| Invasive lobular carcinoma | 57 | 9 (5.77) | 48 (5.88) | |
| Medullary carcinoma | 51 | 8 (5.13) | 43 (5.27) | |
| Other | 74 | 11 (7.05) | 63 (7.72) | |
| Tumor size (cm) | 0.971 | |||
| ≤2 | 241 | 38 (24.36) | 203 (24.88) | |
| 2–5 | 592 | 94 (60.26) | 498 (61.03) | |
| >5 | 139 | 24 (15.38) | 115 (14.09) | |
| Grade (%) | 0.002 | |||
| I–II | 646 | 75 (48.08) | 571 (69.98) | |
| III | 326 | 81 (51.92) | 245 (30.02) | |
| TNM stage | 0.752 | |||
| I | 144 | 22 (14.10) | 122 (14.95) | |
| II | 300 | 54 (34.62) | 246 (30.15) | |
| III–IV | 528 | 80 (51.28) | 448 (54.90) | |
| Axillary lymph node status | 0.009 | |||
| Negative | 534 | 109 (69.87) | 425 (52.08) | |
| Positive | 438 | 47 (30.13) | 391 (47.92) | |
| Metastatic lymph nodes | 0.891 | |||
| 1–3 | 206 | 25 (45.45) | 181 (48.14) | |
| 4–9 | 139 | 19 (34.55) | 120 (31.91) | |
| ≥10 | 86 | 11 (20.00) | 75 (19.95) | |
| Family history | 0.969 | |||
| Negative | 883 | 141 (90.38) | 742 (90.93) | |
| Breast cancer | 63 | 10 (6.41) | 53 (6.50) | |
| Other tumor | 26 | 5 (3.21) | 21 (2.57) | |
TNBC, triple-negative breast cancer.
Table II.
Association between lymph node metastasis and primary tumor size in the TNBC group.
| Lymph node metastasis | |||||
|---|---|---|---|---|---|
|
|
|||||
| T | n | Positive | Negative | χ2 | P-value |
| T1 | 38 | 16 | 22 | - | - |
| T2 | 98 | 40 | 58 | 6.349 | 0.536 |
| T3 | 20 | 14 | 6 | - | - |
| Total | 156 | 70 | 86 | - | - |
TNBC, triple-negative breast cancer.
Recurrence and metastasis
In the TNBC group, a DFS was reported in 120 cases (76.92%), whereas 36 patients (36/156, 23.08%) developed recurrence and metastasis. Common distant metastatic sites were the lung, bone, brain, liver, supraclavicular lymph nodes and pleura. There were significant differences in the metastatic sites between the TNBC and non-TNBC groups (P<0.001). Compared with non-TNBC, TNBC patients exhibited a higher propensity for visceral and brain metastasis (42.11 vs. 6.25%) and a lower incidence of bone metastasis (15.79 vs. 62.50%). Related data are presented in Table III.
Table III.
Recurrence or metastatic status of breast cancer patients according to tumor subgroup (TNBC vs. non-TNBC).
| Subgroup, n (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | TNBC | Non-TNBC | Total (n) | P-value |
| Recurrence or metastasis | 0.866 | |||
| Yes | 36 (23.08) | 180 (22.06) | 216 | |
| No | 120 (76.92) | 636 (77.94) | 756 | |
| Metastatic site | <0.001 | |||
| Bone | 3 (15.79) | 50 (62.50) | 53 | |
| Lung | 1 (5.26) | 2 (2.50) | 3 | |
| Liver | 4 (21.05) | 10 (12.50) | 14 | |
| Brain | 8 (42.11) | 5 (6.25) | 13 | |
| Other | 1 (5.26) | 7 (8.75) | 8 | |
| Multiple | 2 (10.53) | 6 (7.50) | 8 | |
TNBC, triple-negative breast cancer.
Survival analysis
The univariate Cox's regression analysis identified tumor subgroup (TNBC or non-TNBC) as an independent prognostic factor associated with 7-year DFS and OS. Furthermore, tumor size, TNM stage, axillary lymph node status and recurrence or metastasis were also found to exert a statistically significant effect on DFS and OS; however, regarding other variables, including age, menstrual status, pathological type, tumor grade, number of metastatic lymph nodes, metastatic site and family history of breast cancer, there were no significant differences in DFS and OS (Table IV).
Table IV.
Prognostic factors for disease-free survival and overall survival of breast cancer patients in univariate Cox regression analysis.
| Disease-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (≤50 vs. >50 years) | 1.029 | 0.697–1.521 | 0.884 | 1.028 | 0.695–1.519 | 0.891 |
| Menopausal status (prior to vs. after) | 1.016 | 0.684–1.509 | 0.937 | 1.017 | 0.685–1.511 | 0.933 |
| Pathological type (invasive ductal carcinoma vs. other) | 1.019 | 0.822–1.263 | 0.865 | 1.018 | 0.821–1.261 | 0.873 |
| Tumor size (≤2 vs. >2 cm) | 0.099 | 0.038–0.257 | <0.0001 | 0.099 | 0.038–0.257 | <0.0001 |
| Grade (I–II vs. III) | 1.202 | 0.804–1.798 | 0.371 | 1.198 | 0.801–1.792 | 0.379 |
| TNM staging (I–II vs. III–IV) | 1.374 | 1.024–1.841 | 0.034 | 1.371 | 1.023–1.838 | 0.035 |
| Axillary lymph node status (negative vs. positive) | 2.466 | 1.636–3.715 | <0.0001 | 2.438 | 1.618–3.673 | <0.0001 |
| Metastatic lymph nodes (<4 vs. ≥4) | 1.096 | 0.931–1.291 | 0.271 | 1.098 | 0.933–1.294 | 0.260 |
| Recurrence or metastasis (yes vs. no) | 0.639 | 0.420–0.972 | 0.036 | 0.642 | 0.422–0.976 | 0.038 |
| Metastatic site (single vs. multiple) | 0.976 | 0.784–1.215 | 0.828 | 0.976 | 0.784–1.215 | 0.825 |
| Family history (positive vs. negative) | 0.948 | 0.568–1.582 | 0.837 | 0.947 | 0.567–1.580 | 0.834 |
| Tumor subgroups (TNBC vs. non-TNBC) | 0.236 | 0.159–0.350 | <0.0001 | 0.232 | 0.157–0.344 | <0.0001 |
HR, hazard ratio; CI, confidence interval; TNBC, triple-negative breast cancer.
To further analyze the possible factors affecting the prognosis of patients with breast cancer, the multifactor Cox proportional hazards regression model was used. The incorporated factors included tumor size, TNM stage, axillary lymph node status, tumor subgroups and metastatic or recurrence status. The multivariate analysis demonstrated that tumor subgroup (TNBC or non-TNBC) was statistically significant in 7-year DFS and OS. Furthermore, tumor size and axillary lymph node status were independent prognostic factors for TNBC and non-TNBC (Table V).
Table V.
Prognostic factors for disease-free survival and overall survival of breast cancer patients in multivariate Cox regression analysis.
| Disease-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Tumor size (≤2 vs. >2 cm) | 0.115 | 0.044–0.302 | <0.0001 | 0.113 | 0.043–0.298 | <0.0001 |
| TNM stage (I–II vs. III–IV) | 1.252 | 0.939–1.668 | 0.126 | 1.243 | 0.932–1.658 | 0.138 |
| Axillary lymph node status (negative vs. positive) | 2.897 | 1.897–4.423 | <0.0001 | 2.859 | 1.873–4.362 | <0.0001 |
| Recurrence or metastasis (yes vs. no) | 0.649 | 0.426–0.988 | 0.044 | 0.661 | 0.434–1.006 | 0.054 |
| Tumor subgroups (TNBC vs. non-TNBC) | 0.190 | 0.127–0.283 | <0.0001 | 0.188 | 0.126–0.280 | <0.0001 |
HR, hazard ratio; CI, confidence interval; TNBC, triple-negative breast cancer.
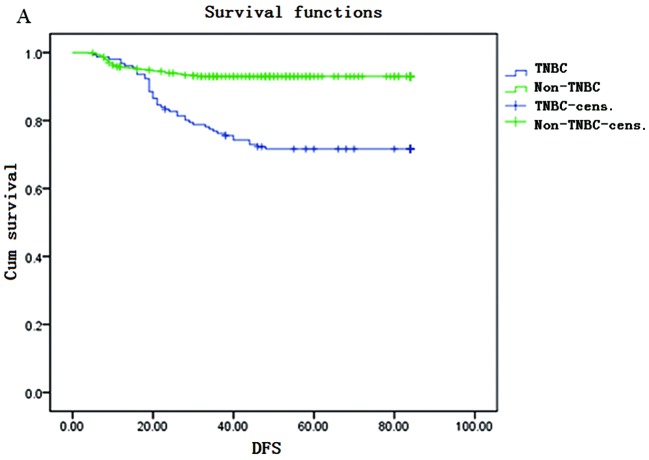
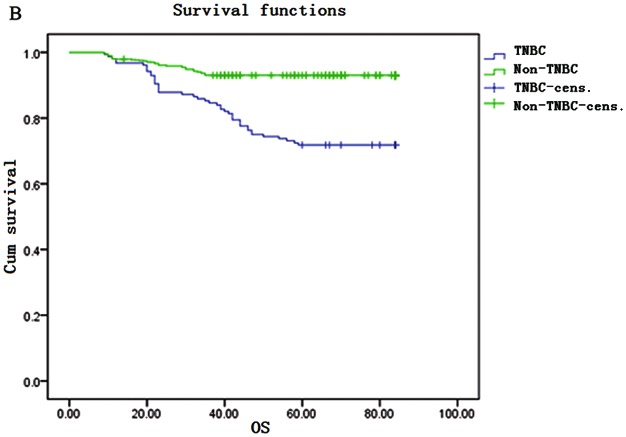
The Kaplan-Meier survival curves are shown in Fig. 1. The DFS rate for TNBC patients was 98, 76, 67 and 64% in the 1st, 3rd, 5th and 7th years, respectively. The OS rate for TNBC patients was 97, 85, 72 and 68% in the 1st, 3rd, 5th and 7th years, respectively. DFS and OS rates were significantly lower in the TNBC compared to those in the non-TNBC group in the 3rd, 5th and 7th years; however, in the 1st year, the DFS rate of the TNBC was higher compared with that in the non-TNBC group (98 vs. 95%).
Figure 1.
Kaplan-Meier survival curves. (A) Disease-free survival (DFS) and (B) overall survival (OS) in triple-negative breast cancer (TNBC) and non-TNBC patients. Cens., censored.
Discussion
TNBC is a special subtype of breast cancer classified according to cell morphology and cell surface receptors. Patients with TNBC exhibit a poor prognosis due to its aggressive biological behavior and lack of effective treatment, as this type of cancer is insensitive to targeted and endocrine therapy. In order to improve the outcomes for TNBC patients, ongoing studies on TNBC are currently conducted clinically and experimentally. In this study, we selected a cohort of 972 patients in order to analyze the clinicopathological characteristics and prognosis of TNBC patients in China. The included patients were selected from two different hospitals in different cities, in order to enhance the reliability of our results.
Numerous studies have demonstrated that premenopausal African-American females were more prone to develop TNBC. Carey et al (11) reported that the morbidity rate of the TNBC subtype among African-American breast cancer patients <50 years old may be as high as 39%, whereas it is only 16% among Caucasian females and 14% among post-menopausal African females. In our study, 16.5% of the included patients had TNBC and it was demonstrated that age and menopausal status did not significantly affect the TNBC incidence in China, which is in accordance with the findings of previous studies, reporting that the prevalence of TNBC among non-African female breast cancer patients ranges between 10 and 17% and it is lower compared to that among pre-menopausal African females (12–14).
Kandel et al (15) demonstrated that the average tumor size of TNBC is 2 cm and 50% of TNBC patients develop lymph node metastases. With regard to pathological characteristics, this type of breast cancer may exhibit three histological grades. However, in the present study, we observed that a tumor size of 2–5 cm represented the largest percentage in the TNBC group and axillary lymph node metastases accounted for 30.13%. Histological grade III accounted for the largest percentage in the TNBC group (P=0.002). It is hypothesized that TNBC in China may differ from that encountered in other countries.
Haffty et al (16) reported that TNBC exhibits a higher proportion of positive family history of breast cancer. In this study, 10 of the 156 TNBC patients (6.41%) had a family history of breast cancer, which was not significantly higher when compared with the non-TNBC subgroup. Furthermore, single- and multifactor analyses did not suggest that TNBC was correlated with a family history of breast cancer. Zhang et al (17) reported that, in China, family history was not statistically different between the TNBC and non-TNBC groups (P=0.180), which is in agreement with our findings. Therefore, we may infer that triple-negative status is not associated with an increased hereditary risk in Chinese females.
The current investigation of the correlation between tumor size and lymph node metastasis has produced varying results. In a controlled study (13), it was suggested that TNBC exhibits a higher tendency for lymph node metastasis, compared to other types of breast cancer. By contrast, other studies have demonstrated that there is no difference in the frequency of lymph node metastasis in TNBC (12,16,18). In an investigation of 292 cases of breast cancer specimens, Haffty et al (16) suggested that tumor size was not associated with lymph node metastasis. In this study, for tumor sizes <2 cm, the lymph node metastatic rate was as high as 42.1%, whereas it was 70.0% for tumor sizes >5 cm (P>0.05). Therefore, tumor size was not found to correlate with lymph node metastasis, which is in agreement with several studies in China and abroad (12,16,18).
In the univariate Cox's regression analysis, our results indicated that tumor size, TNM stage, axillary lymph node status and recurrence or metastasis were prognostic factors for 7-year DFS and OS. Our multivariate Cox's regression analysis demonstrated that tumor size and axillary lymph node status were the main prognostic indicators for 7-year DFS and OS. These findings are in accordance with those of previous studies (8,19). Tumor subgroup (TNBC or non-TNBC) was identified as a significant prognostic factor of breast cancer in the univariate and multivariate survival analysis.
TNBC is prone to local recurrence and distant metastasis. Dent et al (13) observed that, in the 5th year of follow-up, the frequency of distant metastasis was significantly higher among TNBC compared to that among non-TNBC patients (33.9 and 22.4%, respectively) and the risk of distant metastasis was higher in the TNBC group (relative risk=2.6). There was a gradual increase in the risk of distant metastasis in the TNBC group, with a peak in the 2nd and 3rd years, followed by a rapid decline, with a lower risk in the 5th year and no distant metastasis in the 8th year of follow-up. In non-TNBC patients, the risk of distant metastasis during follow-up appeared to remain constant over the follow-up period. TNBC exhibits a propensity for organ-specific metastasis. Rakha et al (12)reported that TNBC is more likely to metastasize to the spinal cord, brain, meninges, liver and lungs, but rarely to the bones. A study conducted at the M.D. Anderson Cancer Center also reported that TNBC was associated with a higher risk of visceral and a lower risk of bone metastases (20). TNBC has a higher risk of local recurrence or distant metastasis following the final diagnosis. This suggests that distant metastasis may exhibit a certain organ tendency in TNBC (21–23) and the specific target organ metastasis may be associated with its specific gene expression (24–26). Consistently, our study demonstrated that, compared to non-TNBC patients, TNBC patients had a higher propensity for visceral metastasis (liver, 21.05 and lung, 5.26%), as well as brain metastasis (42.11%), with a lower incidence of bone metastasis (15.79 vs. 62.50%).
The TNBC subgroup exhibited a more aggressive clinical course and a higher risk of recurrence and mortality when compared to the non-TNBC group, with a 5- and 7-year survival rate of 72 and 68%, respectively. Non-Hispanic females of African descent exhibited the worst prognosis, with a 5-year survival rate of only 14%. Khan et al (20) reported that of 282 African-American female patients with a median age of 57 years, TNBC accounted for 30% of the cases. In this study, in the 1st year, the DFS rate for TNBCs was 98%, which was higher compared with that in the non-TNBC group. This may be attributed to the currently accepted view that TNBC appears to be more sensitive to chemotherapy compared to non-TNBC (27); thus, TNBC patients may achieve higher short-term DFS rates. However, TNBC patients have a worse OS (28) and tend to relapse sooner compared to patients with other breast cancer subtypes. This is mainly due to the shortened disease-free period rendering the tumor more aggressive. Yin et al (7) reported that, in multivariate analysis, TNBC exhibited a significantly increased recurrence rate within 2 years after surgery, which is inconsistent with our findings. TNBC patients have a worse 7-year DFS and OS compared to non-TNBC patients, with the risk of any recurrence increasing sharply from the date of diagnosis, peaking at 1–2 years and decreasing quickly thereafter, which was similar to the findings reported by Dent et al (13).
The Cox model regression analysis results revealed that axillary lymph node status was an independent prognostic factor for TNBC (P=0.001). Our results were in accordance with the majority of the previously published literature.
In conclusion, TNBC has its own unique clinical pathological and molecular characteristics. Due to the lack of specific treatment guidelines following surgery, despite the administration of chemotherapy and radiotherapy, the prognosis of TNBC patients remains poor. Efforts are currently focused on developing a specific therapy for TNBC. It was reported that TNBC may have a specific signal transduction pathway which is crucial in the occurrence and development of breast cancer (29–31), which may provide a novel approach for clinical treatment based on molecular markers and investigation of this specific pathway.
Acknowledgements
The authors would like to thank Professor Borong Pan, Outpatient Department of Oncology, Cancer Institute, Fourth Military Medical University (Xi'an, China), for offering advice regarding the modifications to this study.
Abbreviations
- TNBC
triple-negative breast cancer
- ER
estrogen receptor
- PgR
progesterone receptor
- HER2
human epidermal growth factor receptor 2
References
- 1.Alkis N, Durnali AG, Arslan UY, et al. Optimal timing of adjuvant treatment in patients with early breast cancer. Med Oncol. 2011;28:1255–1259. doi: 10.1007/s12032-010-9566-4. [DOI] [PubMed] [Google Scholar]
- 2.Jha K, Shukla M, Pandey M. Survivin expression and targeting in breast cancer. Surg Oncol. 2012;21:125–131. doi: 10.1016/j.suronc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 5.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 6.Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19:312–321. doi: 10.1016/j.breast.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Yin WJ, Lu JS, Di GH, et al. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat. 2009;115:325–333. doi: 10.1007/s10549-008-0096-0. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Chien SY, Kuo SJ, et al. A 10-year follow-up of triple-negative breast cancer patients in Taiwan. Jpn J Clin Oncol. 2012;42:161–167. doi: 10.1093/jjco/hyr196. [DOI] [PubMed] [Google Scholar]
- 9.Kurebayashi J, Moriya T, Ishida T, et al. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast. 2007;16(Suppl 2):S72–S77. doi: 10.1016/j.breast.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Lin C, Chien SY, Chen LS, Kuo SJ, Chang TW, Chen DR. Triple negative breast carcinoma is a prognostic factor in Taiwanese women. BMC Cancer. 2009;9:192. doi: 10.1186/1471-2407-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 12.Rakha EA, El-Sayed ME, Green AR, Lee AH, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 13.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 14.Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandel MJ, Stadler Z, Masciari S, et al. Prevalence of BRCA1 mutations in triple negative breast cancer (BC) J Clin Oncol. 2006;24:508. [Google Scholar]
- 16.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Hao C, Dong G, Tong Z. Analysis of clinical features and outcome of 356 triple-negative breast cancer patients in China. Breast Care (Basel) 2012;7:13–17. doi: 10.1159/000336539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischkowitz M, Brunet JS, Begin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura R, Arima N. Is triple negative a prognostic factor in breast cancer? Breast Cancer. 2008;15:303–308. doi: 10.1007/s12282-008-0042-3. [DOI] [PubMed] [Google Scholar]
- 20.Khan AM, Sabnani I, Tsang P, et al. Triple-negative early-stage breast cancer in African American women: A fuel to the fire. J Clin Oncol. 2008;26:11039. [Google Scholar]
- 21.Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 22.Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2858. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 26.Fadare O, Wang SA, Hileeto D. The expression of cytokeratin 5/6 in invasive lobular carcinoma of the breast: evidence of a basal-like subset? Hum Pathol. 2008;39:331–336. doi: 10.1016/j.humpath.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan HG, Malmgren JA, Atwood M. T1N0 triple negative breast cancer: risk of recurrence and adjuvant chemotherapy. Breast J. 2009;15:454–460. doi: 10.1111/j.1524-4741.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 29.Yin WJ, Lu JS, Di GH, Lin YP, Zhou LH, Liu GY, Wu J, Shen KW, Han QX, Shen ZZ, Shao ZM. Clinicopathological features of the triple-negative tumors in Chinese breast cancer patients. Breast Cancer Res Treat. 2009;115:325–333. doi: 10.1007/s10549-008-0096-0. [DOI] [PubMed] [Google Scholar]
- 30.Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010;23:123–133. doi: 10.1038/modpathol.2009.145. [DOI] [PubMed] [Google Scholar]
- 31.Shah SN, Cope L, Poh W, et al. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PLoS One. 2013;8:e63419. doi: 10.1371/journal.pone.0063419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maire V, Baldeyron C, Richardson M, et al. TTK/hMPS1 is an attractive therapeutic target for triple-negative breast cancer. PLoS One. 2013;8:e63712. doi: 10.1371/journal.pone.0063712. [DOI] [PMC free article] [PubMed] [Google Scholar]